Abstract
In humans, the pathways of memory T-cell differentiation remain poorly defined. Recently, adoptive cell transfer (ACT) of tumor-reactive T lymphocytes to metastatic melanoma patients after nonmyeloablative chemotherapy has resulted in persistence of functional, tumor-reactive lymphocytes, regression of disease, and induction of melanocyte-directed autoimmunity in some responding patients. In the current study, longitudinal phenotypic analysis was performed on melanoma antigen-specific CD8+ T cells during their transition from in vitro cultured effector cells to long-term persistent memory cells following ACT to 6 responding patients. Tumor-reactive T cells used for therapy were generally late-stage effector cells with a CD27Lo CD28Lo CD45RA- CD62 ligand- (CD62L-) CC chemokine receptor 7- (CCR7-) interleukin-7 receptor αLo (IL-7RαLo) phenotype. After transfer, rapid up-regulation and continued expression of IL-7Rα in vivo suggested an important role for IL-7R in immediate and long-term T-cell survival. Although the tumor antigen-specific T-cell population contracted between 1 and 4 weeks after transfer, stable numbers of CD27+ CD28+ tumor-reactive T cells were maintained, demonstrating their contribution to the development of long-term, melanoma-reactive memory CD8+ T cells in vivo. At 2 months after transfer, melanoma-reactive T cells persisted at high levels and displayed an effector memory phenotype, including a CD27+ CD28+ CD62L- CCR7- profile, which may explain in part their ability to mediate tumor destruction. (Blood. 2005;105:241-250)
Introduction
In the initial phase of the adaptive immune response, selection, differentiation, and expansion of antigen-specific naive T cells occurs, followed by a contraction of effector cell numbers and maintenance of a relatively small memory T-cell population.1,2 Considerable effort has been made to characterize definitive pathways of CD8+ T-cell differentiation in humans as well as the precise mechanisms involved in maintenance of antigen-specific CD8+ T-cell memory. In murine studies, longitudinal gene expression profiling of viral epitope-specific T cells in transition from effector to memory cell stages has identified numerous differentially expressed genes, including genes encoding molecules involved in lymphocyte homeostasis and lymphoid organ homing.3 A subset of antigen-specific effector cells expressing high levels of interleukin-7 receptor α (IL-7Rα, CD127) has been shown to preferentially yield long-lived memory CD8+ T cells,4 a finding consistent with the role of IL-7 as a mediator of memory CD8+ T-cell homeostatic maintenance in vivo.5-12 In addition, memory CD8+ T cells can be maintained as 2 phenotypically distinguishable and functionally distinct subsets in mice and humans.13,14 Central memory T cells (CC chemokine receptor 7+ [CCR7+] CD62 ligand+ [CD62L+]) preferentially home to lymph nodes, produce IL-2, and lack immediate effector function, while effector memory T cells (CCR7- CD62L-) circulate in the peripheral blood and tissues, express perforin, and display immediate effector function.13-15
Discrimination between distinct stages of human CD8+ T-cell differentiation has often relied upon the cell surface expression of CD27 and CD28, costimulatory molecules of distinctive function.16,17 Interaction of CD28 with CD80 (B7-1) and CD86 (B7-2) on antigen-presenting cells (APCs) amplifies T-cell receptor (TCR)-mediated T-cell proliferation and activation through a series of direct effects.18 However, prolonged TCR stimulation results in the subsequent down-regulation of CD28 expression.19 Interaction of CD27 with its ligand, CD70, augments TCR-stimulated proliferation of CD8+ T cells.20 Changes in CD27 expression are less well characterized, although expression is known to increase on naive T cells upon TCR stimulation.21,22 Prolonged T-cell stimulation results in loss of CD27 expression, which appears to be irreversible, and the loss of CD27 expression is thought to define a population of terminally differentiated effector T cells.16,21 CD27 may also be required in part for the generation and maintenance of T-cell memory.23 Based on human viral infection studies, a linear model of T-cell differentiation has been proposed wherein CD27+ CD28+ CD45RA+ naive cells progress through a CD27+ CD28+ CD45RA- early antigen-experienced phenotype to a CD27+ CD28- CD45RA-/+ intermediate phenotype and finally to a CD27- CD28- CD45RA+/- late antigen-experienced phenotype that parallels an increased cytotoxic potential and reduced ability to proliferate.24
Our recent clinical studies have demonstrated the efficacy of nonmyeloablative chemotherapy and subsequent adoptive transfer of ex vivo-expanded tumor-infiltrating lymphocytes (TILs) for the treatment of HLA-A*0201 (HLA-A2) patients with refractory metastatic melanoma.25 Of 13 heavily pretreated patients, refractory to standard therapies, 6 (42%) experienced an objective tumor response. Some patients with significant tumor regression experienced dramatic lymphocytosis after infusion of tumor-reactive TIL. Following an effector cell-like contraction phase, cell therapy resulted in the long-term persistence of a clonal melanoma-reactive CD8+ T-cell population in the peripheral blood. Other patients responding to therapy have experienced similar persistence of circulating tumor antigen-specific CD8+ T-cell clones. The transfer of characterized tumor antigen-specific T cells into patients and the monitoring of their progeny over time in vivo have provided a unique opportunity to study the dynamics of phenotypic change in humans occurring during effector-to-memory transition. In the current study, longitudinal phenotypic analysis was performed on tumor-reactive CD8+ T-cell populations from 6 patients who experienced immunotherapy-associated tumor regression. The data presented here demonstrate the in vivo transition of tumor-reactive CD8+ T-cell clones from a late-stage effector to an effector memory phenotype. The dynamics of homeostatic cytokine receptor and costimulatory molecule expression by persistent tumor antigen-reactive CD8+ T cells in the peripheral blood after adoptive cell transfer (ACT) suggest that expression of these receptors is important for the maintenance and long-term survival of adoptively transferred melanoma-reactive CD8+ T cells in vivo.
Patients, materials, and methods
Patient cell samples
All patients whose cells were used in this study had metastatic melanoma and were entered on institutional review board-approved protocols in the Surgery Branch of the National Cancer Institute. Informed consent was obtained from all subjects. TIL cultures were established as previously described.25,26 Briefly, TIL cultures were grown for 2 to 4 weeks in IL-2, screened to select tumor antigen-reactive cultures, and subsequently expanded using a rapid expansion protocol27 using 30 ng/mL OKT3 (anti-CD3) antibody (Ortho Biotech, Bridgewater, NJ) and 6000 IU/mL IL-2 in the presence of irradiated (50 Gy), allogeneic feeder cells at a 200:1 ratio of feeder cells to TIL cells. After about 14 days, cells were harvested from culture bags and prepared for patient treatment, and aliquots were cryopreserved for future experimental analysis. Peripheral blood lymphocytes were purified on Ficoll-Hypaque step gradients (LSM Lymphocyte Separation Medium; ICN Biochemicals, Aurora, OH) and cryopreserved.
Tetramers, monoclonal antibodies, and flow cytometric immunofluorescence analysis
Phycoerythrin (PE)- or allophycocyanin-labeled MART-1:26-35(27L) (ELA-GIGILTV) peptide/HLA-A*0201 tetramer complexes were obtained from Beckman Coulter (Fullerton, CA). Antibodies specific for the β chain variable regions, Vβ7, Vβ12, and Vβ22, of the T-cell receptor were obtained from Immunotech (Marseille, France). Antihuman CD8 antibody was obtained from BD Biosciences (San Jose, CA). Biotinylated IL-7Rα (CD127) and IL-15Rα antibodies and fluorescein isothiocyanate (FITC)-conjugated CCR7 antibody were obtained from R&D Systems (Minneapolis, MN). Biotinylated antibody was used with streptavidin-allophycocyanin (BD Biosciences) or streptavidin-FITC (BD Pharmingen, San Diego, CA). FITC-conjugated anti-CD27, anti-CD45RA, anti-CD45RO, anti-CD62L, anti-CD25, and allophycocyanin-labeled CD8 and CD28 antibodies were obtained from BD PharMingen. Cryopreserved TIL and peripheral blood leukocytes (PBLs) were thawed into prewarmed human serum AB (Gemini Bio-Products, Woodland, CA), washed, and resuspended in fluorescence-activated cell sorter (FACS) buffer consisting of phosphate-buffered saline (PBS) with 2% fetal bovine serum (FBS; Gemini Bio-Products) at 5 × 106 cells/mL and blocked with 10% normal mouse immunoglobulin (Ig; Caltag Labs, Burlingame, CA) for 10 minutes on ice. Cells (5 × 105) in 100 μL were stained with tetramer and FITC-, PE-, or allophycocyanin-conjugated antibodies at 4°C for 40 minutes in the dark. Cells were washed twice, briefly stained with propidium iodide (PI) for nonviable cell exclusion, and subsequently analyzed in a FACSCalibur (Becton Dickinson, San Jose, CA). For IL-15Rα and IL-7Rα staining, cells were first stained with biotinylated specific antibody, washed, and treated with streptavidin-allophycocyanin or FITC. Subsequently, IL-15Rα and IL-7Rα antibody-stained cells were stained with tetramer and anti-CD8 antibody.
Cytokine release assay
Cryopreserved TIL or PBL samples were thawed and cultured overnight in complete medium (CM) plus recombinant human IL-2 (rhIL-2, 300 IU/mL). Responder cells were washed twice and 105 cells cocultured overnight in CM with 105 HLA-A2+ T2 APCs unpulsed or pulsed with 1 μM or 10 μM MART-1:27-35, gp100:209-217 or gp100:280-288 peptides, or melanoma cell lines 526, 624, 888, and 2098. Coculture supernatants were harvested and assessed for the presence of gamma-interferon (IFN-γ) by enzyme-linked immunosorbent assay (ELISA) in accordance with manufacturer's protocol. Values reflect the average of triplicate measurements and are reported in pg/mL.
Results
Patient selection and treatment
Selected for study were 6 HLA-A2+ patients with metastatic melanoma who had received immunodepleting chemotherapy with cyclophosphamide and fludarabine for 7 days prior to adoptive transfer of highly selected tumor-reactive TIL (Table 1). Patient selection was based upon the ability to identify tumor antigen-specific T-cell populations in TIL and peripheral blood after ACT with melanoma-associated peptide-loaded HLA-A2 tetramer complexes or antibodies specific for the β chain variable region of the T-cell receptor (Vβ TCR). Prior to treatment, patients had progressive disease at various anatomic sites that was refractory to standard therapies, including high-dose IL-2. Patients received between 1.8 × 1010 and 10.7 × 1010 ex vivo-expanded TIL and 5 to 13 doses of IL-2. After ACT of tumor-reactive TIL, 4 patients experienced objective partial responses for durations up to 15 months after infusion and 2 patients demonstrated mixed clinical responses; one with significant shrinkage of one or more lesions but progression at other sites (patient 20) and the other with 38% tumor shrinkage (patient 28). Concomitant with tumor regression, 4 of 5 patients infused with MART-1-reactive TIL experienced melanocyte-associated autoimmunity, including vitiligo and uveitis, further suggestive of in vivo immune activity of the transferred TIL population.
Patient demographics, sites of disease, prior therapies, cell treatments received, and clinical outcome*
Patient . | Age, y/sex . | Sites of metastases . | Prior therapies . | No. cells received, × 1010 . | Ag reactivity . | No. IL-2 doses . | Tumor response (duration, mo) . | Autoimmunity . |
|---|---|---|---|---|---|---|---|---|
| 9 | 57/M | Cutaneous, subcutaneous | S, IT, IFN-α, HD IL-2 | 9.6 | MART-1 | 10 | PR (11) | Vitiligo |
| 10 | 55/M | LN, cutaneous, subcutaneous | S, IT, C, HD IL-2 | 10.7 | MART-1† | 12 | PR (14) | Uveitis |
| 20 | 30/M | LN, lung, adrenal, subcutaneous | S, IT, HD IL-2 | 5.8 | MART-1 | 9 | Mixed | Vitiligo, Uveitis |
| 21 | 48/F | Subcutaneous, lung | S, C, IT, HD IL-2 | 2.8 | Autol Vβ22 | 6 | PR (15+) | None |
| 23 | 45/M | LN, parotid, subcutaneous | S, C, IFN-α, R, IT, HD IL-2 | 1.8 | MART-1 | 13 | Mixed | None |
| 28 | 54/M | LN, brain | S, IT, HD IL-2 | 10.4 | MART-1 | 5 | PR (4) | Uveitis |
Patient . | Age, y/sex . | Sites of metastases . | Prior therapies . | No. cells received, × 1010 . | Ag reactivity . | No. IL-2 doses . | Tumor response (duration, mo) . | Autoimmunity . |
|---|---|---|---|---|---|---|---|---|
| 9 | 57/M | Cutaneous, subcutaneous | S, IT, IFN-α, HD IL-2 | 9.6 | MART-1 | 10 | PR (11) | Vitiligo |
| 10 | 55/M | LN, cutaneous, subcutaneous | S, IT, C, HD IL-2 | 10.7 | MART-1† | 12 | PR (14) | Uveitis |
| 20 | 30/M | LN, lung, adrenal, subcutaneous | S, IT, HD IL-2 | 5.8 | MART-1 | 9 | Mixed | Vitiligo, Uveitis |
| 21 | 48/F | Subcutaneous, lung | S, C, IT, HD IL-2 | 2.8 | Autol Vβ22 | 6 | PR (15+) | None |
| 23 | 45/M | LN, parotid, subcutaneous | S, C, IFN-α, R, IT, HD IL-2 | 1.8 | MART-1 | 13 | Mixed | None |
| 28 | 54/M | LN, brain | S, IT, HD IL-2 | 10.4 | MART-1 | 5 | PR (4) | Uveitis |
LN indicates lymph node; S, surgery; IT, experimental immunotherapy; IFN-α, interferon alpha; HD IL-2, high-dose intravenous interleukin-2; PR, objective partial response, 50% or more decrease in the sum of the products of perpendicular diameters of all measurable lesions for one month or more without increases in any lesion or appearance of new lesions; C, chemotherapy; mixed, mixed response, shrinkage in some lesions but an increase in others; and R, radiation therapy.
Patients 20 and 23 had each received a prior lymphodepleting chemotherapy followed by cell transfer and IL-2. The lymphocytes analyzed in this report (course 2) differed substantially from those administered during their first treatment, and no persistent cell population for course 1 was observed prior to the initiation of course 2 (data not shown).
TIL from patient 10 recognized native MART-1:(27-35) peptide but not modified (27L) peptide.
Characteristics of tumor antigen-specific CD8+ TIL populations in vitro
Antigen specificity of TIL was determined by cytokine release assay prior to patient infusion (Table 2). TIL from 5 patients secreted IFN-γ when stimulated with MART-1-expressing HLAA2+ melanoma cell lines or MART-1:27-35 peptide-pulsed APCs, demonstrating immediate effector function. TIL also exhibited cytolytic activity against MART-1-expressing melanoma cell line and peptide-pulsed targets in vitro (not shown). TIL from patient 21 lacked MART-1 specificity but was reactive against the 2098 melanoma cell line, an autologous HLA-A2+ MART-1-deficient line derived from patient 21.
Specificity and activity of tumor antigen-reactive TIL used for adoptive cell therapy
Patient no. . | T2 . | T2/irrelevant peptide, 1 μM . | T2/MART-1, 1 μM . | T2/MART-1, 10 μM . | 888mel (A2− MART-1+) . | 526mel (A2+ MART-1+) . | 624mel (A2+ MART-1+) . | 2098mel (A2+ MART-1−) . | VβTCR of antigen-reactive clones . |
|---|---|---|---|---|---|---|---|---|---|
| 9 | 59 | 92 | 5216† | ND | 5 | 4898† | 4794† | ND | Vβ12 |
| 10 | 10 | 36* | ND | 595† | 12 | 5580† | 4125† | ND | Vβ7 |
| 20 | 2 | 5 | 1165† | 2516† | 20 | 2061† | 1011† | 76 | ND |
| 21 | 30 | 17 | 15 | 19 | 12 | 25 | 21 | 1598† | Vβ22 |
| 23 | 23 | 22 | 1360† | 3040† | 17 | 1292† | 399† | 53 | Vβ14 |
| 28 | 3 | 7 | 2517† | 2872† | 20 | 2061† | 2148† | 167† | Vβ16 |
Patient no. . | T2 . | T2/irrelevant peptide, 1 μM . | T2/MART-1, 1 μM . | T2/MART-1, 10 μM . | 888mel (A2− MART-1+) . | 526mel (A2+ MART-1+) . | 624mel (A2+ MART-1+) . | 2098mel (A2+ MART-1−) . | VβTCR of antigen-reactive clones . |
|---|---|---|---|---|---|---|---|---|---|
| 9 | 59 | 92 | 5216† | ND | 5 | 4898† | 4794† | ND | Vβ12 |
| 10 | 10 | 36* | ND | 595† | 12 | 5580† | 4125† | ND | Vβ7 |
| 20 | 2 | 5 | 1165† | 2516† | 20 | 2061† | 1011† | 76 | ND |
| 21 | 30 | 17 | 15 | 19 | 12 | 25 | 21 | 1598† | Vβ22 |
| 23 | 23 | 22 | 1360† | 3040† | 17 | 1292† | 399† | 53 | Vβ14 |
| 28 | 3 | 7 | 2517† | 2872† | 20 | 2061† | 2148† | 167† | Vβ16 |
Interferon-gamma secretion (pg/mL) from patient TIL stimulated with T2 cells pulsed with peptide at the indicated concentrations or with melanoma cell lines. Cytokine secretion from TIL was measured in multiple independent assays. Cryopreserved TIL were thawed and rested overnight in CM and rhIL-2 (50 cU/mL) prior to establishment of cocultures. T2 cells were pulsed with either MART-1:27-35 peptide or irrelevant peptide, gp 100:209-27.
ND indicates not determined; Vβ TCR, specific tumor antigen-reactive T-cell clones identified in TIL that persisted in the peripheral blood after adoptive transfer to lymphodepleted patients.
Control gp 100:280-288 peptide was used.
Values represent specific cytokine secretion specified as more than 3-fold increase compared with controls and more than 100 pg/mL concentration.
Clonal populations of MART-1-reactive T cells were identified in the TIL of patients 9, 10, and 21 using TCR V(D)J region-specific nucleotide sequence analysis. MART-1-specific Vβ12+ T-cell clones from TIL of patient 9 and Vβ7+ T-cell clones from TIL of patient 10 have been described.25 The MART-1-specific T-cell population from patient 9 TIL was composed of a Vβ12+ clone, which represented 14% of CD8+ T cells and persisted in vivo after ACT, and a Vβ13+ clone, which failed to persist following ACT and for which TCR-specific antibodies were not available for detection. A clonal tumor-reactive Vβ22+ CD8+ T-cell population with autologous tumor reactivity and unique antigen specificity has been identified in TIL from patient 21.55 TIL from patients 23 and 28 contained MART-1-reactive Vβ14+ and Vβ16+ CD8+ T-cell populations, respectively. The clonality of the MART-1-specific CD8+ T-cell population is not known for patient 20.
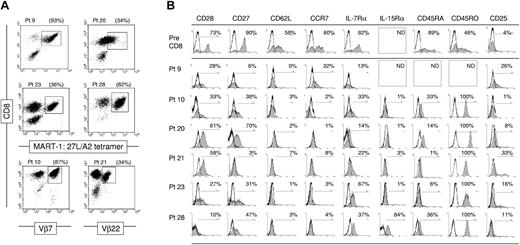
The frequency of tumor antigen-specific CD8+ T cells in TIL was measured using MART-1:26-35(27L) peptide-loaded HLA-A2 tetramer complexes or Vβ TCR-specific antibodies. The frequency of CD8+ lymphocytes ranged between 80% and 99% of viable lymphocytes. Compared with CD8+ T cells, the frequency of CD4+ T cells in TIL was low, ranging between 1% and 19% of lymphocytes (data not shown). MART-1-specific CD8+ T-cell populations were evident in 4 TIL samples, with frequencies ranging between 34% and 93% of CD8+ T cells (Figure 1A). TIL from patient 10 reacted against native MART-1:27-35 peptide but not modified 26-35(27L) peptide,25 therefore MART-1-specific cells could not be detected using commercially available HLA-A2 tetramer complexes that commonly contain the more stable modified MART-1:26-35(27L) peptide. Native MART-1 peptide-specific T cells from patient 10 were instead detected using Vβ7 TCR antibody.
Ex vivo-expanded T cells used for treatment contain tumor antigen-specific clones with late-stage effector phenotype. (A) Tumor antigen-specific CD8+ T cells are detectable in TIL samples using MART-1:26-35(27L) peptide containing HLA-A*0201 tetramer complexes and anti-CD8α antibody. Alternatively, melanoma antigen-specific T-cell clones were identified in patients 9 and 21 TIL using Vβ7- or Vβ22-specific antibodies, respectively. (B) Cell surface expression of costimulatory, lymphoid-homing, and homeostasis-associated molecules on pretreatment PBL and TIL-derived tumor antigen-specific CD8+ T cells prior to ACT. The percentage of marker-positive events within the gated tetramer or Vβ-stained, CD8α+ population are provided. Pt indicates patient; ND, not done.
Ex vivo-expanded T cells used for treatment contain tumor antigen-specific clones with late-stage effector phenotype. (A) Tumor antigen-specific CD8+ T cells are detectable in TIL samples using MART-1:26-35(27L) peptide containing HLA-A*0201 tetramer complexes and anti-CD8α antibody. Alternatively, melanoma antigen-specific T-cell clones were identified in patients 9 and 21 TIL using Vβ7- or Vβ22-specific antibodies, respectively. (B) Cell surface expression of costimulatory, lymphoid-homing, and homeostasis-associated molecules on pretreatment PBL and TIL-derived tumor antigen-specific CD8+ T cells prior to ACT. The percentage of marker-positive events within the gated tetramer or Vβ-stained, CD8α+ population are provided. Pt indicates patient; ND, not done.
Flow cytometric analysis was performed to measure the expression of molecules associated with CD8+ T-cell differentiation, homeostatic proliferation and maintenance, and lymph node homing on tumor antigen-specific CD8+ T cells from ex vivo-expanded TIL (Figure 1B). Expression of CD27 and CD28 costimulatory molecules on tumor antigen-specific CD8+ T cells in TIL revealed frequencies of 3% to 70% and 10% to 61%, respectively. Despite variability in the frequency of costimulatory molecule-expressing cells in TIL, the level at which CD27 and CD28 were expressed on patients' tumor antigen-specific CD8+ T cells was generally low to absent, compared with pretreatment CD8+ T cells. The frequency of tumor-reactive T cells that expressed the migration-associated markers, CD62L and CCR7, was low (0%-7% and 1%-32%, respectively). Consistent with ex vivo TCR stimulation, CD45 isoforms were differentially expressed. CD45RO was expressed at a high level on all cells, while the frequency of CD45RA-expressing TIL was low (1%-36%).
The expression level of alpha chain receptor subunits for common γ chain signaling cytokines, IL-2, IL-7, and IL-15, was also examined on tumor antigen-specific CD8+ T cells in TIL. The frequency of IL-7Rα-expressing cells ranged from 13% to 67%; however, like CD27 and CD28, the level of positive staining was generally low. The frequency of IL-15Rα+ cells ranged between 1% to 3% in 4 of 5 TIL samples tested. Tumor antigen-specific T cells from patient 28 TIL were the exception with 84% expressing IL-15Rα. Small frequencies of T cells expressed CD25 (IL-2Rα, 1%-33%). Despite the low frequencies of CD25 expression, ex vivo-expanded TIL expressed the lymphocyte activation marker CD69 and were negative for CD57, a marker associated with lymphocyte senescence (not shown). The phenotype of the whole CD8+ T-cell population in TIL was generally uniform, as tetramer and Vβ-negative CD8+ T cells displayed similar phenotypic characteristics observed for MART-1- and Vβ-specific T cells. In summary, TIL-derived tumor antigen-specific CD8+ T cells generally expressed a CD27Lo CD28Lo CD45RO+ CD62L- CCR7- IL-7RαLo phenotype. This is most consistent with an effector CD8+ T-cell phenotype, fitting for T cells stimulated about 14 days previously with anti-CD3 antibody and grown in excess IL-2.
Fate of adoptively transferred TIL in vivo
Immediately following lymphodepleting chemotherapy conditioning and prior to ACT, few lymphocytes were detectable in the peripheral blood and thus adoptively transferred T cells could be detected without difficulty (Figure 2A). Approximately 1 week after transfer, when lymphocytes were first detectable in the blood, lymphocytosis developed rapidly in patients 9 and 10. Patients 20 and 23 also demonstrated a rapid elevation in ALC. ALCs from patients 21 and 28 peaked at nearly 1400 and 1100 cells/mm3, respectively. Despite these lower cell numbers, patients 21 and 28 underwent objective tumor regressions in vivo. Over the next 2 months, all patients' ALCs normalized to homeostatic levels. Approximately 1 week after transfer, more than 80% of circulating lymphocytes were CD8+ in 4 patients, recapitulating frequencies observed in the transferred TIL (Figure 2B). Circulating CD8+ T-cell frequencies generally remained elevated over the course of study compared with pretreatment levels, which ranged between 11% and 32% of lymphocytes (Figure 2B).
High levels of tumor antigen-reactive clones persist in the blood of ablated patients after adoptive transfer. (A) Elevated numbers of lymphocytes in the peripheral blood of select patients following cell transfer contract to normal levels. Approximately 1 week after transfer, absolute lymphocyte counts (ALCs) were measured from peripheral blood from patients 9 (▵), 10 (▧), 20 (♦), 21 (•), 23 (x), and 28 (▪). The x-axis represents days relative to cell transfer, with day 0 representing the day of cell infusion. Latest time points analyzed for patients 9, 10, and 23 were days 124, 524, and 161 after infusion, respectively. (B) CD8+ T-cell frequencies are generally elevated after cell transfer and diminish temporally. The frequency of CD8+ cells was measured on gated viable lymphocytes. (C) High frequencies of tumor antigen-specific CD8+ T cells persist in the peripheral blood of select patients. Tumor antigen-specific CD8+ T cells were identified using MART-1: 27-35(27L) peptide containing HLA-A*0201 tetramer complexes or Vβ-specific antibodies. (D) High numbers of melanoma-specific CD8+ T cells persist in the blood of metastatic melanoma patients receiving ACT. Absolute numbers of tumor antigen-specific CD8+ T cells were calculated as number of CD8+ lymphocytes per mm3 times percent tetramer or Vβ-specific antibody positive. (E-F) PBLs from treated patients maintain tumor antigen-specific reactivity. Posttreatment PBL from patients 9 (d 56), 10 (d 524) 20 (d 56), 21 (d 63), 23 (d 28), and 28 (d 97) was tested for IFN-γ secretion in response to T2 targets cells pulsed with 1 μM gp100:209-217 or MART-1:27-35 (M1) peptide, or 10 μM MART-1:27-35 (M10) peptide (E) or melanoma cell lines 888 (HLA-A2- MART-1+), 2098 (HLA-A2+ MART-1-), 526 (HLA-A2+ MART-1+), or 624 (HLA-A2+ MART-1+) by standard ELISA assay (F).
High levels of tumor antigen-reactive clones persist in the blood of ablated patients after adoptive transfer. (A) Elevated numbers of lymphocytes in the peripheral blood of select patients following cell transfer contract to normal levels. Approximately 1 week after transfer, absolute lymphocyte counts (ALCs) were measured from peripheral blood from patients 9 (▵), 10 (▧), 20 (♦), 21 (•), 23 (x), and 28 (▪). The x-axis represents days relative to cell transfer, with day 0 representing the day of cell infusion. Latest time points analyzed for patients 9, 10, and 23 were days 124, 524, and 161 after infusion, respectively. (B) CD8+ T-cell frequencies are generally elevated after cell transfer and diminish temporally. The frequency of CD8+ cells was measured on gated viable lymphocytes. (C) High frequencies of tumor antigen-specific CD8+ T cells persist in the peripheral blood of select patients. Tumor antigen-specific CD8+ T cells were identified using MART-1: 27-35(27L) peptide containing HLA-A*0201 tetramer complexes or Vβ-specific antibodies. (D) High numbers of melanoma-specific CD8+ T cells persist in the blood of metastatic melanoma patients receiving ACT. Absolute numbers of tumor antigen-specific CD8+ T cells were calculated as number of CD8+ lymphocytes per mm3 times percent tetramer or Vβ-specific antibody positive. (E-F) PBLs from treated patients maintain tumor antigen-specific reactivity. Posttreatment PBL from patients 9 (d 56), 10 (d 524) 20 (d 56), 21 (d 63), 23 (d 28), and 28 (d 97) was tested for IFN-γ secretion in response to T2 targets cells pulsed with 1 μM gp100:209-217 or MART-1:27-35 (M1) peptide, or 10 μM MART-1:27-35 (M10) peptide (E) or melanoma cell lines 888 (HLA-A2- MART-1+), 2098 (HLA-A2+ MART-1-), 526 (HLA-A2+ MART-1+), or 624 (HLA-A2+ MART-1+) by standard ELISA assay (F).
Tumor antigen-specific CD8+ T-cell frequencies generally peaked 1 week after infusion, ranging from 18% to 97% of CD8+ T cells (Figure 2C). Compared with the transferred TIL, this frequency was elevated in patients 10, 21, 23, and 28 and decreased in patients 20 and 9. Following early peaks, the percentage of CD8+ T cells that were tumor antigen-specific remained relatively stable with only minor diminution in most patients over time. Absolute numbers of circulating tumor antigen-specific CD8+ T cells generally peaked approximately 1 week after transfer (Figure 2D). High numbers of tumor antigen-specific T cells persisted in the peripheral blood of all patients nearly 2 months after ACT (Figure 2D). At this and later time points, PBLs from treated patients maintained tumor antigen-specificity and reactivity as demonstrated by cytokine secretion following MART-1 peptide (Figure 2E) and HLA-matched, MART-1-expressing tumor stimulation, except for PBL from patient 21 that recognized a unique antigen only on the autologous tumor, 2098mel (Figure 2F). The high number of persistent, clonal CD8+ T cells of known antigen specificity observed in these patients is virtually unprecedented in human immunology and offered a unique opportunity to study their phenotype.
Persistent tumor antigen-specific memory CD8+ T cells express an effector memory phenotype in vivo
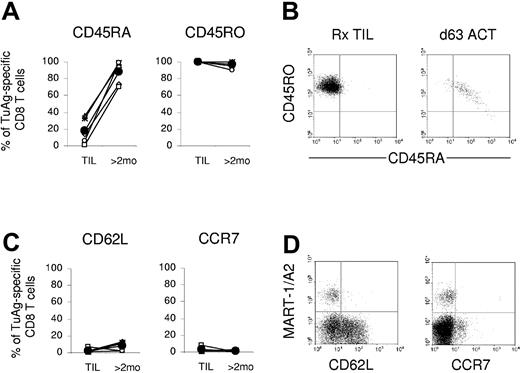
Melanoma-reactive CD8+ T cells were evaluated for the expression of markers associated with memory T-cell differentiation. Prior to administration, tumor-reactive T cells from ex vivo-expanded TIL were uniformly CD45RO+ and had little to no CD45RA cell surface expression (Figure 3A-B). At 2 months after transfer, persisting tumor-reactive cells from all 6 patients showed marked CD45RA up-regulation with concomitant maintenance of CD45RO expression (Figure 3A). Over time, CD45RO expression was sustained, but the intensity of CD45RO staining was modestly reduced in parallel with the observed increase in CD45RA (Figure 3B). Tetramer-negative CD8+ T cells, which represent the reconstituting endogenous repertoire, also displayed a CD45RO+ memory phenotype 2 months after transfer in these patients (not shown). Expression of CD62L and CCR7, which was not detectable in most TIL, was similarly absent on persisting tumor antigen-specific T cells from all 6 patients in vivo (Figure 3C). In these PBL samples, expression of CD62L and CCR7 was readily evident on tetramer-negative CD8+ T cells confirming the activity and sensitivity of these antibodies (Figure 3D). The lack of detectable CD62L and CCR7 expression by persistent transferred TIL is consistent with the long-term maintenance of effector memory CD8+ T cells with tumor antigen specificity in metastatic melanoma patients responding to immunotherapy.
Adoptively transferred tumor antigen-specific CD8+ T cells persist in the peripheral blood of patients as effector memory T cells. (A) Compared with TIL, the frequency of tetramer or Vβ-positive staining CD8+ T cells expressing CD45RA increased in the peripheral blood of all patients more than nearly 2 months after cell infusion, while positive CD45RO expression was maintained. Expression was evaluated on tumor antigen-specific CD8+ T cells from patients 9 (▵), 10 (×), 20 (⋄), 21 (○), 23 (x), and 28 (□), and the average of all values is shown (•). (B) CD45RA expression is temporally reacquired with concomitant CD45RO reduction on tumor antigen-reactive T cells in vivo. Representative dot plots show the expression of CD45 isoforms on gated MART-1+ CD8+ T cells from patient 28 TIL or peripheral blood 63 days after cell administration. (C) TIL and posttransfer PBL-derived tumor antigen-specific CD8+ T cells lack expression of the lymphoid homing markers, CD62L and CCR7. Expression of CD62L and CCR7 was not detected. (D) CD62L and CCR7 expression is absent on gated MART-1+ CD8+ T cells from patient 28 TIL or day-63 posttransfer PBLs and limited to endogenously reconstituted CD8+ T cells. TuAg indicates tumor antigen.
Adoptively transferred tumor antigen-specific CD8+ T cells persist in the peripheral blood of patients as effector memory T cells. (A) Compared with TIL, the frequency of tetramer or Vβ-positive staining CD8+ T cells expressing CD45RA increased in the peripheral blood of all patients more than nearly 2 months after cell infusion, while positive CD45RO expression was maintained. Expression was evaluated on tumor antigen-specific CD8+ T cells from patients 9 (▵), 10 (×), 20 (⋄), 21 (○), 23 (x), and 28 (□), and the average of all values is shown (•). (B) CD45RA expression is temporally reacquired with concomitant CD45RO reduction on tumor antigen-reactive T cells in vivo. Representative dot plots show the expression of CD45 isoforms on gated MART-1+ CD8+ T cells from patient 28 TIL or peripheral blood 63 days after cell administration. (C) TIL and posttransfer PBL-derived tumor antigen-specific CD8+ T cells lack expression of the lymphoid homing markers, CD62L and CCR7. Expression of CD62L and CCR7 was not detected. (D) CD62L and CCR7 expression is absent on gated MART-1+ CD8+ T cells from patient 28 TIL or day-63 posttransfer PBLs and limited to endogenously reconstituted CD8+ T cells. TuAg indicates tumor antigen.
Immediate and continued expression of IL-7Rα and CD28 by circulating antigen-specific clones after transfer
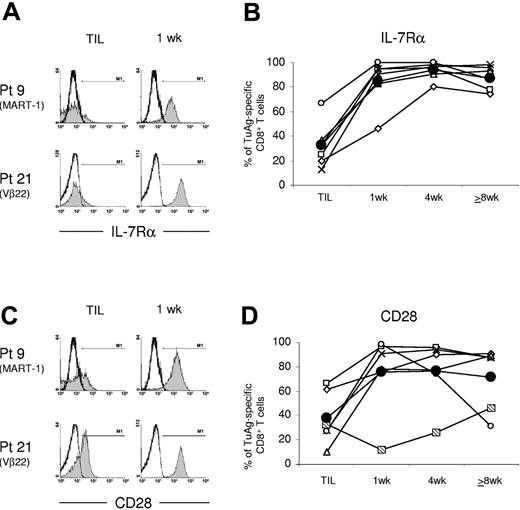
To determine the early and long-term impact of ACT on tumor antigen-specific CD8+ T-cell differentiation, longitudinal cell surface phenotype was evaluated. At the earliest detection of lymphocytes in the blood nearly 1 week after transfer, IL-7Rα, which was expressed at low levels on TIL, was expressed on a high frequency of tumor antigen-specific CD8+ T cells in vivo (Figure 4A). The average frequency of IL-7Rα+ antigen-specific T cells increased from 33% in TIL to 85% in PBLs. Furthermore, the mean fluorescence intensity (MFI) of anti-IL-7Rα staining was augmented on tumor-reactive T cells from all 6 patients 1 week after transfer. (Table 3). In the peripheral blood, IL-7Rα expression was immediate, high in fluorescence intensity, and sustained on a high frequency of antigen-specific T cells in a longitudinal study (Figure 4B). Expression of other common γ chain cytokine receptor alpha subunits, CD25 and IL-15Rα, which were expressed at low levels in TIL samples, was not detected on persisting tumor antigen-specific CD8+ T cells in vivo (not shown).
IL-7Rα and CD28 are highly expressed by melanoma antigen-specific CD8+ T cells in the early aftermath of ACT into lymphoablated metastatic melanoma patients. (A) The frequency of tumor-reactive CD8+ T cells with elevated IL-7Rα expression levels is increased nearly 1 week after cell infusion. Representative histograms show IL-7Rα expression by TIL or day-7 posttransfer PBL-derived MART-1+ or Vβ-22+ CD8+ T cells from patients 9 and 21, respectively. (B) Tumor antigen-specific T-cell clones rapidly and continuously express IL-7Rα after adoptive transfer. IL-7Rα expression was detected on tumor antigen-specific CD8+ T cells from patients 9 (x), 10 (▧), 20 (⋄), 21 (□), 23 (○), and 28 (▵), and the average of all values is shown (•). Expression by persistent tumor antigen-specific CD8+ T cells was assessed about 1, 4, and 8 or more weeks after ACT. (C-D) CD28 expression was immediately enhanced on melanoma-reactive CD8+ T cells from most patients after cell transfer. Although low, CD28 expression was shown to temporally increase on Vβ-7+ CD8+ T cells from patient 10 in vivo in 4 independent flow cytometric analyses.
IL-7Rα and CD28 are highly expressed by melanoma antigen-specific CD8+ T cells in the early aftermath of ACT into lymphoablated metastatic melanoma patients. (A) The frequency of tumor-reactive CD8+ T cells with elevated IL-7Rα expression levels is increased nearly 1 week after cell infusion. Representative histograms show IL-7Rα expression by TIL or day-7 posttransfer PBL-derived MART-1+ or Vβ-22+ CD8+ T cells from patients 9 and 21, respectively. (B) Tumor antigen-specific T-cell clones rapidly and continuously express IL-7Rα after adoptive transfer. IL-7Rα expression was detected on tumor antigen-specific CD8+ T cells from patients 9 (x), 10 (▧), 20 (⋄), 21 (□), 23 (○), and 28 (▵), and the average of all values is shown (•). Expression by persistent tumor antigen-specific CD8+ T cells was assessed about 1, 4, and 8 or more weeks after ACT. (C-D) CD28 expression was immediately enhanced on melanoma-reactive CD8+ T cells from most patients after cell transfer. Although low, CD28 expression was shown to temporally increase on Vβ-7+ CD8+ T cells from patient 10 in vivo in 4 independent flow cytometric analyses.
Antibody staining intensity on melanoma-reactive CD8+ T cells (MFI)
. | . | . | Time relative to cell transfer . | . | . | ||
|---|---|---|---|---|---|---|---|
| Marker, patient . | Before . | TIL . | 1 wk . | 1 mo . | 2 mo or longer . | ||
| CD27 | |||||||
| 9 | ND | 5 | 4 | 5 | 8 | ||
| 10 | 62 | 12 | 6 | 18 | 45 | ||
| 20 | 108 | 30 | 25 | 67 | 49 | ||
| 21 | 424 | 5 | 7 | 17 | 11 | ||
| 23 | 351 | 9 | 7 | 38 | 20 | ||
| 28 | 43 | 10 | 9 | 60 | 41 | ||
| CD28 | |||||||
| 9 | ND | 12 | 93 | 113 | 119 | ||
| 10 | 34 | 7 | 6 | 8 | 21 | ||
| 20 | 55 | 17 | 31 | 41 | 62 | ||
| 21 | 124 | 33 | 254 | 263 | 65 | ||
| 23 | 169 | 8 | 275 | 64 | 7 | ||
| 28 | 21 | 3 | 18 | 24 | 77 | ||
| IL-7Rα | |||||||
| 9 | ND | 3 | 59 | 80 | 100 | ||
| 10 | 75 | 9 | 48 | 66 | 100 | ||
| 20 | 20 | 7 | 16 | 38 | 24 | ||
| 21 | 291 | 12 | 247 | 372 | 69 | ||
| 23 | 15 | 25 | 206 | 246 | 35 | ||
| 28 | 35 | 3 | 10 | 9 | 85 | ||
. | . | . | Time relative to cell transfer . | . | . | ||
|---|---|---|---|---|---|---|---|
| Marker, patient . | Before . | TIL . | 1 wk . | 1 mo . | 2 mo or longer . | ||
| CD27 | |||||||
| 9 | ND | 5 | 4 | 5 | 8 | ||
| 10 | 62 | 12 | 6 | 18 | 45 | ||
| 20 | 108 | 30 | 25 | 67 | 49 | ||
| 21 | 424 | 5 | 7 | 17 | 11 | ||
| 23 | 351 | 9 | 7 | 38 | 20 | ||
| 28 | 43 | 10 | 9 | 60 | 41 | ||
| CD28 | |||||||
| 9 | ND | 12 | 93 | 113 | 119 | ||
| 10 | 34 | 7 | 6 | 8 | 21 | ||
| 20 | 55 | 17 | 31 | 41 | 62 | ||
| 21 | 124 | 33 | 254 | 263 | 65 | ||
| 23 | 169 | 8 | 275 | 64 | 7 | ||
| 28 | 21 | 3 | 18 | 24 | 77 | ||
| IL-7Rα | |||||||
| 9 | ND | 3 | 59 | 80 | 100 | ||
| 10 | 75 | 9 | 48 | 66 | 100 | ||
| 20 | 20 | 7 | 16 | 38 | 24 | ||
| 21 | 291 | 12 | 247 | 372 | 69 | ||
| 23 | 15 | 25 | 206 | 246 | 35 | ||
| 28 | 35 | 3 | 10 | 9 | 85 | ||
Similar to IL-7Rα, the frequency of melanoma antigen-specific CD8+ T cells expressing CD28 costimulatory molecules was sharply increased in 5 of the 6 patients' PBL samples immediately after infusion (Figure 4C-D). The average frequency of antigen-reactive T cells expressing CD28 increased from 38% in TIL to 75% in PBLs about 1 week after transfer (Figure 4D). Nearly log-fold increases in CD28 staining intensity occurred over this time, indicating that up-regulation of CD28 expression by the tumor-reactive cells had occurred in vivo (Figure 4C). The frequency of Vβ7+ CD8+ T cells from patient 10's PBLs that expressed CD28 increased in a more indolent manner over 2 months following transfer in vivo. These tumor-reactive cells were uniformly CD28+ nearly 17 months after initial treatment (not shown). The phenotype of the transferred T-cell clones from patient 23 contrasted these observations. In this patient, the frequency of circulating CD28+ MART-1-specific CD8+ T cells declined following an initial elevation of CD28. It is intriguing that patient 23 initially experienced transient tumor regression over a one-month period, but then experienced progression of melanoma lesions with low MART-1 antigen expression. Circulating tumor antigen-specific CD8+ T cells from the remaining mixed responder, patient 20, maintained expression of CD28 despite the progression of HLA-A2+ MART-1-expressing lesions.
CD27 expression on tumor antigen-specific T cells at the peak of the inflammatory response is predictive for long-term persistence
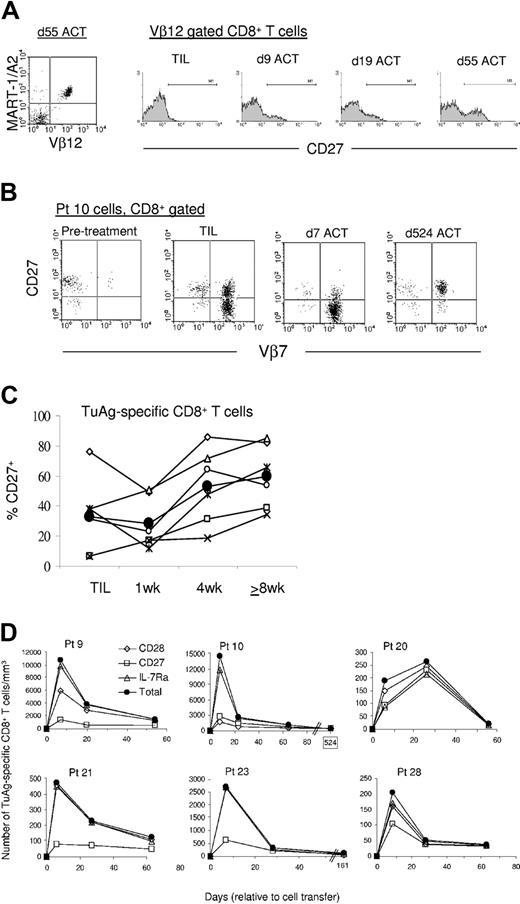
The costimulatory molecule CD27 has been implicated in T-cell differentiation and memory generation,23 therefore CD27 expression was evaluated on persisting melanoma-specific CD8+ T cells. Unlike CD28, a high frequency of CD27-expressing tumor antigen-specific CD8+ T cells was not detected in the peripheral blood 1 week after infusion (Figure 5). Rather, the frequency of melanoma-specific T cells expressing CD27 was minimally increased compared with TIL in 3 patients' PBLs immediately after transfer and slightly diminished in the remaining patients. Figure 5A demonstrates the near absence of CD27 expression on the clonal Vβ12+ MART-1-specific T cells from the TIL of patient 9 (expressed on 6% of cells). The frequency of CD27-expressing Vβ12+ T-cell clones had increased to 17% 9 days after transfer, and 34% at day 55 after ACT. The level of staining intensity on some Vβ12+ T-cell clones from day-9 posttransfer PBLs was visibly higher than levels detected in the TIL, indicating that CD27 re-expression by the tumor-reactive T-cell population had occurred in the interim. In contrast to patient 9, CD27 expression was down-regulated immediately after ACT on melanoma-reactive T cells from 3 patients. For example, 38% of melanoma antigen-specific clones from TIL of patient 10 were CD27+, but declined to 12% 1 week after ACT (Figure 5B). The average frequency of CD27-expressing melanoma-reactive CD8+ T-cell clones in TIL (32%) was unchanged in the blood 1 week after transfer (27%) but escalated subsequently with an indolent progression (Figure 5C). One month after transfer, the average frequency was elevated to 53% and further amplified to 65% 2 or more months after transfer. In addition, the MFI of anti-CD27 staining of tumor antigen-specific CD8+ T cells in the blood one month after transfer was augmented compared with TIL (Table 3). CD27+ cell frequency increased longitudinally such that 95% of the Vβ7+ tumor-reactive clones from patient 10 expressed CD27 524 days after transfer (Figure 5B), demonstrating that CD27 is expressed by the majority of melanoma-reactive memory T cells that persist in vivo following ACT.
CD27 expression by melanoma antigen-specific CD8+ T cells increases in an indolent manner and identifies a subset of memory cell precursors within the effector cell population. (A) The increase in CD27 by tumor-specific CD8+ T cells occurs in an indolent fashion. Dot plot analysis shows that the MART-1:26-35(27L) peptide-specific lymphocyte population from the peripheral blood of patient 9 is composed of Vβ12+ cells 55 days after cell transfer (left). Representative histograms show the steady temporal increase in CD27 expression level and frequency by Vβ12+ CD8+ T cells from patient 9. (B) The frequency of CD27-expressing tumor-reactive T cells decreases in the early aftermath of cell transfer in select patients. Dot plot analysis of CD8+ T cells from patient 10 TIL and PBLs demonstrates the early reduction in CD27 expression by the Vβ7+ tumor-reactive population at day 7 after transfer, compared with TIL, and the uniformity of CD27 expression 524 days after ACT. (C) The frequency of CD27-expressing melanoma-reactive CD8+ T cells increases with continued persistence in vivo. Tumor antigen-specific CD8+ T cells from patients 9 (x), 10 (▧), 20 (⋄), 21 (□), 23 (○), and 28 (▵) were assayed for CD27 expression in the TIL or peripheral blood 1, 4, and 8 or more weeks after ACT. The average of all values is shown (•). (D) CD27 identifies a stable subset of tumor antigen-specific CD8+ effector T cells that give rise to the memory T-cell population in most patients. Longitudinal examination of the absolute number of CD28- (⋄), CD27- (□), or IL-7Rα- (▵) expressing melanoma antigen-specific CD8+ T cells compared with the total number of melanoma antigen-specific CD8+ T cells (•) in the blood of all 6 patients is shown. Values represent the number of tumor antigen-specific CD8+ T cells with the indicated phenotype in the peripheral blood as cells/mm3.
CD27 expression by melanoma antigen-specific CD8+ T cells increases in an indolent manner and identifies a subset of memory cell precursors within the effector cell population. (A) The increase in CD27 by tumor-specific CD8+ T cells occurs in an indolent fashion. Dot plot analysis shows that the MART-1:26-35(27L) peptide-specific lymphocyte population from the peripheral blood of patient 9 is composed of Vβ12+ cells 55 days after cell transfer (left). Representative histograms show the steady temporal increase in CD27 expression level and frequency by Vβ12+ CD8+ T cells from patient 9. (B) The frequency of CD27-expressing tumor-reactive T cells decreases in the early aftermath of cell transfer in select patients. Dot plot analysis of CD8+ T cells from patient 10 TIL and PBLs demonstrates the early reduction in CD27 expression by the Vβ7+ tumor-reactive population at day 7 after transfer, compared with TIL, and the uniformity of CD27 expression 524 days after ACT. (C) The frequency of CD27-expressing melanoma-reactive CD8+ T cells increases with continued persistence in vivo. Tumor antigen-specific CD8+ T cells from patients 9 (x), 10 (▧), 20 (⋄), 21 (□), 23 (○), and 28 (▵) were assayed for CD27 expression in the TIL or peripheral blood 1, 4, and 8 or more weeks after ACT. The average of all values is shown (•). (D) CD27 identifies a stable subset of tumor antigen-specific CD8+ effector T cells that give rise to the memory T-cell population in most patients. Longitudinal examination of the absolute number of CD28- (⋄), CD27- (□), or IL-7Rα- (▵) expressing melanoma antigen-specific CD8+ T cells compared with the total number of melanoma antigen-specific CD8+ T cells (•) in the blood of all 6 patients is shown. Values represent the number of tumor antigen-specific CD8+ T cells with the indicated phenotype in the peripheral blood as cells/mm3.
Calculating the number of cells with distinct phenotypic characteristics can provide a significantly different picture than that observed when studying cell frequency. To evaluate temporal alterations in T-cell number, the absolute number of blood-derived tumor antigen-specific T cells that expressed IL-7Rα, CD28, or CD27 was calculated. The total number of tumor antigen-specific T cells in the blood expanded about 1 week after transfer and subsequently contracted, resulting in the persistence of relatively stable cell numbers (Figures 5D and 2D). In patient 20, the number of melanoma-reactive T-cell clones continued to expand beyond 1 week but dramatically contracted between 1 and 2 months after transfer.
The number of circulating tumor antigen-specific CD8+ T cells that expressed IL-7Rα or CD28 directly paralleled the total number of melanoma antigen-specific T-cell clones in most patients (Figure 5D). Their numbers were elevated about 1 week after transfer but exhibited pronounced contraction over the following weeks, resulting in a relatively stable number of persistent melanoma-specific cells, which expressed IL-7Rα or CD28. Unlike IL-7Rα- or CD28-expressing cells, the absolute number of antigen-specific T cells that expressed CD27 was stably maintained over time in the peripheral blood of most patients. In contrast, transferred cells that lacked immediate CD27 expression demonstrated similar kinetics as those observed for IL-7Rα- and CD28-expressing cells. CD27- tumor antigen-specific CD8+ T cells peaked in number 1 week after transfer but dramatically contracted and failed to persist at high numbers 2 months after transfer.
Coexpression of CD27 and CD28 on persistent tumor-specific memory T cells
Although CD28 signaling amplifies TCR-mediated T-cell proliferation and activation,28 stable numbers of CD28-expressing melanoma-reactive T cells did not persist over time. Prior to chemotherapeutic conditioning, the majority of circulating CD8+ T cells concomitantly expressed cell surface CD27 and CD28 molecules, with smaller frequencies of CD27+ CD28- and CD27- CD28- cells (Figure 6A). Few CD27- CD28+ CD8+ T cells were detected in pretreatment PBL samples. Two months after chemotherapy, the reconstituting endogenous CD8+ T-cell population was composed of CD27 and CD28 expression subsets similar to pretreatment PBLs. In accordance with robust TCR stimulation and expansion ex vivo, the majority of tumor antigen-specific CD8+ T cells in TIL displayed a CD27- CD28- effector phenotype (Figure 6B). However about 1 week after infusion, the preponderance of the transferred T-cell population had transitioned to a CD27- CD28+ phenotype, with small frequencies of CD27+ CD28+ cells. Expression of the CD27- CD28+ phenotype by tumor-reactive T cells was transient as the frequency of CD27+ CD28+ cells generally increased in parallel with a reduction in CD27- CD28+ frequency over time (Figure 6B). Longitudinal determination of T-cell phenotypic frequency measures the changes within a distinct cell population yet fails to adequately represent the effects on circulating T-lymphocyte number. Enumeration of phenotypic subsets revealed temporal reductions in the number of CD27- CD28+ tumor antigen-specific CD8+ T-cell clones with concomitant maintenance of relatively stable numbers of CD27+ CD28+ cells, therefore the high frequency of CD28+ cells that persist over time reflects, in part, the maintenance of stable numbers of CD28+ cells that coexpress CD27 in patients undergoing objective tumor responses (Figure 6C). High numbers of CD27+ CD28- and CD27- CD28- tumor antigen-specific T cells were not detected at late time points after ACT. In summary, these data demonstrate that the long-term melanoma-reactive memory T-cell population is largely composed of CD27+ CD28+ cells. These data further imply that CD27+ CD28+ melanoma-specific CD8+ T-cell clones detectable in the blood soon after ACT may represent precursors to long-term tumor antigen-specific memory T cells in vivo.
Persisting melanoma antigen-specific CD8+ T cells express both CD27 and CD28 in vivo. (A) Expression of CD27 and CD28 by the reconstituting endogenous CD8+ T-cell repertoire. Dot plot analysis of gated CD8+ MART-1/A2 tetramer-negative T cells from patient 28's PBLs obtained prior to and after ACT of TIL reveals that the phenotype of pretreatment CD8 T cells is restored in reconstituting CD8 T-cell population. The frequency of CD28+ CD27- is low in PBLs prior to lymphodepletion and at day 63 after cell infusion. (B) Transfer of predominantly CD27- CD28- TIL results in the generation of a persisting population of CD27+ CD28+ tumor antigen-specific CD8+ T cells. Representative dot plots show the expression of CD27 and CD28 on tumor antigen-specific CD8+ T cells from TIL and posttreatment PBL samples from patients 28 (MART-1+), 23 (MART-1+), 10 (Vβ7+), and 21 (Vβ22+). The tumor antigen-reactive T-cell population transitioned through a CD28+ CD27- 1 week after transfer before becoming CD27+ CD28+ memory T cells. (C) CD28-expressing melanoma-reactive CD8+ T cells that concomitantly express CD27 persist after ACT in vivo. The absolute number of transferred tumor antigen-specific CD8+ T cells expressing both CD27 and CD28 was assessed in PBLs from patients with objective clinical responses (10, 21, and 28) beginning 1 week after infusion and is represented as cells/mm3, depicted in log scale.
Persisting melanoma antigen-specific CD8+ T cells express both CD27 and CD28 in vivo. (A) Expression of CD27 and CD28 by the reconstituting endogenous CD8+ T-cell repertoire. Dot plot analysis of gated CD8+ MART-1/A2 tetramer-negative T cells from patient 28's PBLs obtained prior to and after ACT of TIL reveals that the phenotype of pretreatment CD8 T cells is restored in reconstituting CD8 T-cell population. The frequency of CD28+ CD27- is low in PBLs prior to lymphodepletion and at day 63 after cell infusion. (B) Transfer of predominantly CD27- CD28- TIL results in the generation of a persisting population of CD27+ CD28+ tumor antigen-specific CD8+ T cells. Representative dot plots show the expression of CD27 and CD28 on tumor antigen-specific CD8+ T cells from TIL and posttreatment PBL samples from patients 28 (MART-1+), 23 (MART-1+), 10 (Vβ7+), and 21 (Vβ22+). The tumor antigen-reactive T-cell population transitioned through a CD28+ CD27- 1 week after transfer before becoming CD27+ CD28+ memory T cells. (C) CD28-expressing melanoma-reactive CD8+ T cells that concomitantly express CD27 persist after ACT in vivo. The absolute number of transferred tumor antigen-specific CD8+ T cells expressing both CD27 and CD28 was assessed in PBLs from patients with objective clinical responses (10, 21, and 28) beginning 1 week after infusion and is represented as cells/mm3, depicted in log scale.
Discussion
Recent data from our lab have demonstrated a significant correlation between the persistence of transferred lymphocyte clones and cancer regression in ablated patients receiving cell transfer therapy.29 Based on these findings, we hypothesized that tumor-reactive clones that persist more than one month after ACT are the cells or progeny of cells mediating therapy-based tumor regressions in vivo. The ability to longitudinally monitor and examine autologous tumor antigen-reactive CD8+ T-cell clones after ACT in the context of tumor regression in vivo provided a novel opportunity to characterize the transition of functional tumor-reactive effector CD8+ T cells to a long-lived memory T-cell population. The results herein implicate a series of signature markers for immediate and long-term survival of antigen-reactive CD8+ T cells, which are present in the peripheral blood following ACT and endure effector cell contraction to give rise to a persistent memory T-cell population, and illustrate a model of in vivo memory T-cell development in parallel with ongoing antigen-specific immune responses.
The common γ-chain signaling cytokines, IL-7 and IL-15, have been shown to play key roles in the homeostatic maintenance and proliferation of CD8+ memory T cells,9,12,30-32 and deprivation of such factors has been proposed to be responsible for the loss of effector T cells during the contraction phase of the T-cell response.33-35 The levels of homeostatic cytokines in the serum of patients receiving lymphoablative conditioning are currently under investigation. Nonetheless, cell-intrinsic factors, such the ability of effector cells to express IL-7Rα, appear to be equally important for the generation of the memory population.4,9 The finding that nearly all circulating tumor antigen-reactive CD8+ T cells in patients express IL-7Rα shortly after administration of TIL, which lack or express little IL-7Rα, is suggestive of in vivo up-regulation of the alpha subunit. Accordingly, gene profile array analysis comparing tumor antigen-specific TIL and day-7 postinfusion PBLs from patient 9 has revealed the up-regulation of IL-7Rα mRNA by melanoma-reactive CD8+ T cells in the aftermath of ACT (K. Kerstann, Surgery Branch of the National Cancer Institute, personal oral communication, August 2003). In contrast to IL-7Rα, IL-15Rα was not observed on persisting T cells despite reports of IL-15Rα expression in memory CD8+ T-cell populations. Trans presentation of IL-15 to T cells lacking the alpha subunit is known to occur, and thus IL-15-mediated homeostatic signaling may remain intact in the transferred population in vivo.36,37
Since TCR-mediated signals alone are generally insufficient for proliferation of T cells during immune responses, secondary costimulatory signals are required.38 Compared with TIL, whose CD28 expression was commonly low as measured by fluorescence intensity, the frequency of circulating CD28+ melanoma epitope-reactive CD8+ T cells was augmented 1 week after ACT. Reports of CD28 re-expression by T cells have been few and limited to in vitro studies.39,40 Elevated CD28 expression by persisting transferred cells suggests that CD28 up-regulation can occur in the conditions of ACT after lymphodepletion in vivo. Furthermore, the presence of CD28+ T cells and the lack of CD28- cells immediately following cell infusion in vivo suggest an early survival advantage for CD28+ CD8+ T cells. Preferential expansion of CD28+ melanoma-reactive cells in vivo may also account in part for their increased prevalence. Despite the known prosurvival effects of CD28 and IL-7Rα signaling and coexpression of these molecules by most tumor-reactive T cells in vivo, tumor antigen-specific CD8+ T-cell numbers contracted between 1 and 4 weeks after transfer, demonstrating that while CD28 and IL-7Rα may be important molecules in the early aftermath of ACT, additional cell-intrinsic and -extrinsic factors may be necessary for augmenting the long-term persistence potential of effector CD8+ T cells in vivo.
Signaling through tumor necrosis factor (TNF) receptor family molecules, including CD27, OX-40 (CD134), and 4-1BB (CD137), in association with TNF receptor-associated factor (TRAF) family adaptor proteins, may promote cell survival.41-43 CD27 was generally expressed at low intensity levels on tumor antigen-specific T cells in TIL, consistent with their recent ex vivo activation. Compared with TIL, a reduction in CD27+ T-cell frequency was detected within the circulating melanoma-reactive T-cell populations from 3 patients immediately after ACT. Since prolonged TCR stimulation is known to result in the loss of CD27,45,46 reductions in CD27+ cell frequencies after ACT might reflect recent antigenic stimulation in these patients undergoing immune-mediated tumor regressions. While CD27- melanoma-reactive CD8+ T cells predominated during the inflammatory response in the early aftermath of ACT, CD27- cell numbers severely declined over the following weeks, suggesting that these are terminally differentiated T cells with the ability to eliminate tumor but not persist. Studies have shown that CD27- CD8+ T cells are primarily composed of cytolytic effector T cells, preferentially expressing perforin and exhibiting cytotoxic activity.16 Conversely, the level of CD27 fluorescence intensity was augmented on T-cell clones from patients 9 and 21 in the interim between cell infusion and 1 week after infusion. Thus, it appears that adoptive transfer of effector CD8+ T cells under lymphopenic conditions may promote CD27 re-expression in vivo.
Within the confines of this study, it remains uncertain what mechanisms account for the preferential expression of CD27 by the memory population developing in the peripheral blood of lymphodepleted patients receiving ACT. CD27 loss has been reported to be irreversible in in vitro studies and serves as a marker for terminal differentiation.16,21 Our data are thus most consistent with the selective survival of infused effector T cells that immediately express CD27 after transfer, resulting in the continued maintenance of relatively stable numbers CD27+ T-cell clones in vivo that give rise to a long-lived memory population. Moreover, CD27 signals may function to support the generation of the tumor-reactive memory CD8+ T cells in vivo. The significance of CD27-mediated signals for the generation of memory responses after primary stimulation has been demonstrated in knock-out mice and contributes by stimulating the survival of activated T cells in vivo, even in the absence of CD28.23,41 Alternatively, enduring CD27- effector CD8+ T cells might temporally reacquire CD27 expression in vivo, a phenomenon not previously described.
In the transition to memory, transferred tumor-reactive T cells from these 6 patients generally underwent similar phenotypic dynamics, progressing from a primarily CD27- CD28- CD45RA- effector stage in TIL to a CD27+ CD28+ CD45RAINT memory status in PBLs. CD27- CD28- CD45RA- cells, which failed to persist at high numbers, appear to represent a terminally differentiated T-cell population. Immediately after ACT, melanoma-reactive CD8+ T cells in the blood primarily displayed a distinct CD27- CD28+ CD45RA- phenotype, present at minimal frequencies in healthy individuals,47 which was generally short-lived and underwent pronounced contraction over the ensuing weeks. In contrast, CD27+ CD28+ melanoma-reactive clones were largely stable in number, persisted, and concomitantly augmented CD45RA expression. At 2 months after transfer, most persistent melanoma antigen-specific CD8+ T-cell clones re-expressed CD45RA in vivo, albeit at intermediate levels, with a modest diminishment in CD45RO intensity, a transition described previously.48 Reacquisition of CD45RA expression is postulated to denote terminal CD8+ T-cell differentiation.16,49 The CD27+ CD28+ CD45RAINT phenotype displayed by melanoma-reactive memory CD8+ T cells is consistent with the early antigen-experienced phenotype reported in virus-specific T-cell populations.24
The cause of this preferential phenotype observed in transferred melanoma-reactive memory CD8+ T cells may be multifactoral. Virus-specific memory CD8+ T cells that result from transient influenza infection primarily express CD27 and CD28, while CD27-CD28- virus-specific memory cells predominate in individuals infected with persistent human cytomegalovirus (HCMV),50 suggesting that duration of antigen exposure may influence phenotype. Immunotherapeutic targeting of MART-1 was associated with melanocyte-related autoimmune manifestations in 4 of 5 studied patients, illustrating MART-1-directed immune activity in vivo. Despite the potential for chronic MART-1 antigen exposure, the majority of circulating melanoma-reactive memory CD8+ T cells continued to maintain the early antigen-experienced phenotype, reminiscent of an Epstein-Barr virus (EBV)-specific CD8+ T-cell phenotype observed during acute and chronic infections.24 This suggests that beyond chronic antigen exposure other phenotype influencing factors may exist. Distinct phenotypic subsets may be uniquely susceptible to apoptosis, have essential functional characteristics for tumor destruction, and have unique proliferation capacities or homing capabilities.
Human memory T cells can be divided into 2 distinct pools named central and effector memory T cells.14 Since CCR7+ memory T cells can down-regulate chemokine receptor and gain functional competency by short-term in vitro culturing,14,51 it has been proposed that effector memory cells are derived from a central memory origin.52 Alternatively, these memory subsets may develop as independent progeny of effector cell precursors.53 Ex vivo-expanded TIL lack CCR7 and CD62L expression prior to cell infusion and persist as circulating CCR7- CD62L- effector memory CD8+ T cells in vivo. The maintenance of melanoma-reactive CD8+ T cells with effector memory phenotype in patients lacking detectable melanoma deposits contrasts the CCR7 and CD62L conversion of viral epitope-specific T cells observed in murine viral studies.54 It remains possible that CCR7+ CD62L+ tumor-reactive memory cells were not detectable in the peripheral blood of ACT patients due to preferential homing to lymphoid organs. Alternatively, the maintenance of effector memory CD8+ T cells in patients receiving MART-1-specific TIL may be supported by chronic, low-level MART-1 stimulation by normal melanocytes. CCR7+ CD62L+ memory cells were not detectable in postinfusion blood samples from patient 21, who received TIL containing Vβ22+ T cells with nonshared autologous tumor reactivity. The persistence of Vβ22+ T cells with effector memory phenotype in the absence of clinically detectable disease suggests that human effector memory CD8+ T cells can be maintained in the absence of antigen.
The results herein demonstrate the increased presence of melanoma-reactive CD8+ T cells expressing CD28 and IL-7Rα in the early aftermath of ACT of ex vivo-expanded TIL with selective survival of effector memory T cells that up-regulate CD27 expression and prevail following effector cell contraction, resulting in the preferential long-term persistence of a clonal CD27+ CD28+ CD45RAINT CD45RO+ CD62L- CCR7- IL-7Rα+ melanoma antigen-specific CD8+ T-cell population in patients undergoing tumor regression in vivo. Understanding the mechanism by which adoptively transferred tumor-reactive T cells can mediate tumor destruction and persist in vivo to provide long-term immunity may aid in increasing the efficacy of ACT immunotherapy for the treatment of patients with cancer and serve to further our understanding of human memory T-cell development. Additional analyses of phenotypically distinct CD8+ T-cell subsets, including the examination of gene profiles, proliferative potential, cytokine secretion patterns, cytolytic activity, and susceptibility to apoptosis, are warranted. Furthermore, studies evaluating the phenotypic distinctions between TIL-derived, tumor antigen-specific T cells that persisted in vivo and mediated objective clinical responses versus those that failed to persist may further provide important information for optimizing the efficiency of ACT therapy by the administration of phenotypically distinct subsets of TIL preparations.
Prepublished online as Blood First Edition Paper, September 2, 2004; DOI 10.1182/blood-2004-06-2482.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Arnold Mixon and Shawn Farid from the Surgery Branch FACS lab facility.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal