Antigen-specific cancer immunotherapy targeting minimal residual disease emerges as a novel treatment modality in multiple myeloma. Two candidate target antigens were identified: cancer/testis (CT) antigen NY-ESO-1 and BCMA, a plasma-cell membrane receptor.
The 2 most promising treatment options for patients with multiple myeloma are tandem high-dose chemotherapy followed by autologous stem cell infusion or allogeneic hematopoietic stem cell transplantation after myeloablative therapy or reduced-intensity conditioning. However, cure is rarely achieved due to persistence of minimal residual disease. Thus, there is an urgent need for innovative treatment modalities to stabilize or even eradicate residual tumor cells.
With the discovery and molecular characterization of cancer target antigens, more focused approaches may become available to steer the patient's immune system to recognize and eliminate residual cancer cells after conventional ablative therapies. In this issue of Blood, van Rhee and colleagues and Bellucci and colleagues describe 2 promising target antigens, NY-ESO-1 and B-cell maturation antigen (BCMA), identified in multiple myeloma. Both are recognized by the immune system and may therefore prove to be suitable targets in clinical cancer immunotherapy trials with multiple myeloma patients.
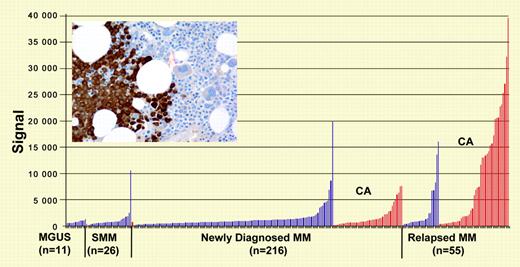
NY-ESO-1 gene and protein expression (inset: immunohistochemistry analysis of myeloma cells in bone marrow). See the related figures in the article beginning on page 3939.
NY-ESO-1 gene and protein expression (inset: immunohistochemistry analysis of myeloma cells in bone marrow). See the related figures in the article beginning on page 3939.
The paper by van Rhee et al identifies NY-ESO-1 as a potential target antigen in poor-prognosis myeloma characterized by cytogenetic abnormalities. In this population NY-ESO-1 is highly expressed and immunogenic. Spontaneous NY-ESO-1–specific humoral and cellular immune responses were observed in up to 50% of patients dependent on tumor load and on chromosomal abnormalities. NY-ESO-1 may likely be the most immunogenic human cancer antigen known to date. It belongs to a category of antigens named cancer/testis (CT) antigens due to their pattern of expression: while silent in most normal tissues with the exception of spermatogonia in testis, this category of antigens is expressed in up to 30% to 40 % of various types of cancer. CT antigens are also thought to play a critical role in regulating gene expression and in early germ cell development.1
Spontaneous NY-ESO-1–specific immune responses are found in up to 50% of patients with NY-ESO-1–expressing cancers. Augmenting or eliciting NY-ESO-1–specific cellular and humoral immune responses by suitable cancer vaccination protocols resulted in more favorable courses of disease and, in some cases, cancer regression.2,3 This study by van Rhee et al is the most comprehensive assessment of NY-ESO-1 expression in multiple myeloma to date. NY-ESO-1's known immunogenicity, inducing both spontaneous cellular and often humoral NY-ESO-1–specific immune responses, and the described pattern of expression in primarily poor prognosis multiple myeloma opens new pathways to explore NY-ESO-1–specific immunotherapy as an additional treatment option for patients with plasma cell disorders.
Another approach to cancer-specific immunotherapy is pursued by exploiting graft-versus-tumor effects after allogeneic stem cell transplantation following suitable conditioning regimens. The documented antitumor effects observed after donor lymphocyte infusion (DLI) as pioneered by Kolb et al4 have been a focus of research, with an emphasis on identifying the relevant target antigens. While minor histocompatibility antigens and possibly attached or presented peptide epitopes have been found as one class of target antigens for tumor-directed T-cell responses, natural killer (NK) cell responses are another antitumor effector mechanism postulated.5
Bellucci et al report in this issue of Blood the induction of a strong lytic antibody response to a differentiation antigen, BCMA (B-cell maturation antigen), secondary to DLI in multiple myeloma patients with persistent disease after allogeneic hematopoietic stem cell transplantation (HSCT). BCMA is a cell surface antigen on normal B cells and on multiple myeloma cells. The induced antibody responses as seen in 2 patients turned out to be lytic for tumor cells in vivo by different mechanisms of antibody-mediated tumor cell lysis.
This is a novel and unique observation. Until now, antibodies induced secondary to HSCT and DLI were primarily found to be directed against intracellular antigens. This now is the first report of antibodies with tumor-lytic properties induced in vivo against a cell surface antigen. As discussed in this paper, humoral immune responses against intracellular antigens may also augment pre-existing or induced T-cell responses to the target antigen through antibody-mediated antigen presentation to dendritic cells.
Reported observations of spontaneous as well as induced humoral and cellular immune responses to NY-ESO-1 secondary to NY-ESO-1–specific vaccination in patients with NY-ESO-1–positive cancers other than multiple myeloma are noteworthy in this context.3,6 NY-ESO-1 is a cancer antigen primarily represented in the intracellular compartment. The biologic function of any spontaneous or induced NY-ESO-1 antibodies in patients with NY-ESO-1–positive cancers is unresolved to date.
The papers by van Rhee et al and by Bellucci et al complement each other, adding to the understanding of cancer antigen expression, prognostic features, and immune responses in multiple myeloma. The work points to the therapeutic potential of steering a coordinated immune response against cancer antigens like NY-ESO-1 or BCMA in multiple myeloma. Inducing or augmenting antigen-specific cellular and humoral immune responses to eliminate minimal residual cancer may become an additional treatment modality to improve the outcome for multiple myeloma patients. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal