Abstract
The presence of a metaphase cytogenetic abnormality (CA) is the key negative predictor of outcome in patients with multiple myeloma (MM). Gene expression profiling (GEP) of such patients showed increased expression of NY-ESO-1 compared to patients with normal cytogenetics (60% versus 31%; P = .004). NY-ESO-1 was also highly expressed in relapsing MM especially patients with CA (100% versus 60.7%; P < .001). GEP findings were confirmed at the protein level by immunostaining of marrow biopsies for NY-ESO-1. We detected spontaneous NY-ESO-1–specific antibodies by enzyme-linked immunosorbent assay in 33% of patients with NY-ESO-1+ MM, especially in CA patients (9 of 13; 70%), but in none of the NY-ESO-1- patients with MM (n = 27) or healthy donors (n = 21). Spontaneous NY-ESO-1157-165–specific T cells (0.2%-0.6% of CD8+ T cells) were found in the peripheral blood of NY-ESO-1+ MM with HLA-A*0201/NY-ESO-1157-165 tetramers. These NY-ESO-1–specific T cells, when expanded, killed primary MM cells (50% lysis, effector-target [E/T] ratio, 10:1). Our data demonstrate that NY-ESO-1 is frequently expressed in MM with CA and is capable of eliciting spontaneous humoral and T-cell immunity. The pool of NY-ESO-1–specific cytotoxic T cells expands easily on NY-ESO-1 peptide stimulation and is functionally active. NY-ESO-1 should therefore be an ideal tumor target antigen for immunotherapy of patients with poor-prognosis MM.
Introduction
During the past 10 years, numerous human tumor-associated antigens (Ags) have been identified, either by screening cDNA libraries with sera derived from cancer patients containing an antibody (Ab) to a tumor-associated Ag (SEREX) or by using T lymphocytes specific for tumor peptides presented in the context of specific HLA alleles.1-11 The most rapidly expanding group of tumor Ags are the cancer/testis (C/T) Ags, which are either not expressed or are present at very low levels in normal tissues except the testes and perhaps the placenta.12,13 Because the testes are not patrolled by the immune system, expression of C/T Ags in this environment is not harmful.
Of the C/T Ags described thus far, NY-ESO-1 is among the most immunogenic with not only well-documented spontaneous14-20 and vaccine-induced immunity, but also clinical responses in a substantial percentage of chemorefractory cancers.19,21 NY-ESO-1 mRNA is found in approximately 20% to 40% of tumors including melanoma, prostate, transitional cell bladder, breast, lung, medullary thyroid, squamous head and neck, and cervical carcinoma.12,14,22-27 Because it is expressed in such a wide variety of tumors, NY-ESO-1 offers a unique opportunity to develop a broad-spectrum tumor-specific cancer vaccine.
High-dose chemotherapy with autologous peripheral blood stem cell transplantation (auto-PBSCT) has significantly improved the outcome of patients with multiple myeloma (MM).28-37 We and others have shown that the presence of cytogenetic abnormalities (CAs) is the most powerful prognosticator for poor outcome.38-45 Intensification of treatment in our Total Therapy II (TTII) protocol has resulted in additional improvement in event-free (EFS) and overall survival (OS) of patients without CAs (67% of patients). However, no such improvement has yet been observed for patients with CAs (33% of patients).41,43,46 Fewer than 10% of patients treated with tandem auto-PBSCT protocols remain in long-term remission and are considered “operationally cured.”40 These data highlight the urgent need for new approaches to improve diseasefree survival in such patients.
We analyzed our database of the transcriptome of primary MM cells compiled by the gene expression profiling (GEP) of 335 patients to search for tumor-specific Ags suitable for immunotherapy in MM. We selected only Ags expressed in more than 20% of patients with MM. A large number of Ags was rejected because expression of these Ags in normal tissues had been documented, thus raising the potential for autoimmune reactions. Moreover, the selected genes had to be immunogenic and encode for peptides capable of binding to common HLA class I and II alleles.47,48 NY-ESO-1 met all the above criteria.
We correlated NY-ESO-1 expression in primary MM samples with disease stage (newly diagnosed versus relapsed MM) and presence or absence of CAs. We found that NY-ESO-1 is expressed at significantly higher levels in patients with CAs. We determined by immunohistochemistry (IHC) that NY-ESO-1 Ag is also present at the protein level in malignant plasma cells (PCs). We next established that patients with NY-ESO-1+ and CA MM have spontaneous humoral and cytotoxic immune responses to NY-ESO-1. Moreover, expanded NY-ESO-1–specific T lymphocytes were capable of killing primary NY-ESO-1+ MM cells.
Patients, materials, and methods
Patients
NY-ESO-1 expression was studied in 374 patients with MM at diagnosis or relapse by GEP (n = 335) or IHC (n = 39). Twenty of these patients were studied by both GEP and IHC for comparison between NY-ESO-1 RNA and protein expression. Of 19 patients studied by IHC, 13 were studied both at diagnosis and at relapse. Enzyme-linked immunosorbent assay (ELISA) studies for NY-ESO-1 Abs were performed on 66 patients with MM. Informed consent was obtained according to the Declaration of Helsinki and the study was approved by the University of Arkansas for Medical Sciences Institutional Review Board.
Detection of NY-ESO-1 gene expression by GEP
Testing for NY-ESO-1 RNA expression in highly purified (> 95%) CD138+ MM PCs by GEP was performed and analyzed as reported previously.49
Immunohistochemical staining for NY-ESO-1 protein
To detect NY-ESO-1 protein, bone marrow (BM) biopsies were fixed in Zenker fluid at the time of collection and stained by IHC using the NY-ESO-1–specific murine IgG1 monoclonal Ab (mAb) B9.8 and the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) per the manufacturer's instructions. Briefly, sections were deparaffinized with xylene and hydrated after passing through descending grades of ethanol, immersed in preheated Ag retrieval solution, steamed for 30 minutes, cooled, and washed. Endogenous peroxidase activity was abolished using peroxidase block (Dako, Carpinteria, CA) for 10 minutes at room temperature (RT) and 10% goat serum in Tris (tris(hydroxymethyl)aminomethane)–buffered saline, pH 7.6, was used for nonspecific protein blocking. After incubation with undiluted primary mAb B9.8 in a humid chamber for 2 hours at RT, the sections were washed and biotinylated using a goat antimouse secondary Ab (diluted 1:400). After incubation with avidin-biotinperoxidase complex (Vector Laboratories), the chromogenic substrate 3, 3′diaminobenzidine tetrahydrochloride (ResGen; Invitrogen, Carlsbad, CA) was added. The slides were counterstained with hematoxylin-2 (Richard Allan Scientific, Kalamazoo, MI). A tumor-free testicular biopsy was used as a positive control. There was intense staining of the seminiferous tubules with strong intratubular staining. The intertubular connective tissue, spermatids, and Sertoli cells were negative by IHC. Appropriate isotype Ab controls were used. Biopsies from 6 patients with MM in remission (< 5% PCs), which tested negative for NY-ESO-1 by GEP at diagnosis, served as negative controls.
ELISA
Recombinant NY-ESO-1 protein (50 ng), highly purified from Escherichia coli in our laboratory, in sodium carbonate coating buffer (15 mM Na2CO3, 30 mM NaHCO3, pH 9.6) was adsorbed to 96-well plates (Nunc, Roskilde, Denmark) for 2 hours at RT. Plates were washed and blocked overnight at 4°C with 2% bovine serum albumin/phosphate-buffered saline (BSA/PBS). The test serum was diluted (1:50, 1:100, 1:400, and 1:1600) in 2% BSA/PBS and incubated for 2 hours at RT with 50 μL/well of each serum dilution in triplicate. After washing, 50 μL/well of diluted goat anti–human horseradish peroxidase (200 μg/0.5 mL; Santa Cruz Biotechnology, Santa Cruz, CA) in 2% BSA/PBS was added and incubated for 1 hour at RT. Following washing, the substrate solution was added (3, 3′, 5, 5′ tetramethyl benzidine liquid; Sigma Aldrich, St Louis, MO) and 25 minutes later the optical density (OD) at 655 nm was read. Sera from 66 patients with MM was collected and tested for Abs to NY-ESO-1. Thirty-nine patients were positive for NY-ESO-1 RNA by GEP, whereas 27 were negative by GEP. The latter were used as controls along with sera from 21 healthy volunteers. Each assay was performed with positive controls that included NY-ESO-1+ sera from 2 patients that previously tested positive against recombinant NY-ESO-1 protein in an outside laboratory (a kind gift from Dr Brad Stone, Benaroya Research Institute, Seattle, WA) and mAb B9.8. A sample was considered positive if the mean OD of the 1:100 serum dilution was higher than the mean OD value of the healthy donor sera plus 3 times the SD. Further negative controls comprised test sera incubated with the DEK oncoprotein, which was expressed in the same E coli system. Each sample was tested in triplicate in separate ELISAs and yielded consistent results. IgG subclass ELISAs were not performed.
Generation of NY-ESO-1–specific CTLs
A total of 2 to 3 × 105 mature, monocyte-derived dendritic cells (DCs), prepared as previously described,50 pulsed with NY-ESO-1157-C165V peptide were irradiated with 2500 cGy prior to coculture with 2 to 3 × 106 autologous CD3+ or CD8+ T cells purified by negative selection from peripheral blood cells with Ab-coated immunomagnetic beads (Dynal, Oslo, Norway). Then, 500 pg/mL interleukin 12 (IL-12; R&D Systems, Minneapolis, MN) was added. The number of cells was adjusted to 106/mL on day 2 and maintained at this concentration. Cultures were fed every third day with 50% fresh media (RPMI supplemented with 10% human AB+ plasma and 20 IU/mL IL-2; Chiron, Emeryville, CA). Cultures were restimulated weekly with irradiated autologous DCs or peripheral blood mononuclear cells (PBMCs) pulsed with NY-ESO-1157-C165V peptide at a ratio of 1 DC to 20 cytotoxic T lymphocytes (CTLs) or 1 PBMC to 5 CTLs.
Immunophenotyping of NY-ESO-1–specific T cells
The CTLs were stained by standard methods and analyzed on a FACScan flow cytometer (Becton Dickinson, San Diego, CA). NY-ESO-1–specific CTLs were visualized with NY-ESO-1157-165/HLA-A*0201 (A2) and NY-ESO-1157-C165V/A2 phycoerythrin (PE)–labeled tetramers (NIAID Tetramer Facility, Bethesda, MD and Beckman Coulter, Fullerton, CA), which stained NY-ESO-1–specific CTLs equally well. Influenza matrix peptide58-66-specific CTLs were stained with influenza matrix peptide58-66/A2 PE tetramers (Beckman Coulter).
Intracellular staining of CTLs
Intracellular staining for interferon γ (IFN-γ), IL-4, perforin, and granzyme production was performed as previously described50 with the exception that the CTLs were stimulated by NY-ESO-1157-C165V-pulsed autologous DCs for 4 hours prior to staining as opposed to nonspecific stimulation. Quadrant assignments were based on isotype controls designed specifically for intracellular staining.
Cytotoxicity assays with NY-ESO-1–specific T cells
NY-ESO-1–specific CTLs were tested for cytotoxicity in triplicate in standard 4-hour 51CrO4 release assays. Targets were more than 95% purified CD138+, A2+, and NY-ESO-1+ primary MM cells, the A2+ and NY-ESO-1+ MM cell line U266, autologous phytohemagglutinin-induced blasts (PHA-blasts) pulsed with NY-ESO-1157-C165V peptide (positive control) or PHA-blasts pulsed with the HLA-A2–binding MAGE-3112-120 peptide (negative control), and the natural killer (NK)–sensitive cell line K562.
Results
NY-ESO-1 is more frequently expressed in patients with CA or in relapse
GEP analysis of cRNA of more than 95% pure PCs from 335 patients showed that high NY-ESO-1 expression was more frequent at diagnosis in MM with CA compared to cytogenetically normal MM (60% versus 31%; P = .004; Table 1). High NY-ESO-1 expression was even more common in relapsed MM and again positively related to the presence of CA (61% versus 100%; P < .001; Table 1; Figure 1). Patients with newly diagnosed NY-ESO-1+ MM were more likely to have chromosome 13 deletion or hypodiploidy (or both) than NY-ESO-1- patients (31% versus 16%; P = .045). The expression frequency of NY-ESO-1 was low in monoclonal gammopathy of undetermined significance (MGUS; n = 22) and smoldering MM (SMM; n = 34), at 4.5% and 5.9%, respectively (P < .001) compared with newly diagnosed MM requiring intensive chemotherapy or relapsed MM.
Expression of NY-ESO-1
. | Normal cytogenetics . | . | Abnormal cytogenetics . | . | . | ||
|---|---|---|---|---|---|---|---|
| MM stage . | Patients studied . | Percent positive . | Patients studied . | Percent positive . | P . | ||
| Diagnosis | 126 | 31 | 35 | 60 | .004 | ||
| Relapse | 28 | 61 | 27 | 100 | < .001 | ||
. | Normal cytogenetics . | . | Abnormal cytogenetics . | . | . | ||
|---|---|---|---|---|---|---|---|
| MM stage . | Patients studied . | Percent positive . | Patients studied . | Percent positive . | P . | ||
| Diagnosis | 126 | 31 | 35 | 60 | .004 | ||
| Relapse | 28 | 61 | 27 | 100 | < .001 | ||
Newly diagnosed and relapsed patients with cytogenetically abnormal MM have increased expression of NY-ESO-1. P was determined with the Pearson χ2 method.
NY-ESO-1 expression by GEP correlates well with protein expression by IHC
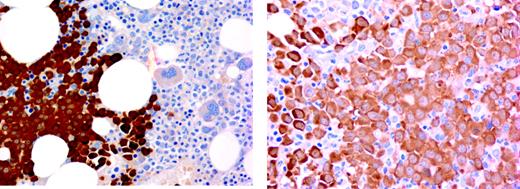
Immunostaining of MM cells by IHC for NY-ESO-1 revealed that NY-ESO-1 was located in the cytoplasm (Figure 2). Simultaneous GEP and IHC data from the same BM aspirate and biopsy were available on 20 patients. All biopsies positive for NY-ESO-1 by GEP also tested positive by IHC, and vice versa. Thus, there is complete concordance between NY-ESO-1 expression at RNA and protein levels.
Because NY-ESO-1 RNA expression by GEP is more frequently increased at relapse than at diagnosis, we next performed IHC to confirm these GEP findings at the protein level. A total of 55 biopsies from 39 patients were available for IHC. NY-ESO-1 protein was detected in all 38 relapse samples compared to 9 of 17 (53%) specimens at diagnosis (P < .001). Sequential studies of IHC-stained biopsies from 13 patients with MM at diagnosis and at relapse further confirmed these findings (diagnosis 5 of 13 versus 13 of 13 NY-ESO-1+; P = .01).
Spontaneous humoral responses are present in poor-prognosis NY-ESO-1+ MM
Ab responses to NY-ESO-1 were detected by ELISA in 13 of 39 (33%) patients who tested NY-ESO-1+ by GEP (Table 2). The antibody responses were titered and remained positive at 1:1600, the highest dilution tested. In contrast, Ab responses to NY-ESO-1 were not detected in any of the 27 NY-ESO-1- patients (P < .001). The mean OD titers in the NY-ESO-1 GEP+ and ELISA+ group, the GEP+ and ELISA- group, and the GEP-NY-ESO-1 group differed significantly at 2.9, 1.2, and 1.2 (P < .001). All 21 healthy donors tested negative. Nine of 13 patients with Ab to NY-ESO-1 had CAs, indicating that patients with poor-prognosis MM were also able to mount a CD4+ T-cell and humoral response to NY-ESO-1.
Antibody responses to NY-ESO-1
Sample type . | No. tested . | NY-ESO-1+ ELISA . |
|---|---|---|
| MM, GEP NY-ESO-1+ | 39 | 13 (39%) |
| MM, GEP NY-ESO-1- | 27 | 0 |
| Healthy donors | 21 | 0 |
Sample type . | No. tested . | NY-ESO-1+ ELISA . |
|---|---|---|
| MM, GEP NY-ESO-1+ | 39 | 13 (39%) |
| MM, GEP NY-ESO-1- | 27 | 0 |
| Healthy donors | 21 | 0 |
A humoral response to NY-ESO-1 protein is detected in one third of patients with NY-ESO-1+ myeloma (P = .003).
NY-ESO-1 gene expression. Gene expression profiling shows that NY-ESO-1 is expressed at increased frequency in patients with abnormal cytogenetics (a positive score is more than mean + 3 SD [90] of the GEP score of normal human PCs). SMM indicates smoldering MM.
NY-ESO-1 gene expression. Gene expression profiling shows that NY-ESO-1 is expressed at increased frequency in patients with abnormal cytogenetics (a positive score is more than mean + 3 SD [90] of the GEP score of normal human PCs). SMM indicates smoldering MM.
BM biopsy stained with B9.8 shows strong expression of NY-ESO-1 protein in myeloma plasma cells. Images (left panel, original magnification × 10, numerical aperture 0.3; right panel, original magnification × 20, numerical aperture 0.46) were visualized with an Olympus microscope, type BH2-RFCA. Images were acquired with a SPOT camera (Diagnostics Instruments, Sterling Heights, MI) and SPOT Advanced version 3.5.6 software without image manipulation.
BM biopsy stained with B9.8 shows strong expression of NY-ESO-1 protein in myeloma plasma cells. Images (left panel, original magnification × 10, numerical aperture 0.3; right panel, original magnification × 20, numerical aperture 0.46) were visualized with an Olympus microscope, type BH2-RFCA. Images were acquired with a SPOT camera (Diagnostics Instruments, Sterling Heights, MI) and SPOT Advanced version 3.5.6 software without image manipulation.
Of the 19 patients studied in the presence of frank MM (diagnosis, n = 11; progressive/relapsed disease, n = 8), 9 patients (47%) had Ab to NY-ESO-1. In contrast, only 4 of 20 (20%) NY-ESO-1+ patients achieving either a major tumor reduction after therapy (n = 13) or (near) complete remission (CR; n = 7) had NY-ESO-1 Abs detectable (P = .08). This suggests a relationship between tumor load and the presence of NY-ESO-1 aAs. There was no relationship between ELISA positivity and absolute CD3+, CD4+, and CD19+ counts per microliter (data not shown).
Spontaneous NY-ESO-1–specific T cells to NY-ESO-1 are present in patients with NY-ESO-1+ poor-prognosis MM
We investigated banked PBMNCs from 3 patients who had NY-ESO-1+ MM with CA and who were HLA-A2+. This allowed for the enumeration in the peripheral blood by tetramer analysis of spontaneously present CD8+ T cells specific for the immunodominant peptide NY-ESO-1157-165 in the context of HLA-A2. Spontaneous NY-ESO-1–specific T cells comprised 0.2% and 0.6%, respectively, of the CD8+ population in 2 patients. The third patient had been heavily pretreated including tandem auto-PBSCT. Control cultures with the HLA-A2–binding influenza matrix peptide (IM58-66) were performed in all 3 patients both to check the specificity of the tetramers and to examine the immunocompetence of the patients. In patients 1 and 2 we could readily expand IM58-66-specific T cells, but this was not possible in patient 3 indicating that the immune system of this patient may have been exhausted.
NY-ESO-1–specific CTLs kill primary MM cells
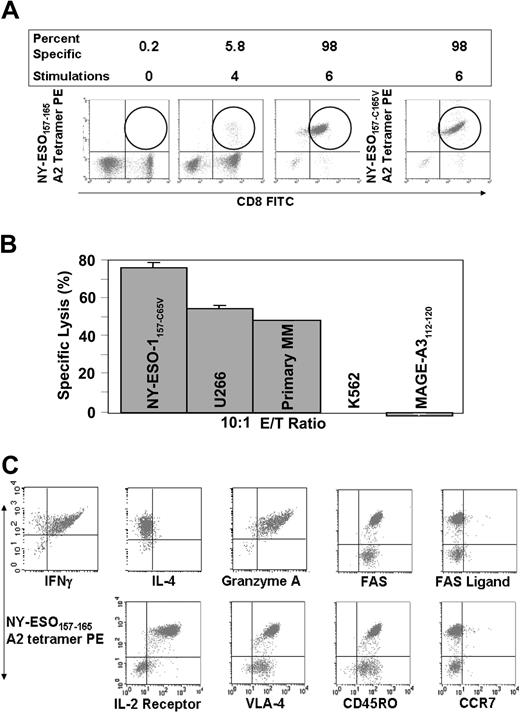
We expanded NY-ESO-1–specific T cells by restimulation with autologous antigen-presenting cells (APCs) pulsed with the NY-ESO-1157-C165V analog peptide, which is more immunogenic due to better binding to HLA-A2 and is also easier to work with in vitro due to its superior stability. The expansion of NY-ESO-1–specific CTLs allowed for detailed investigation of their functional capacity. After expansion, CD8+ NY-ESO-1157-C165V–specific T cells comprised 98% of the CD8+ T-cell population. HLA-A2 tetramers loaded with either wild-type NY-ESO-1157-165 or analog NY-ESO-1157-C165V peptide recognized the NY-ESO-1–specific CTLs equally well (Figure 3A). We were unable to generate NY-ESO-1+ CTLs from 3 NY-ESO-1- healthy donors suggesting that pre-existing immunity to NY-ESO-1 is a prerequisite for expansion of NY-ESO-1–specific CTLs.
The CD8+ NY-ESO-1–specific CTLs killed primary MM, the NY-ESO-1+ and HLA-A2+ MM cell line U266, and the patient's normal cells pulsed with NY-ESO-1157-165 peptide but not the patient's normal cells pulsed with the HLA-A2–binding MAGE-3112-120 (irrelevant peptide) or the NK cell target K562 (Figure 3B). The CTLs were of Tc1 type (IFN-γ + and IL-4-), activated (IL-2 receptor expressed), and had abundant granzymes in their cytoplasm suggesting killing via the perforin/granzyme pathway (Figure 3C). The CTLs did not express CCR7, the lymph node homing receptor, but did express CD45RO, and not CD45RA, and therefore predominantly belonged to the memory effector compartment. Staining with annexin V and 7-amino-actinomycin D (7-AAD) showed little apoptosis and cell death in vitro (data not shown). Fas was abundantly expressed on the specific CTLs, but there was no expression of Fas ligand on CTLs suggesting that there was no homotypic killing of NY-ESO-1–specific CTLs.
Expansion of NY-ESO-1–specific CTLs. (A) NY-ESO-1–specific T cells are spontaneously present and can be progressively expanded by stimulation with APCs pulsed with NY-ESO-1157-C165V analog peptide. Note that there is equivalent staining with NY-ESO-1157-165 wild-type and NY-ESO-1157-C165V analog peptide-loaded A2 tetramers. (B) NY-ESO-1–specific CTLs kill both primary, the HLA-A2+ U266 myeloma cell line, and autologous PHA-blasts pulsed with NY-ESO-1157-C165V, and not control PHA-blasts pulsed with a MAGE-3112-120 A2-binding peptide or K562 cells. (C) NY-ESO-1–specific CTLs produce IFN-γ and not IL-4 (Tc1 type), contain cytolytic granules, and are of memory-effector type. VLA indicates very late antigen; FITC, fluorescein isothiocyanate.
Expansion of NY-ESO-1–specific CTLs. (A) NY-ESO-1–specific T cells are spontaneously present and can be progressively expanded by stimulation with APCs pulsed with NY-ESO-1157-C165V analog peptide. Note that there is equivalent staining with NY-ESO-1157-165 wild-type and NY-ESO-1157-C165V analog peptide-loaded A2 tetramers. (B) NY-ESO-1–specific CTLs kill both primary, the HLA-A2+ U266 myeloma cell line, and autologous PHA-blasts pulsed with NY-ESO-1157-C165V, and not control PHA-blasts pulsed with a MAGE-3112-120 A2-binding peptide or K562 cells. (C) NY-ESO-1–specific CTLs produce IFN-γ and not IL-4 (Tc1 type), contain cytolytic granules, and are of memory-effector type. VLA indicates very late antigen; FITC, fluorescein isothiocyanate.
Discussion
Using GEP in a very large number of patients, we have confirmed previous reports on limited myeloma cases, showing a high frequency of C/T Ag expression in MM.51-54 Our present GEP and cytogenetic findings clearly show that NY-ESO-1 is more frequently expressed in MM with CA, including patients who have deletion of chromosome 13. At diagnosis approximately 60% of patients with CA express NY-ESO-1, whereas at relapse 100% of patients with CA expressed NY-ESO-1. In contrast, NY-ESO-1 was expressed in only 5% to 11% of patients with MGUS or smoldering MM.
We and others have documented that NY-ESO-1 is not expressed, or is at low levels, in normal tissues, including CD34+ cells and normal PCs (data not shown). NY-ESO-1 is therefore an obvious candidate Ag for true MM-specific immunotherapy to supplement the effects of tandem transplants in poor-prognosis patients. These patients attain a CR or near CR with the same frequency as patients with normal cytogenetics, but these responses are not durable.
We examined BM biopsies at the protein level and found that all samples that were positive for NY-ESO-1 RNA by GEP were also positive by IHC. It was important to confirm RNA findings at the protein level because there have been reports that NY-ESO-1 expression by reverse transcription-polymerase chain reaction (RT-PCR) is not always confirmed by IHC, presumably due to the low level of RNA expression in these patients.14,23 We confirmed the GEP finding that NY-ESO-1 protein is more frequently expressed at relapse than at diagnosis (P < .001). We have also studied the C/T Ag MAGE-A3 and our findings mirrored the observations made with NY-ESO-1; MAGE-A3 is also frequently expressed in patients at diagnosis and relapse with abnormal cytogenetics (data not shown).
Other investigators have reported in small series that expression of C/T Ags both at the RNA or protein level is more prevalent in advanced MM based on the Salmon-Durie staging or in the presence of extramedullary plasmacytomata.51-53 Our findings and the reports of other investigators support the notion that the expression of NY-ESO-1 is related to the clonal evolution of MM.51,55 These data in MM are consistent with reports that NY-ESO-1 is expressed with increasing frequency during progression of malignancies in general, probably by gene demethylation.22,26,27
The presence of spontaneous antibody responses to NY-ESO-1 is important because this suggests that there is already a reservoir of NY-ESO-1–specific CD4+ T cells relatively early in the disease that could be expanded and used for the immunotherapy of advanced MM by repeated vaccinations with NY-ESO-1 peptides or proteins. We demonstrated that NY-ESO-1 GEP+ patients were able to mount spontaneous humoral, NY-ESO-1–specific immune responses. The presence of a NY-ESO-1–specific Ab response is an excellent surrogate marker for the presence of a CD4+ NY-ESO-1– specific T-cell response, which will help to prime, expand, and most importantly, sustain CD8+ NY-ESO-1–specific T-cell responses. The CD4+ population may also exert effector functions both via macrophage and eosinophil activation and via cytokines such as IFN-γ that up-regulate expression of MHC class I and II complexes on many tumor cells.56-59 A caveat is that IgG subclass ELISAs were not performed and it can therefore not be completely excluded that some Ab+ patients may have type 2 cellular immune responses.
A high frequency of NY-ESO-1–specific Ab has also been detected in patients with advanced stage IV, NY-ESO-1+ melanoma.16,65 Interestingly, NY-ESO-1–specific Abs were detected less frequently in our patients who had obtained an excellent response to therapy. Our data are consistent with others who found in melanoma that a specific humoral immune response appears to be Ag-driven and removal or regression of NY-ESO-1+ tumor results in loss of Ab.60
Spontaneous NY-ESO-1–specific CD8+ T cells were detected by tetramer staining of the blood of NY-ESO-1+ patients with MM, at levels from 0.2% to 0.6% prior to any restimulation. The NY-ESO-1–specific cells were detected both by NY-ESO-1157-165/HLA-A2 and HLA-A2 tetramers loaded with the more immunogenic NY-ESO-1157-C165V analog peptide. Substitution of cysteine for valine at position 165 of the NY-ESO-1 peptide reduces dimerization of the carboxy terminus cysteine and improves binding affinity for HLA-A2, resulting in enhanced antigenicity.61,62 We were able to significantly expand NY-ESO-1–specific CTLs by repeated stimulation with autologous mature DCs or PBMCs pulsed with NY-ESO-1 peptide from NY-ESO-1+/HLA-A2 patients with MM. These expanded CTLs killed primary MM cells effectively, which indicates that CTLs were not only present but also functional and able to recognize naturally processed and presented NY-ESO-1 protein and displayed NY-ESO-1157-165 peptide in the context of HLA-A2 at the cell surface. We were unable to generate and expand NY-ESO-1–specific CTLs lines in vitro from NY-ESO-1- patients with MM or from healthy individuals. This suggests that stimulation with NY-ESO-1 peptide does not lead to de novo priming of NY-ESO-1–specific CTLs. A good correlation between humoral and cellular immune responses has been observed mainly in advanced melanoma both spontaneously and after peptide vaccination.18,19 Vaccine-induced T-cell responses to NY-ESO-1 have been observed and in one study all patients with humoral responses to NY-ESO-1 showed a strong CD8+ T-cell response, with not only strong concordance between enzyme-linked immunospot assay (ELISPOT), tetramer, and cytotoxicity assays for CD8+ T-cell reactivity to NY-ESO-1, but also stabilization of disease or regression of a single metastasis.21,63
Our data support the suitability of NY-ESO-1 immunotherapy in patients with poor-prognosis MM, preferably in combination with chemotherapy and auto-PBSCT to attain a state of minimal residual disease, which should be optimal for effective immunotherapy. We already have data showing the feasibility of combining auto-PBSCT with immunotherapy, by early (ie, prior to transplantation) vaccination with DCs loaded with tumor lysate and collecting and freezing of specific T cells prior to transplantation. After transplantation, the primed T cells are infused and immediately boosted with further vaccinations. Early vaccination expands memory cells, which are much more resistant even to high-dose chemotherapy than naive T cells and recover more promptly after transplantation. We will test the novel strategy of priming anti-MM T cells before auto-PBSCT and protecting the primed T cells from high-dose chemotherapy by apheresis and cryopreservation of the primed cells in a new clinical trial for high-risk MM using vaccinations with NY-ESO-1 Ag.
Finally, we expect that analysis of the MM transcriptome or protein profile or both will allow us to predict in the near future with high confidence which patients with MGUS or smoldering MM are likely to progress to frank MM and thus require high-dose chemotherapy. We are considering prophylactic vaccination with an optimized NY-ESO-1 vaccine64,65 for these patients to prevent disease progression by eradicating emerging more malignant MM clones, which are likely to be NY-ESO-1+.66
Prepublished online as Blood First Edition Paper, January 25, 2005; DOI 10.1182/blood-2004-09-3707.
Supported by the 2002 Multiple Myeloma Research Foundation (MMRF) Senior Award no. 12716, 2002 MMRF Junior Award no. 07941, and grant no. CA55819-08 (PO1) from the National Institutes of Health. S.M.S. and F.Z. contributed equally to the paper.
An Inside Blood analysis of this article appears at the front of the issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to acknowledge Professor David Mason, Leukemia Research Foundation, Immunodiagnostic Unit, John Radcliffe Hospital, Oxford, United Kingdom, for his expert advice regarding IHC staining. Professor Lloyd Old, Ludwig Cancer Institute, New York, NY, is acknowledged for helpful discussion. Use of the Digital and Confocal Microscopy Laboratory facilities of the Department of Pathology in the Arkansas Cancer Research Center, supported by National Institutes of Health (NIH) grants 1 P20 RR 16460 and PAR-98-092, 1-R24 CA8289, is acknowledged. We further acknowledge the NIH Tetramer Facility (Bethesda, MD) as the source of the NY-ESO-1157-C165V/A2 tetramer reagent.

![Figure 1. NY-ESO-1 gene expression. Gene expression profiling shows that NY-ESO-1 is expressed at increased frequency in patients with abnormal cytogenetics (a positive score is more than mean + 3 SD [90] of the GEP score of normal human PCs). SMM indicates smoldering MM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/10/10.1182_blood-2004-09-3707/6/m_zh80100578580001.jpeg?Expires=1769087710&Signature=mJbEzYkSJgrKIiqc19LTdZAfdvqg~bbmScokqDbF75pcCervcu9sGovZvBpSnbntYsdEDlRYdtspvg4dIGfCexrip1lNpPX-EfBQYSdkA4WKw6QgwfXzYTVc~Fh-ibZc9lAja05MjMcVSRwgvimB3oHb3dPfB~EE7oshyEDMM2Tge~CcHklCZjfQLVu~umMdc0Y2qoKq4enyoaYaHojmoKUZeH9nIzoVVjYuq6jmr-ajt66d4WhKUxGr0CUwIplYiTe3-~wiY480W9AKwzRN6H3XJHZR7gMKBnypMq1cU5pt2R1Vb4~n-5vUDdAc2YqcDc7Drt4vQBs8x~9-HMrrLg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal