Abstract
Phosphoinositide 3-kinase (PI3K) is activated by transmembrane tyrosine kinases such as vascular endothelial growth factor (VEGF) receptors and Tie2 (tunica intima endothelial kinase 2), both of which are key regulators of vascular development. However, the in vivo role of PI3K during developmental vascularization remains to be defined. Here we demonstrate that mice deficient in the p110α catalytic subunit of PI3K display multiple vascular defects, including dilated vessels in the head, reduced branching morphogenesis in the endocardium, lack of hierarchical order of large and small branches in the yolk sac, and impaired development of anterior cardinal veins. These vascular defects are strikingly similar to those in mice defective in the Tie2 signaling pathway. Indeed, Tie2 protein levels were significantly lower in p110α-deficient mice. Furthermore, RNA interference of p110α in cultured endothelial cells significantly reduced Tie2 protein levels. These findings raise the possibility that PI3K may function as an upstream regulator of Tie2 expression during mouse development.
Introduction
Phosphoinositide 3-kinases (PI3Ks) are important mediators of many transmembrane signaling receptors, including tyrosine kinases and 7-transmembrane G-protein–coupled receptors. The class Ia PI3K (abbreviated as PI3K from here on) typically contains p85/p55 as a regulatory subunit and p110α, p110β, or p110δ as the catalytic subunit, and their activities are regulated by Ras and tyrosine kinases (for a review, see Fruman et al1 ). The p110α catalytic subunit is widely expressed in a variety of cell types, including endothelial cells.2-6 Numerous studies have demonstrated that PI3K plays important roles in regulating endothelial proliferation, migration, and survival.7-12 Although these studies indicated that the transmembrane tyrosine kinase Flk-1/VEGF-A receptor-2/KDR plays a crucial role in regulating PI3K activity in endothelial cells,9,13 Tie2 (tunica intima endothelial kinase 2), a tyrosine kinase receptor for angiopoietin-1 (Ang-1) and related molecules, also signals through PI3K to regulate endothelial properties.14-19
Targeted disruption of the gene encoding the p110α subunit (Pik3ca) of PI3K in mice resulted in embryonic lethality in homozygous mutants between embryonic day 9.5 (E9.5) and E10.5,20 but it is unclear whether PI3K is important for vascular development. In this paper, we report that these mice experience significant reductions in Tie2 expression and multiple vascular defects that are highly reminiscent of those in Tie2 mutant mice.
Study design
Gene targeting for Pik3ca, which encodes p110α, has been described previously.20 Stocks of heterozygous mice (Pik3ca+/del [Pik3ca+/-]) were maintained in both 129X1 and CD1 strains, but embryos from CD1 background were used in this study. Genotypes were determined by polymerase chain reaction (PCR) of tail DNA samples using oligonucleotide primers: 5′-CAA GCC TGG GGA GAT GGT CA-3′ and 5′-CCC GAA GAT GGT CGT GGA GG-3′ for wild-type allele (431 base pairs [bp]); 5′-CAA GCC TGG GGA GAT GGT CA-3′ and 5′-CCC GAA GAT GGT CGT GGA GG-3′ for the mutant allele (526 bp). To introduce an endothelial cell–specific reporter gene, Pik3ca+/- mice were crossed with transgenic mice expressing LacZ under the control of Tie2 regulatory sequences21 (T. Sato/Jackson Laboratory, Bar Harbor, ME). X-gal and immunohistochemical (IHC) staining reactions were performed as described.22-24 Primary antibodies included anti–Flk-1 (clone Avas 12a1) (BD Biosciences, PharMingen, San Diego, CA), anti-CD31 (clone Mec 13.3) (BD Biosciences), and anti-Tie2 (clone TEK4, raised against mouse Tie2 extracellular domain) (eBioscience, San Diego, CA).
For Western blotting, embryos proper or yolk sacs were lysed in RIPA buffer (1 × phosphate-buffered saline [PBS]; 1% NP-40; 0.5% sodium deoxycholate; 0.1% sodium dodecyl sulfate [SDS]) containing protease inhibitors (Complete; Roche Applied Science, Indianapolis, IN). Proteins from cleared lysates were subject to Western blotting analyses with the following antibodies: anti-Tie2 (clone 33, raised against human Tie2 extracellular domain) (BD Biosciences), antiphospho-Akt (Cell Signaling Technology, Beverly, MA), anti-Akt (BD Biosciences), or anti–β-actin (Santa Cruz, Santa Cruz, CA). Signals were revealed using horseradish peroxidase (HRP)–conjugated secondary antibodies and chemoluminescence detection system (Amersham Biosciences, Piscataway, NJ). Band intensities were determined using the National Institutes of Health (NIH) Image program.
For p110α knockdown experiments, MS1 endothelial cells25 (American Type Culture Collection [ATCC], Manassas, VA) were cultured in Dulbecco modified Eagle medium (DMEM; Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS; HyClone, Logan, UT) and were transfected with specific siRNA directed against mouse p110α (SmartPool; Dharmacon, Lafayette, CO). The specificity of p110α SmartPool siRNA was established by the supplier. In addition, we included LacZ siRNA as a control for nonspecific effects. Transfections were performed using Lipofectamine 2000 (Invitrogen) following the manufacturer's recommendations. Seventy-two hours after transfection, cells were lysed in ice-cold RIPA buffer as described for embryonic tissues. Proteins in cleared lysates were analyzed by Western blotting. Anti-p110α was affinity purified rabbit polyclonal antibodies from Cell Signaling Technology (Beverly, MA).
Results and discussion
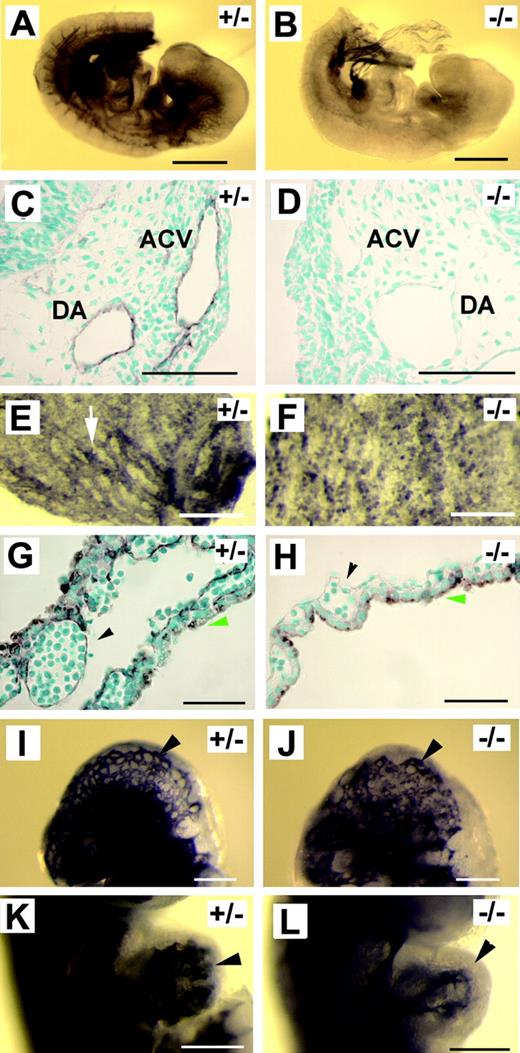
In CD1 strain background, Pik3ca-/- embryos were similar to those in 129X1, but they survived a few hours longer and were still viable at E9.5, as indicated by beating hearts. Dissection at E9.5 first revealed that yolk sacs of Pik3ca-/- embryos were invariably pale, and blood congestion was apparent in the head (Figure 1A-D). To document vascular defects in Pik3ca-/- mice, we visualized the vascular system by X-gal staining after crossing the Pik3ca+/- mice with a transgenic mouse line expressing the Escherichia coli lacZ reporter gene under the control of Tie2 promoter/enhancer,21 which is essentially endothelial specific. In Figure 1E and F, we show that normal and mutant embryos developed dorsal aortae (red arrowheads). In Figure 1E, the anterior cardinal vein (ACV) is clearly visible (white arrowheads). In the mutant, only patches of X-gal–positive signals are seen where the ACV normally forms (Figure 1F), suggesting incomplete development of the ACV. In transverse sections of normal embryos, ACVs are seen as prominent lumens flanking the dorsal aortae (Figure 1G). In mutants, ACV lumens are significantly smaller (Figure 1H). These observations further confirm that ACV was incompletely formed in Pik3ca-/- embryos. Defective development of the ACV may be responsible for the accumulation of blood in the head because blood return from the head to the atrium may be blocked as a result of ACV abnormality.
p110α-deficient embryos develop Tie2-/-–like vascular defects at E9.5. (A-B) Yolk sacs. Arrowhead points to a major branch of vitelline vessels. (C-D) Embryos proper. Arrowheads indicate blood congestion. (E-F) Mid-trunk region of Pik3ca+/- and Pik3ca-/- embryos. White arrowheads point to ACV (E) or to the corresponding position in the mutant at which the ACV is poorly assembled (F). Red arrowheads point to dorsal aortae. Images are focused at the level of the ACV. (G-H) Transverse sections at the level of branchial arches. Arrowheads point to ACV lumens. (I-J) X-gal–stained hearts. (K-L) Transverse sections through the center of the heart. Black arrowheads point to endocardial linings. (M-N) Whole-mount, X-gal–stained yolk sacs. Arrowhead in panel M indicates a large vessel, and arrow points to a small branch. (O-P) Histologic sections of X-gal–stained yolk sacs. Arrowhead in panel O points to a large vascular lumen. (Q-R) Transverse sections at the forebrain region of X-gal–stained embryos. Note that vascular lumens in panel R are significantly dilated and that endothelial cells are only weakly stained (arrowhead). (S-T) X-gal–stained embryos at E9.5. Arrowhead in panel T indicates weak X-gal staining signal. Images for whole-mount tissues (A-F, I, J, M, N, S, and T) were taken with a CoolSNAP CCD digital camera (Photometrics-Roger Scientific, Tucson, AZ) attached to a Leica MZFLIII stereomicroscope (Leica Microsystems, Heerbrugg, Switzerland). A single objective lens (Leica Plan 1.0, 10× magnification, Leica Microsystems) was used to obtain the images. Openlab 3.0 (Improvision, Lexington, MA) was used for initial image acquisition, and Photoshop 5.0 (Adobe Systems, San Jose, CA) was used for subsequent processing. Images for histologic sections (G, H, K, L, O, and P-R) were photographed using Carl Zeiss manufactured images products (Carl Zeiss, Göttingen, Germany), including an AxioCam CR color digital camera, AxioCam image acquisition software, a Zeiss Apochromat objective lens (63× for G, H, K, L, Q, and R; 20× for O and P), and an AxioPlan2 binocular microscope. Images in panels A-D were taken from freshly dissected embryos without staining; other specimens were stained with X-gal. All whole-mount embryo/yolk sac photographs were taken with specimens completely submerged in phosphate-buffered saline. Histological sections were counterstained with nuclear fast red. Scale bars: (A-D) 1 mm; (E-F, M-N) 400 μm; (G-H, K-L) 60 μm; (I-J) 250 μm; (O-P) 120 μm; (Q-R) 50 μm; (S-T) 500 μm.
p110α-deficient embryos develop Tie2-/-–like vascular defects at E9.5. (A-B) Yolk sacs. Arrowhead points to a major branch of vitelline vessels. (C-D) Embryos proper. Arrowheads indicate blood congestion. (E-F) Mid-trunk region of Pik3ca+/- and Pik3ca-/- embryos. White arrowheads point to ACV (E) or to the corresponding position in the mutant at which the ACV is poorly assembled (F). Red arrowheads point to dorsal aortae. Images are focused at the level of the ACV. (G-H) Transverse sections at the level of branchial arches. Arrowheads point to ACV lumens. (I-J) X-gal–stained hearts. (K-L) Transverse sections through the center of the heart. Black arrowheads point to endocardial linings. (M-N) Whole-mount, X-gal–stained yolk sacs. Arrowhead in panel M indicates a large vessel, and arrow points to a small branch. (O-P) Histologic sections of X-gal–stained yolk sacs. Arrowhead in panel O points to a large vascular lumen. (Q-R) Transverse sections at the forebrain region of X-gal–stained embryos. Note that vascular lumens in panel R are significantly dilated and that endothelial cells are only weakly stained (arrowhead). (S-T) X-gal–stained embryos at E9.5. Arrowhead in panel T indicates weak X-gal staining signal. Images for whole-mount tissues (A-F, I, J, M, N, S, and T) were taken with a CoolSNAP CCD digital camera (Photometrics-Roger Scientific, Tucson, AZ) attached to a Leica MZFLIII stereomicroscope (Leica Microsystems, Heerbrugg, Switzerland). A single objective lens (Leica Plan 1.0, 10× magnification, Leica Microsystems) was used to obtain the images. Openlab 3.0 (Improvision, Lexington, MA) was used for initial image acquisition, and Photoshop 5.0 (Adobe Systems, San Jose, CA) was used for subsequent processing. Images for histologic sections (G, H, K, L, O, and P-R) were photographed using Carl Zeiss manufactured images products (Carl Zeiss, Göttingen, Germany), including an AxioCam CR color digital camera, AxioCam image acquisition software, a Zeiss Apochromat objective lens (63× for G, H, K, L, Q, and R; 20× for O and P), and an AxioPlan2 binocular microscope. Images in panels A-D were taken from freshly dissected embryos without staining; other specimens were stained with X-gal. All whole-mount embryo/yolk sac photographs were taken with specimens completely submerged in phosphate-buffered saline. Histological sections were counterstained with nuclear fast red. Scale bars: (A-D) 1 mm; (E-F, M-N) 400 μm; (G-H, K-L) 60 μm; (I-J) 250 μm; (O-P) 120 μm; (Q-R) 50 μm; (S-T) 500 μm.
X-gal staining also revealed a significant reduction in endocardial branching resulting from the Pik3ca-/- mutation (Figure 1I-J). We compared a large number of serial sections and found that endocardial lumens appeared highly complex in normal embryos but that they were much simpler in mutants (Figure 1K-L). This observation is consistent with the whole-mount staining data shown in Figure 1I and J, which demonstrate that the difference in endocardial organization is global throughout the heart. In the yolk sac, the differentiation of large vessels failed in mutant embryos (Figure 1M-N), and lumens were generally small in histologic sections (Figure 1O-P). In addition to these defects, vessels in the forebrain region were dilated (Figure 1Q-R), and the expression of the LacZ reporter was significantly reduced at the anterior end of Pik3ca-/- embryos (Figure 1S-T).
Morphologic manifestations of Pik3ca-/- mice were strikingly similar to those in Tie2-/- embryos,22,26 or embryos in which Tie2 signaling is disrupted by a targeted disruption of angiopoietin-1 or by the overexpression of Ang-2.23,24 Therefore, we wondered whether Tie2 expression was compromised in p110α-deficient embryos. Because Tie2–LacZ reporter gene expression was similar between Pik3ca-/- mutants and controls (except for the forebrain region), Tie2 promoter activity was probably not drastically affected by Pik3ca-/- mutation. Consistent with this notion, we were unable to detect significant reductions of Tie2 expression by multiple repeats of semiquantitative reverse transcription–PCR (RT-PCR) (data not shown). However, from more than 40 embryos examined by anti-Tie2 immunohistochemical staining, we found that though blood vessels in wild-type and heterozygous embryos were consistently positive for staining, none of the Pik3ca-/- embryos showed vascular-specific positive staining (Figure 2A-D), except for some ventral tissues at the posterior end. A similar observation was also made in yolk sacs (Figure 2E-H). To exclude the possibility that reduced Tie2 protein level in Pik3ca-/- embryos might be caused by a nonspecific and global failure of gene expression in mutant endothelial cells, we performed anti–Flk-1 and anti-CD31 staining. These antibodies stained positively in normal and mutant embryos, and examples from anti–Flk-1 staining are shown in Figure 2I to L.
Tie2 protein level is significantly reduced in p110α-deficient mice. (A-H) Anti–Tie2 IHC staining. (A-B) Whole-mount anti-Tie2 IHC of E9.5 embryos. (C-D) Transverse sections of anti-Tie2–stained embryos (at the level of branchial arches). DA indicates dorsal aorta. (E-F) E9.5 yolk sac membranes after whole-mount anti-Tie2 staining. Arrow in panel E points to a Tie2-positive blood vessel. (G-H) Histologic sections of anti-Tie2–stained yolk sac membranes. Arrowheads in panels G and H point to endothelial linings of vascular lumens. Green arrows mark the endoderm. Staining signals on the endoderm were the result of nonspecific antibody binding. (I-L) Anti–Flk-1 IHC staining. (I-J) Head vasculature (arrowheads). (K-L) Embryonic hearts. Arrowheads point to endocardial linings. Images were captured as described in Figure 1, except that methyl green was used as counterstaining dye for panels C, D, G, and H. Scale bars: (A-B) 500 μm; (C-D, G-H) 50 μm; (E-F, I-L) 200 μm.
Tie2 protein level is significantly reduced in p110α-deficient mice. (A-H) Anti–Tie2 IHC staining. (A-B) Whole-mount anti-Tie2 IHC of E9.5 embryos. (C-D) Transverse sections of anti-Tie2–stained embryos (at the level of branchial arches). DA indicates dorsal aorta. (E-F) E9.5 yolk sac membranes after whole-mount anti-Tie2 staining. Arrow in panel E points to a Tie2-positive blood vessel. (G-H) Histologic sections of anti-Tie2–stained yolk sac membranes. Arrowheads in panels G and H point to endothelial linings of vascular lumens. Green arrows mark the endoderm. Staining signals on the endoderm were the result of nonspecific antibody binding. (I-L) Anti–Flk-1 IHC staining. (I-J) Head vasculature (arrowheads). (K-L) Embryonic hearts. Arrowheads point to endocardial linings. Images were captured as described in Figure 1, except that methyl green was used as counterstaining dye for panels C, D, G, and H. Scale bars: (A-B) 500 μm; (C-D, G-H) 50 μm; (E-F, I-L) 200 μm.
Analyses of Tie2 protein levels in embryos and cultured endothelial cells by Western blotting. (A) Analyses of Tie2 protein levels in wild-type (wt) and Pik3ca-/- (-/-) tissues. Tie2 is significantly reduced in Pik3ca-/- embryos and yolk sacs. Akt expression is unaltered, but its phosphorylation level (P-Akt) is reduced in Pik3ca-/- tissues. Western blots are representative of 3 repeats. (B) Effect of p110α knock-down on Tie2 expression. Lane 1, LacZ siRNA as control; lane 2, 80 pmol/mL of p110α SmartPool siRNA; lane 3, 20 pmol/mL of p110α SmartPool siRNA. (C) Bar graph representation of results shown in panel B. p110α and Tie2 protein levels are shown as percentages of remaining protein levels relative to lacZ siRNA control. Numbers on the horizontal axis correspond to lane numbers in panel B.
Analyses of Tie2 protein levels in embryos and cultured endothelial cells by Western blotting. (A) Analyses of Tie2 protein levels in wild-type (wt) and Pik3ca-/- (-/-) tissues. Tie2 is significantly reduced in Pik3ca-/- embryos and yolk sacs. Akt expression is unaltered, but its phosphorylation level (P-Akt) is reduced in Pik3ca-/- tissues. Western blots are representative of 3 repeats. (B) Effect of p110α knock-down on Tie2 expression. Lane 1, LacZ siRNA as control; lane 2, 80 pmol/mL of p110α SmartPool siRNA; lane 3, 20 pmol/mL of p110α SmartPool siRNA. (C) Bar graph representation of results shown in panel B. p110α and Tie2 protein levels are shown as percentages of remaining protein levels relative to lacZ siRNA control. Numbers on the horizontal axis correspond to lane numbers in panel B.
To further evaluate the effect of p110α deficiency on Tie2 protein levels, we analyzed yolk sacs and embryos by Western blotting. As shown in Figure 3A, Tie2 levels were significantly lower in p110α-deficient samples (42% and 31% of normal levels in the yolk sac and embryo proper, respectively). In addition, we also verified that p110α deficiency did not affect the expression of Akt but resulted in significantly reduced phosphorylation of Akt, suggesting that p110α is the major isoform of catalytic subunit mediating PI3K activity in E9.5 mouse embryos. To further establish the relationship between defective PI3K signaling and reduced Tie2 expression, we analyzed the effect of siRNA-mediated knockdown of p110α in cultured endothelial cells. In MS1 cells transfected with 80 pmol/mL of p110α-specific siRNA, p110α was expressed only at 16% of the normal level, indicating successful knockdown. At 20 pmol/mL, p110α was expressed at 34%. The levels of Tie2 protein correlated with the abundance of p110α and were present at 37% and 72% of normal levels, respectively.
These data clearly indicate that PI3K in endothelial cells is required for normal expression of Tie2, an important regulator for vascular development. PI3K might directly regulate the expression or activity of a factor required for the control of Tie2 protein level. Alternatively, PI3K might be a regulator of another signaling pathway that influences Tie2 expression. In cultured endothelial cells, many studies have shown that PI3K is a downstream effector of Tie2 signaling.14-19 However, our observation does not contradict a role for PI3K as a Tie2 effector. Our data do not exclude the intriguing possibility that PI3K might help maintain a normal Tie2 protein level by its interaction with Tie2. The dual roles of PI3K in downstream Tie2 signaling and in maintaining Tie2 protein levels may help explain the development of Tie2-/-–like vascular defects in Pik3ca-/- mice. Given the severity of developmental defects and early embryonic lethality of p110α-deficient embryos, additional mechanisms are likely involved as well. For example, of 4 classes of vascular defects found in p110α-deficient mice, 2 of them (yolk sac defects and lack of ACV) are also present in Notch1-/-/Notch4-/- double knockout mice.27 Further studies are needed to investigate the relevance between these individual signaling pathways and the observed vascular defects in PI3K-deficient mice.
Prepublished online as Blood First Edition Paper, February 1, 2005; DOI 10.1182/blood-2004-10-3955.
This work was supported by U.S. Public Health Grants 5RO1HL68168 and 5PO1HL70694. E.L. was partially supported by a postdoctoral fellowship from la Fondation pour la Recherche Médicale, France.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Nancy Ryan for preparation of the histology sections.
Dr Etienne Lelievre's current address is CNRS UMR 8526, Institut de Biologie de Lille, 1 rue Calmette, 59021 Lille Cedex, France.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal