Abstract
The stem-cell leukemia gene (SCL/tal1) is essential for the formation of all blood lineages. SCL is first expressed in mesodermal cells that give rise to embryonic blood cells, and continues to be expressed in fetal and adult hematopoietic stem cells (HSCs). However, SCL is not required for the maintenance of established long-term repopulating (LTR) HSCs in the adult. The time point at which HSC development becomes SCL independent has not been defined. Tyrosine kinase with immunoglobulin and epidermal growth factor homology domains–2 (Tie2) expression appears in hemogenic and vasculogenic sites shortly after SCL. We therefore used the Tie2Cre mouse to inactivate SCL early during embryonic and fetal hematopoiesis. Tie2Cre completely inactivated SCL in yolk sac, the aortagonad-mesonephros (AGM) region, and fetal liver hematopoietic cells and circulating blood cells. However, the fetal liver was colonized by functional LTR-HSCs. Yet SCL remained crucial for proper differentiation of both primitive and definitive red cells and megakaryocytes. These results indicate that the SCL-dependent phase of HSC development ends before Tie2Cre-mediated gene ablation becomes effective.
Introduction
Blood-cell production is sustained through the lifetime of an individual by hematopoietic stem cells (HSCs). The definitive HSC pool is established during fetal development through a complex process that involves several anatomically and temporally restricted sites. Fetal hematopoiesis is characterized by the formation of short-lived progenitors and immature HSC precursors in the yolk sac and the aorta-gonad-mesonephros (AGM) region, followed by maturation of the precursor cells into functional HSCs capable of repopulating the adult bone marrow (BM), and migration of these cells to the fetal liver for expansion and differentiation, until hematopoiesis shifts to the BM at term.1-4 The first step in this cascade of events is the specification of the hematopoietic lineage from ventral mesoderm/the hemangioblast, a process that is critically dependent on the basic helix-loop-helix (bHLH) transcription factor/T-cell oncogene SCL/tal1.5-8 Additional cell-autonomous functions of SCL during development were uncovered in extraembryonic endothelial cells during the remodeling of the yolk sac vasculature.9 However, endothelial SCL expression is quickly down-regulated in normal embryos after embryonic day 8.5 (E8.5), as blood vessels mature.10,11 When expressed with its partners LIM only protein 2 (LMO2) and GATA binding factor 1 (GATA1), SCL induces ectopic blood cell development.12 These findings establish SCL as a principal hematopoietic regulatory gene during embryonic development. Surprisingly, despite continued expression in adult BM long-term repopulating (LTR) HSCs,13 SCL is not essential for self-renewal and multipotency of these cells, but remains critical for proper megakaryocyte and erythroid development in adult BM.14,15 However, these studies have not provided information about the requirement of SCL for unique events during fetal hematopoiesis when the definitive HSC pool is established. By analyzing hematopoietic potential of yolk sac, AGM, and fetal liver progenitor and stem cells in which the SCL gene was inactivated through Tie2Cre-mediated gene recombination, we discovered that SCL is not required for establishment of the fetal liver HSC pool, after the initial SCL-dependent commitment to the hematopoietic lineage. Our studies define the temporal window of a functional requirement of SCL during development of HSCs at the onset of tyrosine kinase with immunoglobulin and epidermal growth factor homology domains–2 (Tie2) expression, a known marker of endothelial cells and HSCs.
Study design
SCL knock-in ES-cell in vitro differentiation
R1 embryonic stem (ES) cells that express an inactive form of human CD4 from the SCL locus16 were differentiated in vitro as described.17 Embryoid bodies (EBs) were harvested at days 3.6, 4.0, and 4.6 of differentiation, and analyzed for expression of SCL (human CD4-biotin; Caltag-Laboratories, Burlingame, CA); and streptavidin-allophycocyanin (SAV-APC; Pharmingen, San Diego, CA), Tie2 (phycoerythrin [PE]–conjugated; eBiosciences, San Diego, CA) and CD41 (fluorescein isothiocyanate [FITC]–conjugated; Pharmingen).
Mice and fetal hematopoietic tissues
To inactivate SCL in the embryo, mice with a conditionally targeted SCL gene14 were bred with Tie2Cre mice.18 Fetal livers were dissociated mechanically by pipetting, whereas yolk sac and AGM/caudal half of the embryo were first treated with 0.12% collagenase for 1.5 hours. Hematopoietic cells were visualized using a Nikon E800 microscope equipped with a 40 ×/0.85 objective lens (Nikon, Tokyo, Japan) and were photographed with a SPOT-RT Slider camera (Diagnostic Instruments, Sterling Heights, MI). Images were acquired and processed using SPOT 3.5.6 (Diagnostic Instruments) and Adobe Photoshop 7.0 (Adobe, San Jose, CA) software.
Clonogenic progenitor and HSC assays
Clonogenic progenitors from SCLfl/fl Tie2Cre and SCLfl/wt Tie2Cre fetal hematopoietic organs (yolk sac, AGM, blood, and fetal liver, E10.5-E12.5) were assayed in 1% methylcellulose with interleukin-3 and -6, Kit ligand, erythropoietin, and thrombopoietin as described.14 Fetal liver cells (E12.5; CD45.2; one-quarter or one-eighth embryo equivalent) were transplanted with CD45.1/CD45.2 (F1 from C57Bl6.sjl and C57Bl6 mating) competitor BM cells (2 × 105 or 1 × 105, respectively) into lethally irradiated adult recipients (CD45.1, C57Bl6.sjl; Taconic, Germantown, NY). Contribution of fetal cells to peripheral blood myeloid and lymphoid cells was analyzed at 3 to 4 weeks and more than 5 months after transplantation as described.14 All assays at each time point were performed for hematopoietic organs from at least 5 donor embryos per genotype.
Genomic and reverse transcriptase–polymerase chain reaction (RT-PCR) analysis
Genomic deletion of the SCL gene in tissues or individual colonies was analyzed as described14 by 2- or 3-primer PCR that detects floxed (fl)/wild-type (wt) alleles, or fl/wt/excised (ex) alleles, respectively. Total RNA (8.6 μg) was reverse-transcribed using Superscript RTranscriptase II (Invitrogen, Carlsbad, CA) and 5% of the product was PCR-amplified for 20, 25, and 30 cycles (SCL, 25-35 cycles). Details are available upon request.
Results and discussion
Tie2 is coexpressed with SCL at the onset of hematopoietic development, as shown in Figure 1A, which displays embryoid bodies at day 3.6, a stage at which the common precursors of blood and endothelial cells are formed. CD41, the earliest marker of the hematopoietic lineage,17,19 appears by day 4.6 in cells that express SCL and Tie2 (Figure 1B-D). Others have shown that nascent multipotential progenitors and HSCs in mouse embryos express Tie2.20-22 Tie2 gene regulatory elements are thus expected to be transiently active in hematopoietic precursors upon initiation of the hematopoietic program, and we therefore predicted that a Tie2Cre transgene would cause early gene recombination in hematopoietic cells. Efficient Cre-mediated genomic recombination of a conditional allele of the SCL gene was indeed observed in embryonic peripheral blood and fetal liver cells (Figure 1E), and no intact SCL RNA was expressed in E12.5 fetal livers (Figure 2A).
Inactivation of the conditionally targeted SCL gene during fetal development by Tie2Cre expression. (A-D) FACS analysis of Tie2, SCL, and CD41 expression in SCL/hCD4 ES cell–derived EBs at 3.6 (A) and 4.6 days of differentiation (B-D). Numbers indicate the percentage of cells in the gated area. Human CD4 is expressed from the endogenous SCL locus as a reporter of SCL gene activity. Panel C shows a gated CD41+ subpopulation of day 4.6 cells. (E) Two-primer genomic PCR analysis of SCLfl/wt Tie2Cre+ and SCLfl/wt Tie2Cre+ embryonic tissues. EM indicates whole embryo; FL, fetal liver; PB, peripheral blood. (F-G) CD31/PECAM whole-mount staining of vascular endothelial cells of E10.0 SCLfl/wt Tie2Cre+ and SCLfl/fl Tie2Cre+ embryos (7× and 3× original magnification, respectively, on a Leica MZ6 stereomicroscope). (H-I) E13.5 SCLfl/wt Tie2Cre+ and SCLfl/fl Tie2Cre+ embryos. (J-K) May-Grünwald-Giemsa (MGG) staining of peripheral blood cytospins from E13.5 SCLfl/wt Tie2Cre+ and SCLfl/fl Tie2Cre+ embryos (40×). (L-M) FACS analysis of erythropoiesis in SCLfl/wt Tie2Cre+ and SCLfl/fl Tie2Cre+ E12.5 fetal livers.
Inactivation of the conditionally targeted SCL gene during fetal development by Tie2Cre expression. (A-D) FACS analysis of Tie2, SCL, and CD41 expression in SCL/hCD4 ES cell–derived EBs at 3.6 (A) and 4.6 days of differentiation (B-D). Numbers indicate the percentage of cells in the gated area. Human CD4 is expressed from the endogenous SCL locus as a reporter of SCL gene activity. Panel C shows a gated CD41+ subpopulation of day 4.6 cells. (E) Two-primer genomic PCR analysis of SCLfl/wt Tie2Cre+ and SCLfl/wt Tie2Cre+ embryonic tissues. EM indicates whole embryo; FL, fetal liver; PB, peripheral blood. (F-G) CD31/PECAM whole-mount staining of vascular endothelial cells of E10.0 SCLfl/wt Tie2Cre+ and SCLfl/fl Tie2Cre+ embryos (7× and 3× original magnification, respectively, on a Leica MZ6 stereomicroscope). (H-I) E13.5 SCLfl/wt Tie2Cre+ and SCLfl/fl Tie2Cre+ embryos. (J-K) May-Grünwald-Giemsa (MGG) staining of peripheral blood cytospins from E13.5 SCLfl/wt Tie2Cre+ and SCLfl/fl Tie2Cre+ embryos (40×). (L-M) FACS analysis of erythropoiesis in SCLfl/wt Tie2Cre+ and SCLfl/fl Tie2Cre+ E12.5 fetal livers.
In contrast to SCL-/- embryos that died before E9.5 due to absence of blood cells and vitelline vessels,9,23,24 SCLfl/fl Tie2Cre homozygous embryos survived longer, had circulating primitive erythrocytes, and formed an apparently normal vasculature that is connected to the yolk sac vasculature (Figure 1F-G, and data not shown), arguing that expression of SCL prior to the completion of Tie2Cre-mediated gene inactivation was sufficient to rescue the early-lethal hematopoietic and vascular phenotypes. However, the yolk sac vasculature was still abnormal, showing delayed and incomplete remodeling (T.M.S. and S.H.O., unpublished observation, December 2002), a phenotype that is reminiscent of the one observed in SCL-/- embryos. The Tie2Cre SCLfl/fl embryos later succumbed to systemic edema and hemorrhage, a phenotype often observed as a consequence of defective hematopoiesis, and all fetuses were dead by E13.5 to E14.5 (Figure 1H-I). Peripheral blood cytospin analysis (Figure 1J-K) exposed a maturation defect of primitive erythroid cells, characterized by an increase in the occurrence of mitotic and binucleated cells, large nucleus-to-cytoplasm ratio, and a comparatively basophilic cytoplasm. Fluorescence-activated cell sorting (FACS) analysis of E12.5 fetal liver cells (Figure 1L-M) showed a relative accumulation of CD71 (transferrin receptor)high-Ter119low-med cells, and a paucity of more mature CD71+Ter119high cells, indicating a developmental impediment at the level of basophilic erythroblasts.25
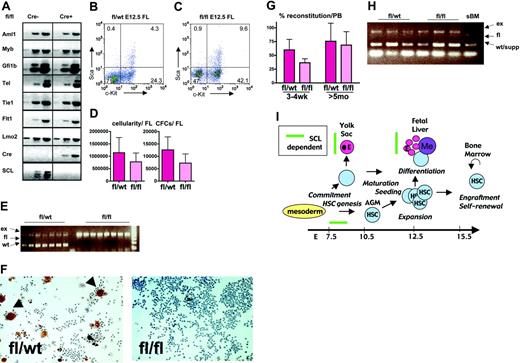
Effect of Tie2Cre-mediated deletion of SCL gene in the development and function of definitive hematopoietic stem cells. (A) Total RNA RT-PCR analysis of E12.5 SCLfl/fl Tie2Cre- and SCLfl/fl Tie2Cre+ fetal liver cells. (B-C) FACS analysis of hematopoietic progenitors and stem cells in fetal livers of E12.5 SCLfl/wt Tie2Cre+ and SCLfl/fl Tie2Cre+ fetuses. (D) Total cellularity and hematopoietic colony-forming potential in SCLfl/wt Tie2Cre+ and SCLfl/fl Tie2Cre+ E12.5 fetal livers (experiments: n = 3; embryos fl/wt: n = 5; embryos fl/fl: n = 6; cellularity: P < .08; CFC: P < .05; t test). Data are presented as means and standard deviations. (E) Single-colony 3-primer PCR analysis of SCL gene excision in SCLfl/wt Tie2Cre+ and SCLfl/fl Tie2Cre+ E12.5 fetal liver–derived colonies. (F) Acetylcholinesterase staining of SCLfl/wt Tie2Cre+ and SCLfl/fl Tie2Cre+ fetal liver cultures 10×. Data are presented as means and standard deviations. (G) Percentage contribution of fetal liver–derived CD45+ cells in the peripheral blood of lethally irradiated recipients at 3 to 4 weeks and more than 5 months after transplantation (experiments: n = 3; donor embryos: n = 6 per genotype; recipients: n = 17 per genotype; P value at 3 to 4 weeks < .01; P value at more than 5 months > .25; t test). Data are presented as means and standard deviations. (H) Three-primer PCR analysis of SCL gene excision in peripheral blood from recipient animals, 5 months after transplantation with E12.5 SCLfl/wt Tie2Cre+ and SCLfl/flTie2Cre+ E12.5 fetal liver cells. Note that the wt band in recipients that received fl/fl fetal liver cells is derived from support BM cells. sBM indicates recipients that received support BM only. (I) SCL is vital for the commitment of mesoderm to the hematopoietic lineage, but thereafter is no longer essential for the establishment of the LTR-HSC pool in the fetal liver. Yet SCL is required for proper differentiation of primitive and definitive erythroid (E) cells and megakaryocytes (Me) in the yolk sac and the fetal liver.
Effect of Tie2Cre-mediated deletion of SCL gene in the development and function of definitive hematopoietic stem cells. (A) Total RNA RT-PCR analysis of E12.5 SCLfl/fl Tie2Cre- and SCLfl/fl Tie2Cre+ fetal liver cells. (B-C) FACS analysis of hematopoietic progenitors and stem cells in fetal livers of E12.5 SCLfl/wt Tie2Cre+ and SCLfl/fl Tie2Cre+ fetuses. (D) Total cellularity and hematopoietic colony-forming potential in SCLfl/wt Tie2Cre+ and SCLfl/fl Tie2Cre+ E12.5 fetal livers (experiments: n = 3; embryos fl/wt: n = 5; embryos fl/fl: n = 6; cellularity: P < .08; CFC: P < .05; t test). Data are presented as means and standard deviations. (E) Single-colony 3-primer PCR analysis of SCL gene excision in SCLfl/wt Tie2Cre+ and SCLfl/fl Tie2Cre+ E12.5 fetal liver–derived colonies. (F) Acetylcholinesterase staining of SCLfl/wt Tie2Cre+ and SCLfl/fl Tie2Cre+ fetal liver cultures 10×. Data are presented as means and standard deviations. (G) Percentage contribution of fetal liver–derived CD45+ cells in the peripheral blood of lethally irradiated recipients at 3 to 4 weeks and more than 5 months after transplantation (experiments: n = 3; donor embryos: n = 6 per genotype; recipients: n = 17 per genotype; P value at 3 to 4 weeks < .01; P value at more than 5 months > .25; t test). Data are presented as means and standard deviations. (H) Three-primer PCR analysis of SCL gene excision in peripheral blood from recipient animals, 5 months after transplantation with E12.5 SCLfl/wt Tie2Cre+ and SCLfl/flTie2Cre+ E12.5 fetal liver cells. Note that the wt band in recipients that received fl/fl fetal liver cells is derived from support BM cells. sBM indicates recipients that received support BM only. (I) SCL is vital for the commitment of mesoderm to the hematopoietic lineage, but thereafter is no longer essential for the establishment of the LTR-HSC pool in the fetal liver. Yet SCL is required for proper differentiation of primitive and definitive erythroid (E) cells and megakaryocytes (Me) in the yolk sac and the fetal liver.
We next turned our attention to the analysis of definitive progenitors and HSCs. Expression of several key regulatory genes that are required for definitive hematopoiesis was unaffected in the fetal livers in spite of Tie2Cre-mediated loss of SCL (Figure 2A). Furthermore, c-Kit+Sca1+ and c-Kit+Sca1- HSCs and progenitors were present and even increased relative to more mature cells (Figure 2B-C). These progenitor cells readily formed colonies in methylcellulose cultures. The number of colony-forming cells (CFCs), as well as the total number of cells, was only slightly reduced in E12.5 SCL-deficient livers (Figure 2D). Genomic PCR was used to confirm the lack of a functional SCL allele in all individual colonies (Figure 2E). However, differentiation of both megakaryocytic and erythroid cells was still affected, as indicated by a severe reduction of the number of acetylcholine esterase+ and benzidine+ cells, respectively (Figure 2F, and data not shown). Furthermore, Tie2Cre-mediated excision of the SCL gene was complete in circulating blood cells as well as clonogenic progenitors derived from E10.5 yolk sac and AGM, before seeding of the fetal liver (data not shown).
Finally, to study whether fetal livers were colonized with functional adult-type HSCs, we tested the ability of SCL-deficient fetal liver cells to rescue hematopoiesis in lethally irradiated adult mice. As shown in Figure 2G, Tie2Cre SCLfl/fl cells were capable of long-term reconstitution of the recipient blood system. Although there was a significant reduction in the short-term competitiveness of SCL-deficient fetal liver cells at 3 to 4 weeks after transplantation, the decrease in contribution of SCL-deficient cells was only minor after more than 5 months, arguing that ongoing expression of SCL is not required for function of fetal liver LTR-HSCs. Furthermore, SCL-deficient cells were able to differentiate into both lymphoid and myeloid cells, although Tie2Cre-mediated inactivation of the SCL allele was complete (Figure 2H, and data not shown).
Our data show that fetal LTR-HSCs do not require SCL for engraftment into adult BM and permanent reconstitution of myeloid and lymphoid lineages (Figure 2I). Rather, SCL is essential for the initial specification of the hematopoietic lineage during a short window of time in the developing embryo that begins with SCL expression in the hemangioblast, and ends before Tie2Cre-mediated knock-down of SCL becomes effective. Furthermore, SCL transgene, driven by Tie2 regulatory elements, efficiently rescues commitment to both primitive and definitive lineages in SCL-/- embryos (T.M.S. and S.H.O., unpublished observation, December 2002). This finding independently suggests that the window during which cells need to respond to SCL in order to initiate the hematopoietic program overlaps with the onset of Tie2 expression. Our results are also in agreement with, and extend, previous in vitro findings that suggest that SCL expression is necessary for hematopoietic development prior to the onset of vascular endothelial (VE)-cadherin expression on hemangioblasts and hemogenic endothelial cells.26 In contrast to its transient requirement in hematopoietic specification, SCL remains essential for proper development of erythroid and megakaryocytic cells throughout embryonic, fetal, and adult hematopoiesis. Finally, it remains to be addressed whether SCL plays more prominent roles in intra-embryonic endothelial cells before Tie2Cre-mediated excision becomes effective, or at later stages of development.
Prepublished online as Blood First Edition Paper, January 27, 2005; DOI 10.1182/blood-2004-11-4467.
Supported in part by a Center of Excellence Award in Molecular Hematology from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). S.H.O. is an Investigator of the HHMI. H.K.A.M. received support from the Finnish Cultural Foundation.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr M. Yanagisawa for Tie2Cre mice; Dr K. C. Choi for providing the SCL-hCD4 knock-in ES cells; Dr C. Walkley for help with ES cell in vitro differentiation; Dr Y. Fujiwara for generating the TieSCL transgenic line; and O. Ogai for help with breeding the animals.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal