Abstract
We assessed the cerebrospinal fluid (CSF) by flow cytometry and cytology in 51 newly diagnosed and 9 treated aggressive B-cell lymphomas at risk for central nervous system (CNS) involvement to examine the utility of flow cytometry, incidence of CSF disease, and clinical surrogates of CNS spread. Multicolor flow cytometry using multiple antibody panels for light chains and B- and T-cell antigens identified neoplastic clones that constituted as little as 0.2% of total CSF lymphocytes. Among 51 newly diagnosed patients, 11 (22%) had occult CSF involvement. All 11 were detected by flow cytometry but only 1 by cytology (P = .002). Among 9 treated patients, CSF involvement was detected by flow cytometry alone in 2 and also by cytology in 1 case. CSF chemistry and cell counts were similar in patients with and without CSF lymphoma. Only the number of extranodal sites was associated with occult CSF lymphoma in newly diagnosed patients by univariate (P = .006) or logistic regression analysis (P = .012). We hypothesize that the biologic phenotype associated with colonization of extranodal sites leads to CNS spread, possibly related to the microenvironment. Patients at risk for CNS spread should undergo staging CSF evaluation by flow cytometry.
Introduction
Secondary involvement of the central nervous system (CNS) by aggressive B-cell lymphomas is an infrequent but nearly always fatal complication.1 Although prophylactic treatment likely reduces the incidence of central nervous system relapse, it increases the toxicity of systemic chemotherapy and is unnecessary in most patients.1-3 This has led to the development of clinical risk paradigms to identify patients who might benefit from central nervous system prophylaxis, but even among these patients, only some ever develop central nervous system disease.1 The risk of central nervous system spread appears to be particularly high among patients with acquired immunodeficiency–related lymphoma (ARL), where it is a major cause of treatment failure and has led some investigators to recommend routine prophylaxis in all patients.4,5 Nevertheless, like other aggressive B-cell lymphomas, only some ARLs spread to the central nervous system and are not accurately predicted by clinical risk paradigms.
These findings indicate a need to develop and validate sensitive analytical methods for occult central nervous system lymphoma to ensure optimal treatment while reducing unnecessary prophylactic treatment. Indeed, successful management of central nervous system involvement requires early detection and treatment as well as reliable measures of treatment response.6 Unfortunately, cytologic examination of the cerebrospinal fluid, the diagnostic “gold standard,” has a low sensitivity, with a reported false-negative rate of 20% to 60%.7,8 This has been ascribed to the paucity of tumor cells in the cerebrospinal fluid of patients with minimal disease and to the presence of confounding reactive lymphocytes. These results suggest that patients with low-volume disease, who are likely to benefit most from treatment, are more likely to be falsely negative.
Flow cytometry is an objective and quantitative method ideally suited to identifying small populations of cells with aberrant phenotypes.9 Methodologically, flow cytometry can detect aberrant B cells that constitute as few as 0.01% of total lymphocytes, a sensitivity capable of detecting occult cerebrospinal fluid involvement.10 To help validate flow cytometry and to assess the clinical correlates of central nervous system involvement, we assessed all newly diagnosed and relapsed aggressive B-cell lymphomas at risk for central nervous system lymphoma by cerebrospinal fluid cytology and flow cytometry and brain imaging since 1999. Herein, we report our findings on the incidence and clinical risk factors for occult cerebrospinal fluid involvement and compare the sensitivity of flow cytometry and cytology.
Patients, materials, and methods
Patients and treatment
Beginning in 1999, flow cytometry was employed for the analysis of all cerebrospinal fluid specimens obtained from patients with aggressive B-cell lymphoma. Patients' histology and cytology were reviewed by E.S.J. and A.A. and associates, respectively, and all patients were treated on an Institutional Review Board–approved protocol and gave written informed consent. In this study, we defined “occult” involvement of the cerebrospinal fluid by lymphoma as specimens negative by conventional cytology but positive by flow cytometry.
Between 1999 and 2004, 92 patients with newly diagnosed aggressive B-cell lymphomas of diffuse large B-cell (DLBCL) and Burkitt histologies were treated at the National Cancer Institute. Of these patients, 51 (55%) were considered to be at high risk for central nervous system disease, as defined in “Results,” and underwent central nervous system evaluation. An additional 9 patients with relapsed/treated DLBCL also underwent evaluation due to symptoms or high risk of central nervous system involvement. The cerebrospinal fluid was obtained by lumbar puncture and was evaluated using standard methods, including glucose, total protein, evaluation for infectious organisms as appropriate, and cytology. The central nervous system was imaged by magnetic resonance imaging (MRI) or computed tomography (CT).
Patients newly diagnosed with DLBCL or Burkitt lymphoma were entered on 1 of 3 different protocols containing dose-adjusted (DA)–EPOCH (infusional etoposide, vincristine, and doxorubicin, with bolus prednisone and cyclophosphamide) or DA-EPOCH with rituximab, as previously reported, depending on the treatment date and HIV status.5,11 Patients with recurrent lymphomas received salvage treatment appropriate for the clinical setting. The newly diagnosed patients who were at risk for central nervous system lymphoma but had no evidence of disease received prophylactic treatment with 12 mg intrathecal methotrexate on days 1 and 5 of cycles 3 to 6 (total, 12 doses) of DA-EPOCH. Patients requiring active treatment for leptomeningeal lymphoma received 12 mg intrathecal methotrexate on the following schedule: induction: twice weekly until 2 weeks past the last negative cerebrospinal fluid flow cytometry with a minimum of 4 weeks of treatment; consolidation: 6 weekly treatments; maintenance: 4 monthly treatments. Modifications of this treatment plan, which included the use of cytarabine and/or cranial radiation, were made as clinically indicated in patients who did not adequately respond, had excess toxicity, and/or had parenchymal brain lesions. Among the newly diagnosed patients with evidence of cerebrospinal fluid lymphoma, treatment was administered via lumbar puncture in 7 and via an Ommaya reservoir in 3 patients. All 3 relapsed patients received treatment via an Ommaya reservoir.
Cytology analysis
Cerebrospinal fluid samples were prepared in a manner to optimize cellular preservation and cellular concentration while minimizing peripheral blood contamination. Fresh cerebrospinal fluid specimens were refrigerated and processed within several hours of collection as follows: 0.5 mL of straight specimen was cytocentrifuged at 700 rpm for 15 minutes and then utilized for a preliminary cytospin, which was air-dried and stained with Diff-Quik (Dade Behring, Newark, DE). Based on the concentration of cells on the cytospin, samples were concentrated or diluted in RPMI 1640 (Gibco, Grand Island, NY).
Each case was interpreted by a cytopathologist (A.F. and A.A.). Results were reported as negative, positive, atypical, or suspicious for malignant lymphoma based on cellular morphology and were independently reviewed from the flow cytometry results. Samples reported as “negative for malignant cells” contained benign-appearing lymphocytes and monocytes. Samples reported as showing “atypical lymphocytes” contained lymphocytes with abnormal nuclear-cytoplasmic ratios, irregular nuclear morphology, and possibly nucleoli. In these cases, the morphology was indeterminate between reactive and a neoplastic etiology. “Suspicious for malignant lymphoma” was reported when the lymphocytes were large, with increased nuclear-cytoplasmic ratios, irregular nuclear contours, and prominent nucleoli. In these instances, although a malignant etiology was favored, either due to the small number of cells or the remote possibility that these cells could be of a reactive etiology, the samples were not definitively called malignant. The diagnosis of “malignant lymphoma” was used when the cells exhibited the characteristic malignant morphology in keeping with the known histology. Immunohistochemistry was not employed in the negative or suspicious cases because the number of cells was too low for this technique to be useful.
Flow cytometry analysis
The cerebrospinal fluid volumes ranged from 1.2 to 5.0 mL. The samples were processed immediately on receipt in the laboratory. Specimens were washed with phosphate-buffered saline (PBS) to remove cytophilic antibodies prior to determining cell number. Cellularity was determined manually by a hemocytometer and viability by trypan blue uptake. Briefly, the cells were pelleted by centrifugation to 200 μL volume. Ten microliters of concentrated cells was diluted in 40 μL trypan blue (50 μL total volume) and examined using a hemocytometer to determine cell number and viability. If no cells were observed in the hemocytometer, the entire sample was placed in a single tube and only 1 set of monoclonal antibodies was used to stain the cells. If 1 cell was observed, 2 tubes and 2 sets of antibodies could be used. Additional tubes were added for the more cellular specimens. Specimens were stained for 30 minutes at room temperature, as previously described, with a cocktail of 3 to 4 antibodies at a concentration according to the manufacturer's recommendations.12 If red cells were observed, the cells were lysed after staining using Immunolyse (Beckman Coulter, Miami, FL) according to the manufacturers' instructions for whole blood lysis. All cells were fixed in 1.0% paraformaldehyde and stored at 4°C for up to 12 hours before acquisition.
The antibody panels were chosen based on the number of cells, previous histologic diagnosis, and available clinical history and involved single, double, or triple antibody panels as shown in Table 1. CD20 was not studied in patients known to have received rituximab. Where possible, the panel included a tube with CD3 for T cells, CD56 and CD16 for natural killer cells, CD19 or CD20 for B cells and, in selected cases, CD45 to separate leukocytes from debris and other cells. Anti-κ and anti-λ light chain antibodies were paired with CD19, CD20, CD22, CD38, CD45, CD5, and CD10, based on the histologic diagnosis. Normal cells within the specimens were analyzed as controls (internal controls).
Immunoglobulin antibody panels
Antibodies . | No. of patients . |
|---|---|
| Single antibody panels | |
| Anti-κ-FITC, anti-λ-PE, anti-CD20-PerCP* | 10 |
| Anti-κ-FITC, anti-λ-PE, anti-CD19-PC5† | 3 |
| Anti-κ-FITC, anti-λ-PE, anti-CD3-PerCP | 1 |
| Anti-κ-FITC, anti-λ-PE, anti-CD45-PerCP | 3 |
| Double antibody panels | |
| Anti-κ-FITC, anti-CD22-PE, anti-CD20-PerCP‡ | 14 |
| Anti-λ-FITC, anti-CD22-PE, anti-CD20-PerCP‡ | |
| Anti-κ-FITC, anti-CD22-PE, anti-CD45-PerCP, anti-CD19-APC | 5 |
| Anti-λ-FITC, anti-CD22-PE, anti-CD45-PerCP, anti-CD19-APC | |
| Anti-κ-FITC, anti-CD10-PE, anti-CD20-PerCP, anti-CD45-APC | 1 |
| Anti-λ-FITC, anti-CD10-PE, anti-CD20-PerCP, anti-CD45-APC | |
| Anti-κ-FITC, anti-CD22-PE, anti-CD45-PerCP, anti-CD10-APC | 1 |
| Anti-λ-FITC, anti-CD22-PE, anti-CD45-PerCP, anti-CD10-APC | |
| Anti-κ-FITC, anti-CD22-PE, anti-CD19-PE, anti-CD45-PerCP, anti-CD14-APC | 3 |
| Anti-λ-FITC, anti-CD22-PE, anti-CD19-PE, anti-CD45-PerCP, anti-CD14-APC | |
| Anti-κ-FITC, anti-CD38-PE, anti-CD20-PerCP | 1 |
| Anti-λ-FITC, anti-CD38-PE, anti-CD20-PerCP | |
| Triple antibody panels | |
| Anti-κ-FITC, anti-λ-PE, anti-CD19-PC5, anti-CD10-APC | 2 |
| Anti-κ-FITC, anti-CD22-PE, anti-CD20-PerCP, anti-CD10-APC | |
| Anti-λ-FITC, anti-CD22-PE, anti-CD20-PerCP, anti-CD10-APC |
Antibodies . | No. of patients . |
|---|---|
| Single antibody panels | |
| Anti-κ-FITC, anti-λ-PE, anti-CD20-PerCP* | 10 |
| Anti-κ-FITC, anti-λ-PE, anti-CD19-PC5† | 3 |
| Anti-κ-FITC, anti-λ-PE, anti-CD3-PerCP | 1 |
| Anti-κ-FITC, anti-λ-PE, anti-CD45-PerCP | 3 |
| Double antibody panels | |
| Anti-κ-FITC, anti-CD22-PE, anti-CD20-PerCP‡ | 14 |
| Anti-λ-FITC, anti-CD22-PE, anti-CD20-PerCP‡ | |
| Anti-κ-FITC, anti-CD22-PE, anti-CD45-PerCP, anti-CD19-APC | 5 |
| Anti-λ-FITC, anti-CD22-PE, anti-CD45-PerCP, anti-CD19-APC | |
| Anti-κ-FITC, anti-CD10-PE, anti-CD20-PerCP, anti-CD45-APC | 1 |
| Anti-λ-FITC, anti-CD10-PE, anti-CD20-PerCP, anti-CD45-APC | |
| Anti-κ-FITC, anti-CD22-PE, anti-CD45-PerCP, anti-CD10-APC | 1 |
| Anti-λ-FITC, anti-CD22-PE, anti-CD45-PerCP, anti-CD10-APC | |
| Anti-κ-FITC, anti-CD22-PE, anti-CD19-PE, anti-CD45-PerCP, anti-CD14-APC | 3 |
| Anti-λ-FITC, anti-CD22-PE, anti-CD19-PE, anti-CD45-PerCP, anti-CD14-APC | |
| Anti-κ-FITC, anti-CD38-PE, anti-CD20-PerCP | 1 |
| Anti-λ-FITC, anti-CD38-PE, anti-CD20-PerCP | |
| Triple antibody panels | |
| Anti-κ-FITC, anti-λ-PE, anti-CD19-PC5, anti-CD10-APC | 2 |
| Anti-κ-FITC, anti-CD22-PE, anti-CD20-PerCP, anti-CD10-APC | |
| Anti-λ-FITC, anti-CD22-PE, anti-CD20-PerCP, anti-CD10-APC |
Additional tube with antibody to detect T and natural killer (NK) cells was added in 4 cases but did not affect diagnosis.
FITC indicates fluorescein isothiocyanate; PE, phycoerythrin; PerCP, peridinin chlorophyll protein; APC, allophycoerythrin.
Had a fourth APC-conjugated antibody in some cases: * 7 cases no fourth antibody, 1 case anti-CD19-APC, 1 case anti-CD10-APC, 1 case anti-CD45-APC; † 1 case no fourth antibody, 1 case anti-CD10-APC, 1 case anti-CD45-APC; ‡ 11 cases no fourth antibody, 2 cases anti-CD19-APC, 1 case anti-CD45-APC.
Three- and 4-color cytometry was performed with a BD (Becton Dickinson, San Jose, CA) FACSCalibur flow cytometer. The sensitivity of fluorescent detectors was set and monitored using Calibrite beads (Becton Dickinson). For analysis, cell populations were analyzed by gating on forward scatter (FSC), side scatter (SSC), CD45, CD19, CD20, and/or CD22. Staining for κ and λ light chains in CD19, CD20, CD22, or CD45-positive cells was determined as previously described.12 B-cell data were analyzed for a clustering of cells with an abnormal pattern of antigen expression as well as light scatter characteristics. Clusters of cells thus identified were analyzed for light chain expression.
Statistical methods
Comparisons of dichotomous parameters were made between cerebrospinal fluid–positive and –negative patients using the Fisher exact test, while ordered categorical parameters were evaluated for the statistical significance of their association with cerebrospinal fluid–positive or –negative outcomes using the Cochran-Armitage trend test.13 Continuously measured parameters were compared between groups using the Wilcoxon rank sum test. Logistic regression analysis was used to further evaluate whether any factors were jointly associated with a classification to positive or negative results. Finally, the McNemar test for paired categorical data was used to determine the degree to which there is concordance or discordance between flow cytometry and cytology results on the same individual. All P values are 2-tailed and have not been adjusted for multiple comparisons.
Results
Patient characteristics
Of 92 consecutive patients with newly diagnosed aggressive lymphoma, 51 (55%) underwent evaluation of the cerebrospinal fluid based on clinical features associated with an increased risk of central nervous system involvement by lymphoma (Table 2).1 These clinical features included a diagnosis of AIDS-related lymphoma (ARL) in 23 of 51 (45%) patients and, among 28 HIV-negative patients, a diagnosis of Burkitt lymphoma in 6 (21%) or a diagnosis of DLBCL with at least 2 extranodal disease sites and elevated lactate dehydrogenase (LDH) or bone marrow involvement in 22 (79%) patients. Expectedly, there was a modest enrichment of adverse prognostic factors among patients undergoing cerebrospinal fluid evaluation, compared with the entire cohort of patients with aggressive lymphomas (Table 2). These factors include elevated LDH in 75% and at least 2 extranodal sites in 53% of patients, which constitute previously identified risk factors for central nervous system spread.1,14
Characteristics of newly diagnosed patients
. | . | Patients at CNS risk with spinal fluid sampling . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| Characteristic . | All patients entered on study . | Total . | CSF positive . | CSF negative . | P* . | |||
| Total no. of patients | 92 | 51 (55%) | 11 (22%) | 40 (78%) | — | |||
| Sex: male | 60 (65%) | 41 (80%) | 7 (64%) | 34 (85%) | .19 | |||
| Histology | ||||||||
| Diffuse large B cell | 84 (91%) | 43 (84%) | 11 (100%) | 32 (80%) | .18 | |||
| Burkitt | 8 (9%) | 8 (15%) | 0 (0%) | 8 (20%) | — | |||
| HIV positive | 23 (25%) | 23 (45%) | 7 (64%) | 16 (40%) | .19 | |||
| Median age, y (range) | 43 (9-85) | 38 (9-81) | 43 (32-75) | 38 (9-81) | .30 | |||
| Performance status: ECOG higher or equal to 2 | 25 (27%) | 18 (35%) | 5 (45%) | 13 (33%) | .49 | |||
| Disease stage III/IV | 63 (68%) | 43 (84%) | 10 (91%) | 33 (83%) | .67 | |||
| Lactate dehydrogenase (LDH) level, U/μL higher than normal | 56 (61%) | 38 (75%) | 9 (82%) | 29 (73%) | .71 | |||
| Two or more extranodal sites | 32 (35%) | 27 (53%) | 10 (91%) | 17 (43%) | .0057 | |||
| IPI score | ||||||||
| Low: 0-2 | 54 (59%) | 20 (38%) | 2 (18%) | 18 (44%) | .17 | |||
| High: 3-5 | 38 (41%) | 32 (62%) | 9 (82%) | 22 (56%) | ||||
. | . | Patients at CNS risk with spinal fluid sampling . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| Characteristic . | All patients entered on study . | Total . | CSF positive . | CSF negative . | P* . | |||
| Total no. of patients | 92 | 51 (55%) | 11 (22%) | 40 (78%) | — | |||
| Sex: male | 60 (65%) | 41 (80%) | 7 (64%) | 34 (85%) | .19 | |||
| Histology | ||||||||
| Diffuse large B cell | 84 (91%) | 43 (84%) | 11 (100%) | 32 (80%) | .18 | |||
| Burkitt | 8 (9%) | 8 (15%) | 0 (0%) | 8 (20%) | — | |||
| HIV positive | 23 (25%) | 23 (45%) | 7 (64%) | 16 (40%) | .19 | |||
| Median age, y (range) | 43 (9-85) | 38 (9-81) | 43 (32-75) | 38 (9-81) | .30 | |||
| Performance status: ECOG higher or equal to 2 | 25 (27%) | 18 (35%) | 5 (45%) | 13 (33%) | .49 | |||
| Disease stage III/IV | 63 (68%) | 43 (84%) | 10 (91%) | 33 (83%) | .67 | |||
| Lactate dehydrogenase (LDH) level, U/μL higher than normal | 56 (61%) | 38 (75%) | 9 (82%) | 29 (73%) | .71 | |||
| Two or more extranodal sites | 32 (35%) | 27 (53%) | 10 (91%) | 17 (43%) | .0057 | |||
| IPI score | ||||||||
| Low: 0-2 | 54 (59%) | 20 (38%) | 2 (18%) | 18 (44%) | .17 | |||
| High: 3-5 | 38 (41%) | 32 (62%) | 9 (82%) | 22 (56%) | ||||
CSF-positive versus -negative groups.
We also examined a separate group of 9 patients with relapsed/treated DLBCL who underwent cerebrospinal fluid evaluation due to suspected central nervous system disease or were considered to be at high risk (Table 3). The distribution of adverse prognostic features was similar to that found in the entire cohort of untreated patients with the exception of an increased number of AIDS-related lymphomas. Overall, 3 patients with relapsed disease had positive cerebrospinal fluid findings, all of whom were HIV positive and had extranodal disease sites at initial diagnosis.
Characteristics of relapsed patients
Characteristic . | Total no. . |
|---|---|
| Total no. of patients | 9 |
| Cerebrospinal fluid tumor | |
| Positive | 3 (33%) |
| Negative | 6 (67%) |
| Sex: male | 12 (100%) |
| Histology: diffuse large B cell | 12 (100%) |
| HIV positive | 8 (89%) |
| Median age, y (range) | 40 (35-58) |
| Performance status: ECOG higher or equal to 2 | 0 (0%) |
| Disease stage III/IV | 5 (56%) |
| Lactate dehydrogenase (LDH) level, U/μL higher than normal | 6 (67%) |
| Two or more extranodal sites | 4 (44%) |
| IPI score | |
| Low: 0-2 | 5 (56%) |
| High: 3-5 | 4 (44%) |
Characteristic . | Total no. . |
|---|---|
| Total no. of patients | 9 |
| Cerebrospinal fluid tumor | |
| Positive | 3 (33%) |
| Negative | 6 (67%) |
| Sex: male | 12 (100%) |
| Histology: diffuse large B cell | 12 (100%) |
| HIV positive | 8 (89%) |
| Median age, y (range) | 40 (35-58) |
| Performance status: ECOG higher or equal to 2 | 0 (0%) |
| Disease stage III/IV | 5 (56%) |
| Lactate dehydrogenase (LDH) level, U/μL higher than normal | 6 (67%) |
| Two or more extranodal sites | 4 (44%) |
| IPI score | |
| Low: 0-2 | 5 (56%) |
| High: 3-5 | 4 (44%) |
Cerebrospinal fluid analysis
We examined the cerebrospinal fluid laboratory characteristics and analyzed their relationship with the detection of cerebrospinal fluid lymphoma (Table 4). Among the 51 newly diagnosed patients, there was no association between the cerebrospinal fluid total protein, white blood cell (WBC) count, and red blood cell (RBC) count and the presence or absence of cerebrospinal fluid lymphoma. Notably, the cerebrospinal fluid total protein was elevated (more than 45 mg/dL) in 40% and 30% of patients with and without cerebrospinal fluid involvement, respectively, and hence was not a discriminating measure. Similarly, the total WBC count was not useful, even on an individual patient basis, and was increased above 0.010 × 109/L (10/μL) in 3 patients with and in 1 patient without cerebrospinal fluid involvement. While recognizing that the number of patients with relapsed/treated disease is small, the laboratory values in this group were also not significantly discriminating (Table 4).
Cerebrospinal fluid characteristics
. | Newly diagnosed patients . | . | . | Relapsed patients . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic of CSF . | CSF positive . | CSF negative . | P . | CSF positive . | CSF negative . | P . | ||||
| Total patients* | 11 | 40 | — | 3 | 6 | — | ||||
| Median total protein, mg/dL (range) | 38 (17-109) | 30 (17-84) | .35 | 40 (34-54) | 39.5 (23-57) | 1.0 | ||||
| Median WBC count per microliter (range) | 2 (0-222) | 1 (0-26) | .06 | 1 (1-50) | 0.5 (0-5) | .35 | ||||
| Median RBC count per microliter (range) | 0 (0-274) | 1 (0-2700) | .29 | 5 (3-10) | 0 (0-2) | .012 | ||||
| Flow cytometry | ||||||||||
| Median lymphocytes per milliliter (range) | 1750 (5.75-236 320) | 1291 (280-30000) | .96 | 62350 (700-124000) | 1700 (933-20000) | 1.0 | ||||
| Median percent tumor cells (range) | 7 (0.2-99) | 0 (0-0) | < .0001 | 6 (3-45) | 0 (0-0) | .012 | ||||
. | Newly diagnosed patients . | . | . | Relapsed patients . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic of CSF . | CSF positive . | CSF negative . | P . | CSF positive . | CSF negative . | P . | ||||
| Total patients* | 11 | 40 | — | 3 | 6 | — | ||||
| Median total protein, mg/dL (range) | 38 (17-109) | 30 (17-84) | .35 | 40 (34-54) | 39.5 (23-57) | 1.0 | ||||
| Median WBC count per microliter (range) | 2 (0-222) | 1 (0-26) | .06 | 1 (1-50) | 0.5 (0-5) | .35 | ||||
| Median RBC count per microliter (range) | 0 (0-274) | 1 (0-2700) | .29 | 5 (3-10) | 0 (0-2) | .012 | ||||
| Flow cytometry | ||||||||||
| Median lymphocytes per milliliter (range) | 1750 (5.75-236 320) | 1291 (280-30000) | .96 | 62350 (700-124000) | 1700 (933-20000) | 1.0 | ||||
| Median percent tumor cells (range) | 7 (0.2-99) | 0 (0-0) | < .0001 | 6 (3-45) | 0 (0-0) | .012 | ||||
Data missing in up to 3 patients depending on column.
Disease detection by flow cytometry versus cytology
We analyzed the cerebrospinal fluid by cytology and flow cytometry for the presence of lymphoma cells in the 51 newly diagnosed patients (Table 5). In these cases, cerebrospinal fluid staging evaluation was performed based on a clinical central nervous system risk paradigm, although 2 patients each in the positive and negative groups also had neurologic symptoms. Flow cytometry detected an abnormal B-cell population, consistent with lymphoma, in 11 of 51 (22%) patients, whereas cytology alone detected an abnormal population in only 1 of these 11 patients (P = .0020) (Table 5). The low detection rate of cytology in these patients reflects the low median percent of tumor cells (7%; range, 0.2% to 99%) within the concentrated cerebrospinal fluid specimen of these mostly asymptomatic patients (Table 4). Of note, the single patient detected by both cytology and flow cytometry was asymptomatic but had the highest concentration of tumor cells, which constituted 99% of the cells.
Flow cytometry versus cytology for detection of cerebrospinal fluid tumor
. | Newly diagnosed patients . | . | . | . | Relapsed/treated patients . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flow cytometry . | Cytology, negative . | Cytology, positive . | Total . | P . | Cytology, negative . | Cytology, positive . | Total . | |||||
| Negative | 40 | 0 | 40 (78%) | .002 | 6 | 0 | 6 (67%) | |||||
| Positive | 10 | 1 | 11 (22%) | — | 2 | 1 | 3 (33%) | |||||
| Total | 50 (98%) | 1 (2%) | 51 | — | 8 (89%) | 1 (11%) | 9 | |||||
. | Newly diagnosed patients . | . | . | . | Relapsed/treated patients . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flow cytometry . | Cytology, negative . | Cytology, positive . | Total . | P . | Cytology, negative . | Cytology, positive . | Total . | |||||
| Negative | 40 | 0 | 40 (78%) | .002 | 6 | 0 | 6 (67%) | |||||
| Positive | 10 | 1 | 11 (22%) | — | 2 | 1 | 3 (33%) | |||||
| Total | 50 (98%) | 1 (2%) | 51 | — | 8 (89%) | 1 (11%) | 9 | |||||
Two-tailed exact McNemar test.
The cerebrospinal fluid from the relapsed/treated patients was also analyzed by both flow cytometry and cytology for evidence of lymphoma (Table 5). In these cases, flow cytometry was also more sensitive than cytology and was positive in 3 cases, whereas cytology was positive in 1 case and suspicious in another case (Table 6). Compared with the newly diagnosed patients, these patients had a high median concentration of 62 350 (range, 700 to 124 000) lymphoma cells per milliliter within the concentrated cerebrospinal fluid specimen. This increased number of cells reflects the presence of symptomatic leptomeningeal disease in some of these patients and accounts for the ability of cytology, which is less sensitive than flow cytometry, to detect disease in some of these cases.
Tumor immunophenotype and cytology results
. | CSF flow cytometry . | . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Case no. . | Lymphocytes per milliliter . | Tumor cells, % . | Immunophenotype . | Tumor immunohistochemistry . | Cytology . | ||
| 1 | 236 320 | 99 | CD20+, CD10+, κ+, λ- | Gastric biopsy: CD20+, CD10+ | Positive | ||
| 2 | 555 | 19 | CD19+, CD20+, λ+, κ- | Kidney biopsy: CD20+, CD10- | Rare atypical cells | ||
| 3 | 2500 | 0.20 | CD20+, κ+, λ- | Liver biopsy: none available | Negative | ||
| 4 | 800 | 7 | CD45+, κ+, λ- | Bone marrow: CD20+, CD10+ | Negative | ||
| Flow cytometry: CD20+, CD10+, κ+ | |||||||
| 5 | <850 | 7 | CD19+, CD22+, CD45+, λ+, κ- | Liver biopsy: CD20+, CD10+ | Nondiagnostic | ||
| 6 | None available | 1 | CD20+, CD22+, λ+, κ- | Lymph node biopsy: CD19+, CD20+, CD10+, λ+, κ- | Negative | ||
| 7 | 3000 | 7 | CD20+, λ+, κ- | Intestinal biopsy: CD20+, CD30+ | Negative | ||
| 8 | 3125 | 13 | CD19+, CD22+, CD20-, κ-, λ-, surface IgG- | Lymph node biopsy: CD20+, C30-, CD10- | Negative | ||
| 9 | 1500 | 2 | CD19+, λ+, κ- | Bone marrow: CD19+, CD20+, λ+, κ- | Negative | ||
| 10 | 2000 | 1.94 | CD20+, λ+, κ- | Lymph node biopsy: none available (Burkitt) | Negative | ||
| 11 | 5750 | 4 | CD19+, CD22+, CD14-, CD45+, λ-, κ-, surface IgG- | Parotid gland: CD20+, CD10+ | Negative with lymphocytosis | ||
| 12 | None available | 6 | CD20+, CD22+, κ+, λ- | Subcutaneous tissue biopsy: CD20+, CD10+, κ+ | Suspicious | ||
| 13 | 700 | 3 | K+, λ- | Gastric biopsy: CD20+, CD45+ | Negative | ||
| 14 | 124 000 | 45 | CD45+, CD20-, κ-, λ- | Soft tissue biopsy: CD20+, CD10+, κ+ | Positive | ||
. | CSF flow cytometry . | . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Case no. . | Lymphocytes per milliliter . | Tumor cells, % . | Immunophenotype . | Tumor immunohistochemistry . | Cytology . | ||
| 1 | 236 320 | 99 | CD20+, CD10+, κ+, λ- | Gastric biopsy: CD20+, CD10+ | Positive | ||
| 2 | 555 | 19 | CD19+, CD20+, λ+, κ- | Kidney biopsy: CD20+, CD10- | Rare atypical cells | ||
| 3 | 2500 | 0.20 | CD20+, κ+, λ- | Liver biopsy: none available | Negative | ||
| 4 | 800 | 7 | CD45+, κ+, λ- | Bone marrow: CD20+, CD10+ | Negative | ||
| Flow cytometry: CD20+, CD10+, κ+ | |||||||
| 5 | <850 | 7 | CD19+, CD22+, CD45+, λ+, κ- | Liver biopsy: CD20+, CD10+ | Nondiagnostic | ||
| 6 | None available | 1 | CD20+, CD22+, λ+, κ- | Lymph node biopsy: CD19+, CD20+, CD10+, λ+, κ- | Negative | ||
| 7 | 3000 | 7 | CD20+, λ+, κ- | Intestinal biopsy: CD20+, CD30+ | Negative | ||
| 8 | 3125 | 13 | CD19+, CD22+, CD20-, κ-, λ-, surface IgG- | Lymph node biopsy: CD20+, C30-, CD10- | Negative | ||
| 9 | 1500 | 2 | CD19+, λ+, κ- | Bone marrow: CD19+, CD20+, λ+, κ- | Negative | ||
| 10 | 2000 | 1.94 | CD20+, λ+, κ- | Lymph node biopsy: none available (Burkitt) | Negative | ||
| 11 | 5750 | 4 | CD19+, CD22+, CD14-, CD45+, λ-, κ-, surface IgG- | Parotid gland: CD20+, CD10+ | Negative with lymphocytosis | ||
| 12 | None available | 6 | CD20+, CD22+, κ+, λ- | Subcutaneous tissue biopsy: CD20+, CD10+, κ+ | Suspicious | ||
| 13 | 700 | 3 | K+, λ- | Gastric biopsy: CD20+, CD45+ | Negative | ||
| 14 | 124 000 | 45 | CD45+, CD20-, κ-, λ- | Soft tissue biopsy: CD20+, CD10+, κ+ | Positive | ||
To help assess the potential impact of the flow cytometry results on the cytology results and for quality assurance, the same cytologists (A.F. and A.A.) undertook a retrospective review of the cerebrospinal fluid cytology from the 11 newly diagnosed patients with leptomeningeal disease. Overall, the cytology was changed for 1 case from negative to positive and was changed for 1 case from showing atypical cells to “highly suspicious.” These results indicate that the concentration of tumor cells in asymptomatic patients is generally below the level of adequate detection by cytology and that flow cytometry is required for early disease detection.
Flow cytometry analysis of cerebrospinal fluid
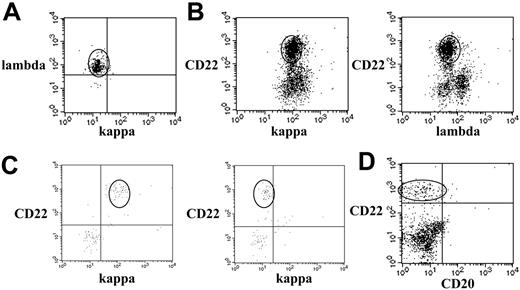
Cerebrospinal fluid flow cytometry should be interpreted within the context of the patient's histologic diagnosis and is dependent on the analysis of light chain restriction and/or aberrant antigen expression.15-17 Light chain restriction alone is sufficient to establish the presence of a monoclonal population and was present in 11 (79%) of the 14 positive cases from the current series (Table 6). Furthermore, among these 11 cases, the tumor biopsies showed the same light chain restriction in 4 (36%) and were unavailable in the 7 remaining cases. As shown for case 10, expression of a single immunoglobulin light chain produces a readily identifiable unimodal staining pattern (Figure 1A). It is not uncommon, however, for aggressive B-cell lymphomas to lack surface immunoglobulin expression, which in itself is a highly abnormal finding.16,18 In these instances, demonstration of a homogeneous population of large-surface light chain–negative B cells in the cerebrospinal fluid is adequate for diagnosis of lymphoma, as shown by cases 11 (Figures 1B), 8, and 14 (Table 6).18 Case 8 had a primary mediastinal (thymic) diffuse large B-cell lymphoma, which usually lacks surface immunoglobulin. In case 14, the lymphoma cells in the cerebrospinal fluid were negative for light chain restriction but positive for κ restriction in the tumor biopsy, a finding that was secondary to cytoplasmic localization of the light chain.
Flow cytometric dot plots of cerebrospinal fluid specimens demonstrating lymphoma populations. Each dot represents a single cell. The units on the x- and y-axes are relative fluorescent intensity. (A) Monoclonal B-cell population. All lymphoma cells in the oval area are λ-positive (y-axis) and κ-negative (x-axis). (B) Surface immunoglobulin-negative or dim B-cell lymphoma. The B cells in the oval area are CD22+ (y-axis) and negative for κ on the left and λ on the right (x-axis). (C) Small population of monoclonal B cells in a specimen with low cellularity. The B cells in the oval area are CD22+ (y-axis) and κ-positive (left, x-axis) and λ-negative (right, x-axis). (D) CD20– B-cell lymphoma. The B cells in the oval area are CD20– (x-axis) and CD22+ (y-axis) and monoclonal (not shown). The CD22– and CD20– cells are T cells.
Flow cytometric dot plots of cerebrospinal fluid specimens demonstrating lymphoma populations. Each dot represents a single cell. The units on the x- and y-axes are relative fluorescent intensity. (A) Monoclonal B-cell population. All lymphoma cells in the oval area are λ-positive (y-axis) and κ-negative (x-axis). (B) Surface immunoglobulin-negative or dim B-cell lymphoma. The B cells in the oval area are CD22+ (y-axis) and negative for κ on the left and λ on the right (x-axis). (C) Small population of monoclonal B cells in a specimen with low cellularity. The B cells in the oval area are CD22+ (y-axis) and κ-positive (left, x-axis) and λ-negative (right, x-axis). (D) CD20– B-cell lymphoma. The B cells in the oval area are CD20– (x-axis) and CD22+ (y-axis) and monoclonal (not shown). The CD22– and CD20– cells are T cells.
The high sensitivity of flow cytometry comes from its ability to detect monoclonal B-cell populations in samples with sparse cellularity or B-cell lymphopenia but in the presence of normal polyclonal B cells or cells with cytophilic antibody (passively adsorbed immunoglobulins from the plasma in vivo), as demonstrated by case 12 (Figure 1C).12 In samples containing admixed monoclonal and polyclonal B cells, the simultaneous analysis of cell size using forward scatter, antigen expression intensity, and differential or aberrant antigen expression allows the detection of lymphoma cells. Delineating an abnormal antigen expression pattern in B cells, such as absent CD19, CD20, or CD22 expression or abnormal antigen expression intensity, can be diagnostic and may allow subclassification into discrete diagnostic categories as shown by case 8 (Figure 1D).12,16,18
Prognostic factors and clinical outcome
To identify the clinical characteristics associated with cerebrospinal fluid involvement in the 51 patients at risk for central nervous system involvement, we performed a univariate and logistic regression analysis of the characteristics listed in Table 2. The presence of 2 or more extranodal sites was the only evaluated parameter that was associated with cerebrospinal fluid involvement (P = .0057; Table 2), and the risk was slightly enhanced with increasing number of sites (P = .0055; data not shown). In a logistic regression model, only the number of multiple extranodal sites was associated with cerebrospinal fluid involvement (P = .012). Lactate dehydrogenase, a previously identified factor for risk of central nervous system disease, along with multiple extranodal sites, was not identified as a significant parameter in the univariate (P = .71) or logistic model (P = .86). There was a trend between a high International Prognostic Index (IPI) score and risk of cerebrospinal fluid involvement in both the univariate (P = .062) and logistic regression analyses (P = .066), but this, of course, also reflects the presence of multiple extranodal sites. Importantly, although the presence of multiple extranodal sites was the best predictor of a positive cerebrospinal fluid, its positive predictive value was relatively low, as 17 of 27 (63%) of such patients had a negative cerebrospinal fluid evaluation.
We evaluated the long-term clinical outcome of all 92 newly diagnosed patients for the development of central nervous system relapse. Therapeutically, among the 11 newly diagnosed patients with evidence of occult cerebrospinal fluid lymphoma by flow cytometry, 9 received active treatment, 1 received prophylactic treatment, and 1 sought treatment at another institution. Of these patients, 5 (45%) relapsed in the clinical central nervous system and died despite their having initially received active treatment. The 40 patients at increased risk for central nervous system involvement but with negative cerebrospinal fluid by flow cytometry received only prophylactic treatment. Of these patients, 3 (8%) relapsed in the central nervous system and died. In contrast, there were no central nervous system relapses among the 41 patients who were at low risk for central nervous system disease, and none of these patients received prophylactic treatment. Among the relapsed/treated patients, only the 3 patients with evidence of cerebrospinal fluid lymphoma by flow cytometry/cytology had central nervous system disease.
Discussion
In the present report, flow cytometry was able to detect occult cerebrospinal fluid lymphoma in 11 of 51 (22%) newly diagnosed aggressive B-cell lymphoma patients who were at increased clinical risk for developing central nervous system involvement. In contrast, cytology was not sufficiently sensitive to detect this low level involvement by neoplastic cells. Quantitatively, flow cytometry detected a neoplastic clone that constituted as little as 0.2% of the concentrated cellular pellet in the present study, whereas cytology generally requires that neoplastic cells constitute at least 5% of cells.10,17,19,20 Of course, this is dependent on the tumor phenotype and histology for these respective methods.17 Perhaps not surprisingly, there was no difference in the cerebrospinal fluid laboratory characteristics among patients with or without occult cerebrospinal fluid involvement, indicating that low concentrations of neoplastic cells do not significantly impact these parameters and highlighting their low utility in this setting. On the other hand, cytology was able to detect cerebrospinal fluid involvement in patients with relapsed/treated disease, where the median percent of neoplastic cells was higher than that found in the newly diagnosed patients.
These results provide scientific validation for clinical risk paradigms in large B-cell lymphomas and insight into the biology of central nervous system involvement. van Besien et al reported a 15.4% incidence of central nervous system recurrence at 1 year among patients at clinical risk for central nervous system lymphoma, which is similar to the 22% incidence of occult disease found at disease presentation in our study.1 Importantly, our study demonstrated that the number of extranodal sites was the only variable independently associated with occult central nervous system involvement, raising the hypothesis that the biologic phenotype associated with colonization of extranodal sites by large B-cell lymphoma is what primarily drives central nervous system spread. Furthermore, the finding of occult cerebrospinal fluid involvement at presentation indicates this phenotype is intrinsic to the tumor cell and independent of biologic evolution or central nervous system contamination over time. Indeed, the clinical behavior suggests this phenotype may derive from alterations in the microenvironment requirements of the tumor cell with an ability to survival within non-nodal sites, or less likely due to changes in cellular trafficking. The absence of other independent clinical risk factors, such as an elevated LDH, in our series is also consistent with this hypothesis. The finding by van Besien et al, however, that an elevated LDH is a covariate with extranodal sites may be due to its ability to discriminate among different extranodal tissue sites and/or number of sites, both of which impact the risk of central nervous system spread.1 Notably, there was no independent association between the International Prognostic Index and risk of cerebrospinal fluid spread in either the present or van Besien et al's studies, suggesting that this index is not the appropriate clinical surrogate to determine central nervous system prophylaxis.21 To be sure, the International Prognostic Index is not a surrogate of a biologic phenotype but does include the presence of extranodal sites.
Cytology is considered the method of choice for detection of B-cell neoplasms in the cerebrospinal fluid, but 2 small studies have shown flow cytometry can increase detection sensitivity when combined with cytology.10,20 In these 2 studies of 10 and 9 positive cases, respectively, 3 cases each were detected by flow cytometry alone with the remaining cases also detected by cytology. In the present study, however, 10 of 11 cases from newly diagnosed patients were detected by flow cytometry alone, indicating that cytology is inadequate for cerebrospinal fluid staging of newly diagnosed aggressive B-cell lymphomas. The higher rate of concordance between cytology and flow cytometry in the 2 published studies almost certainly reflects the clinical practice of sampling cerebrospinal fluid in symptomatic patients and not for staging purposes. In the former setting, the tumor cell concentration will be relatively high and, hence, usually detectable by both cytology and flow cytometry, as we found in our relapsed/treated patients.
These results raise important questions regarding the optimal management of patients at increased risk for central nervous system involvement by aggressive B-cell lymphoma. The phenomenon of central nervous system involvement by diffuse large B-cell lymphomas is incontrovertible and is clinically observed in 5% of unselected patients and 8% of high-risk patients who do not receive central nervous system–penetrating agents.1,3,22 In a recent randomized study of CHOP (doxorubicin, cyclophosphamide, vincristine, and prednisone) versus ACVBP (doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone) in poor-prognosis aggressive lymphomas, for example, the absence of central nervous system–penetrating agents in the CHOP arm resulted in a significantly increased incidence of central nervous system disease, suggesting that central nervous system–directed therapy can reduce central nervous system failures.3 Because most patients have little if any risk of central nervous system involvement, prophylaxis should be confined to those at increased risk. Clinically, both our results and those by van Besien et al indicate that the presence of multiple extranodal sites is the best clinical surrogate of risk and should be used to select patients for central nervous system prophylaxis. Alternatively, prophylaxis could be based on the presence of cerebrospinal fluid involvement by flow cytometry at initial staging, which our results suggest can identify most patients at risk. This, however, requires further study, because 3 patients with a negative flow cytometry on initial staging ultimately developed central nervous system lymphoma. Notably, despite active treatment, 5 of 9 patients progressed in the central nervous system, reinforcing the need for more effective therapy.
It is likely that as the treatment of aggressive B-cell lymphomas improves, the impact of central nervous system involvement on overall treatment failure will rise, as seen when the treatment of acute lymphocytic leukemia improved.23 Knowledge of cerebrospinal fluid involvement early in the disease course will allow more rational approaches and will, it is hoped, improve outcome. Indeed, early results with intrathecal rituximab suggest it may represent an important step in the treatment of this sanctuary site.24 We feel it is important to study this question in clinical trials and that, presently, patients at increased risk for central nervous system involvement by aggressive B-cell lymphoma as defined by at least 2 extranodal disease sites or bone marrow involvement undergo staging evaluation of the cerebrospinal fluid by flow cytometry and cytology.
Prepublished online as Blood First Edition Paper, September 9, 2004; DOI 10.1182/blood-2004-05-1982.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal