Abstract
The CD34+CD38– phenotype identifies a population in the bone marrow that is enriched in the steady state for hematopoietic stem cells (HSCs). Following ex vivo culture of CD34+ cells, HSC content is difficult to measure since committed CD34+CD38+ progenitors down-regulate CD38 surface expression during culture. In this study, we sought to define the phenotype of human HSCs following ex vivo culture under conditions that support the expansion of human cells capable of repopulating non-obese diabetic/severe combined immunodeficiency (SCID)–repopulating cells (SRCs). Contact coculture of fluorescence-activated cell sorter (FACS)–sorted bone marrow (BM) CD34+CD38– cells with human brain endothelial cells (HUBECs) supported a 4.4-fold increase in CD34+CD38– cells with a concordant 3.6-fold increase in SRCs over 7 days. Noncontact HUBEC cultures and the addition of thrombopoietin, stem cell factor (SCF), and macrophage colony stimulating factor I receptor (Fms)–like tyrosine kinase 3 (Flt-3) ligand supported further increases in CD34+CD38– cells (6.4-fold and 13.1-fold), which correlated with significant increases in SRC activity. Moreover, cell-sorting studies performed on HUBEC-cultured populations demonstrated that SRCs were significantly enriched within the CD34+CD38– subset compared with the CD34–CD38– population after culture. These results indicate that human HSCs can be identified and characterized by phenotype following expansion culture. These studies also demonstrate that HUBEC-elaborated soluble factors mediate a unique and potent expansion of human HSCs.
Introduction
Cell surface expression of the CD34 antigen is a reliable indicator of enrichment for hematopoietic progenitor and stem cells.1,2 However, cells within the CD34+ compartment are heterogeneous and include committed CD34+CD38+ progenitors that lack stem cell activity.3,4 The CD34+CD38– fraction makes up 1% to 10% of the CD34+ population and is highly enriched for both extended long-term culture-initiating cells (ELTC-ICs) and the most primitive assayable cells that are capable of repopulating nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice (SCID-repopulating cells [SRCs]).3-7 Using fluorescence-activated cell sorting to collect steady-state cord blood (CB) CD34+CD38– cells, Bhatia et al4 demonstrated a frequency of 1 SRC per 617 CB CD34+CD38– cells and no detectable SRCs within the CD34+CD38+ fraction.4 Bhatia et al8 also showed, via a limiting dilution analysis, that purified human CB CD34+CD38– cells could be cultivated ex vivo with cytokines resulting in a 2- to 4-fold increase in SRCs at day 4 followed by loss of SRCs by day 9. A subsequent study by Glimm and Eaves9 further demonstrated that self-renewal divisions occur within primitive CB–repopulating cells during 5-day cytokine suspension cultures. Other studies have suggested that human CB SRCs can be maintained ex vivo in liquid suspension cultures from 1 to 12 weeks.10-12 Conversely, ex vivo culture of adult (bone marrow [BM], peripheral blood) sources of human CD34+ stem cells with cytokines, with and without stroma, has been reproducibly associated with the loss of primitive repopulating cells over time.13-17 Investigations into the proliferative capacity and SRC content of purified human BM CD34+CD38– cells have been more limited in part due to a lack of culture conditions that support the maintenance or expansion of adult hematopoietic stem cells (HSCs). 8,18
Recent studies have indicated that expression of CD38 antigen on CD34+CD38+ hematopoietic progenitors may be down-modulated during ex vivo culture with cytokines, calling into question the reliability of the CD34+CD38– phenotype as an indicator of HSC content during or after culture.19,20 As evidence that the CD34+CD38– phenotype did not correlate with primitive stem cell content, Dorrell et al19 demonstrated that surface expression of myeloid maturation antigens, CD33 and CD13, increased more than 2-fold on CB CD34+CD38– cells during short-term ex vivo culture. This down-regulation of surface CD38 expression on committed progenitors has been attributed to a depletion of retinoids that occurs over time during in vitro culture.21,22 We recently reported that coculture of adult human BM CD34+ cells with primary human brain endothelial cells (HUBECs) induced a 4.1-fold increase in SRC frequency,15 but we also observed a significantly larger expansion (212-fold) of cells bearing the CD34+CD38– phenotype.15 Conversely, we found that the loss of phenotypic CD34+CD38– cells following liquid suspension culture with granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin 3 (IL-3), IL-6, stem cell factor (SCF), and macrophage colony stimulating factor I receptor (Fms)–like tyrosine kinase 3 (Flt-3) ligand alone was associated with a complete loss of SRCs.15 Taken together, these data suggested that SRCs may have been contained within the CD34+CD38– fraction following HUBEC culture, but this was not measurable since heterogeneous CD34+ cells were used in these studies. Since the ability to conveniently monitor HSC content during/after ex vivo culture would be advantageous for both research and clinical purposes, we sought to define the phenotype of adult HSCs that had replicated under HSC-supportive culture conditions. Via rigorous cell-sorting experiments, we found that SRC expansion correlates with amplification of BM CD34+CD38– cells and this result appears to be mediated by soluble factors elaborated by HUBECs.
Materials and methods
Isolation of human BM CD34+CD38– cells prior to ex vivo culture
Human BM CD34+ cells were acquired from Biowhittaker (Gaithersburg, MD). CD34+ cells (> 95% purity) were thawed and placed in complete culture medium containing Iscove modified Dulbecco medium (IMDM; Invitrogen, Carlsbad, CA), 10% fetal bovine serum (FBS; Hyclone, Logan, UT), 100 U/mL penicillin, and 100 μg/mL streptomycin (1% penicillin/streptomycin [pcn/strp]) at 37°C. Cells were then pelleted and resuspended in phosphate-buffered saline (PBS), counted, and stained with anti-CD34–fluorescein isothiocyanate (FITC) and anti-CD38–phycoerythrin (PE; Becton Dickinson, San Jose, CA). After 30 minutes on ice, the cells were washed twice and resuspended in PBS containing 10% heat-inactivated FBS, 1% pcn/strp. Samples were the analyzed and sorted using a MoFlo cell sorter (DakoCytomation, Carpinteria, CA) to isolate CD34+CD38– and CD34+CD38+ subsets. The CD34+CD38– sort gate was set to collect only those events falling in the lowest 2% of PE fluorescence within the total CD34+ population, to ensure acquisition of highly purified CD34+CD38– cells.
Ex vivo culture of CD34+, CD34+CD38–, CD34+CD38+ cells
HUBEC monolayers were established in culture as previously described.15 Briefly, 1 × 105 HUBECs were cultured on gelatin-coated 6-well plates (Costar, Cambridge, MA) in complete endothelial cell culture medium (5 mL/well) containing M199 (Invitrogen), 10% FBS, 100 μg/mL L-glutamine (Invitrogen), 50 μg/mL heparin, 60 μg/mL endothelial cell growth supplement (Sigma, St Louis, MO), and 1% pcn/strp at 37°C in 5% CO2 atmosphere. After 48 hours, HUBECs were washed twice with PBS, and the media was replaced with BM expansion medium (5 mL/well) containing IMDM, 10% FBS, pcn/strp, 2 ng/mL granulocyte-macrophagecolony stimulating factor (GM-CSF), 5 ng/mL interleukin 3 (IL-3), 5 ng/mL IL-6, 120 ng/mL stem cell factor (SCF), and 50 ng/mL Flt-3 ligand (GM36SF; R&D Systems, Minneapolis, MN). BM CD34+CD38– sorted cells were then added at 1 × 103 to 4 × 104 cells/well and maintained at 37°C in 5% CO2 atmosphere. After 7 days of HUBEC coculture, nonadherent cells were harvested by washing the monolayers gently with warm IMDM. For comparison, BM CD34+CD38+ sorted cells were cultured at 1 × 105 cells per well under identical conditions. In order to determine the effect of HUBEC-soluble activity, we also cultured fluorescence-activated cell sorter (FACS)–sorted BM CD34+CD38– cells with HUBECs separated by a 0.4-μm transwell insert (Costar). The effect of HUBEC coculture of BM CD34+CD38– cells supplemented with 20 ng/mL thrombopoietin (TPO), 120 ng/mL SCF, and 20 ng/mL thrombopoietin/stem cell factor/flt-3 ligand (TSF) was also measured in 7-day cultures. As controls, FACS-sorted BM CD34+CD38– cells were also plated in liquid suspension cultures with the GM36SF and TSF. Hemacytometer counts were performed to determine cellular expansion. Multiple donor BM CD34+ cells were combined prior to FACS sorting of BM CD34+CD38– cells so that identical cell populations were used for initiation of all comparative experiments.
Immunophenotype analysis and colony-forming assays of BM CD34+CD38– cells before and after culture
Freshly sorted BM CD34+CD38– cells and BM CD34+CD38+ cells cultured either with HUBEC monolayers or liquid suspension culture were analyzed for phenotypic changes at day 4 and day 7 of culture. The cells were stained with CD34-FITC and CD38-PE for 30 minutes on ice and compared with appropriate isotype control antibody staining. Samples were analyzed using a MoFlo cell sorter with Summit software (DakoCytomation). Colony-forming assays of day-0 BM CD34+CD38– cells and the progeny of BM CD34+CD38– cells cultured with HUBECs + GM36SF or GM36SF alone were performed as previously described.15 Cells (5 × 102 to 50 × 102) were cultured in 35-mm culture dishes (Miles Laboratories, Naperville, IL) in media consisting of 1 mL of IMDM, 1% methylcellulose, 30% FBS, 5 U/mL erythropoietin, 2 ng/mL GM-CSF, 10 ng/mL IL-3, and 120 ng/mL SCF. At day 14, triplicate cultures were evaluated to determine the number of colonies (> 50 cells) per dish.
NOD/SCID transplantation studies
NOD/SCID mice23 received transplants of either FACS-sorted BM CD34+CD38– cells or the progeny of BM CD34+CD38– cells cultured with HUBEC monolayers supplemented with GM36SF over a range of doses. Cells were transplanted via tail vein injection after irradiating NOD/SCID mice with 300 cGy using a linear accelerator source as previously described.15 Mice that received transplants of day-0 BM CD34+CD38– cells received cotransplants of 2 × 105 CD34– accessory cells to facilitate engraftment as previously described.4,24 Mice that received transplants of the progeny of BM CD34+CD38– cells following 7 days of HUBEC culture received no CD34– accessory cells or exogenous cytokines to facilitate engraftment. Additional groups of mice received transplants of FACS-sorted subsets of HUBEC progeny at day 7. All mice in each group were killed at week 8 and marrow samples were obtained by flushing their femurs with IMDM at 4°C. Red cells were lysed using red cell lysis buffer (Sigma) and flow cytometric analysis of human hematopoietic engraftment was performed as previously described using commercially available monoclonal antibodies against human leukocyte differentiation antigens to identify engrafted human leukocytes and discriminate their hematopoietic lineages.15,25
FACS sorting and transplantation of HUBEC-cultured subpopulations
In order to define the phenotype of amplified SRCs following HUBEC culture, BM CD34+CD38– cells that had been cultured for 7 days with HUBECs + GM36SF were collected, washed in IMDM, centrifuged, and then stained with CD34-FITC and CD38-PE. An aliquot of cells was also stained with immunoglobulin G (IgG)–FITC and IgG-PE antibodies for control staining. After 30 minutes on ice, the cells were resuspended in PBS with 10% FBS/1% pcn/strp, centrifuged, and resuspended in IMDM + 10% FBS. Samples were then analyzed and sorted using a MoFlo cell sorter. Since HUBEC-cultured cells showed predominance of either the CD34+CD38– or CD34–CD38– phenotype, we collected the CD34+CD38– and the CD34–CD38– populations after culture. The sorted populations were centrifuged, resuspended in PBS, and transplanted via tail vein injection into NOD/SCID mice for measurement of repopulating capacity. Each mouse received a transplant of the cultured product of 40 000 FACS-sorted BM CD34+CD38– cells per condition.
Statistical analysis and SRC frequency measurements
For purposes of our limiting dilution analysis, a mouse that received a transplant was scored as positively engrafted if at least 0.1% of the marrow cells expressed human CD45 via high-resolution FACS analysis. This criterion is consistent with previously published criteria for human cell repopulation in NOD/SCID mice.9,19 SRC frequency in each cell source was calculated using the maximum likelihood estimator as described previously by Taswell26 for the single-hit Poisson model.27,28 The χ2 test provides a measure of the legitimacy of using pooled data and of the validity of applying the single-hit model.27 We calculated confidence intervals for the frequencies using the profile likelihood method, and we used the likelihood ratio test to confirm the fit of the model. As a confirmation of the maximum likelihood estimator, we also applied a minimum χ2 estimator to the pooled data.
Results
HUBEC coculture increases total cells, CD34+ cells, and CD34+CD38– cells
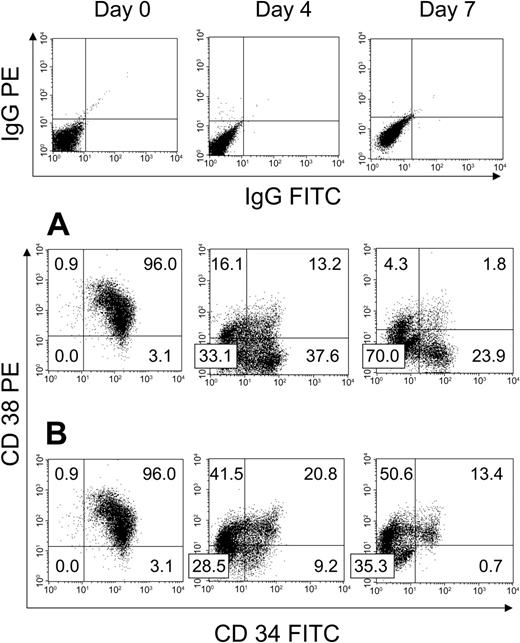
We first examined the effect of HUBEC culture + GM36SF and GM36SF alone on the expansion of human BM CD34+ cells (N = 3). Figure 1A shows a representative BM CD34+ cell sample from day 0 through day 7 of HUBEC culture. Input cells were 99.1% ± 0.1% CD34+, with 96% ± 0.1% of these cells being CD34+CD38+ and 3.2% ± 0.1% being CD34+CD38–, defined as CD34+ cells showing PE fluorescence less than that of the IgG-PE isotype control. By day 4 of HUBEC culture + GM36SF, 36.7% ± 0.7% of the cells became CD34+CD38–, and by day 7, 22.6% ± 0.1% of the cultured cells were CD34+CD38–. At day 7 of HUBEC culture + GM36SF, we calculated a mean 16-fold increase in total cells, a 3.9-fold increase in CD34+ cells, and a 115-fold increase in CD34+CD38– cells (Table 1). For comparison, liquid suspension cultures of BM CD34+ cells with GM36SF alone caused a mean 19.3-fold increase in total cells and a 2.6-fold increase in CD34+ cells at day 7, but the CD34+CD38– population was nearly completely lost during 7-day culture (9.8% ± 0.3% CD34+CD38– cells at day 4, < 1% at day 7). Figure 1B shows representative phenotypic changes within BM CD34+ cells during culture with GM36SF alone over 7 days.
Phenotypic analysis of unsorted BM CD34+ cells at day 0 and following HUBEC culture versus GM36SF alone. Human BM CD34+ cells were cultured with either HUBEC monolayers supplemented with GM36SF or GM36SF alone for 7 days (N = 3). (A) Representative phenotype of BM CD34+ cells at day 0, day 4, and day 7 of HUBEC culture, demonstrating a high percentage of CD34+CD38– cells persistent after culture. (B) Representative phenotype of BM CD34+ cells at day 0, day 4, and day 7 of culture with GM36SF alone, demonstrating nearly complete loss of CD34+CD38– cells. All cell populations were stained with anti-CD34–FITC and anti-CD38–PE antibodies and analyzed by flow cytometry. Isotype control staining for each time point is shown at top. Numbers indicate the percent of cells in each quadrant.
Phenotypic analysis of unsorted BM CD34+ cells at day 0 and following HUBEC culture versus GM36SF alone. Human BM CD34+ cells were cultured with either HUBEC monolayers supplemented with GM36SF or GM36SF alone for 7 days (N = 3). (A) Representative phenotype of BM CD34+ cells at day 0, day 4, and day 7 of HUBEC culture, demonstrating a high percentage of CD34+CD38– cells persistent after culture. (B) Representative phenotype of BM CD34+ cells at day 0, day 4, and day 7 of culture with GM36SF alone, demonstrating nearly complete loss of CD34+CD38– cells. All cell populations were stained with anti-CD34–FITC and anti-CD38–PE antibodies and analyzed by flow cytometry. Isotype control staining for each time point is shown at top. Numbers indicate the percent of cells in each quadrant.
Expansion of purified BM CD34+ subsets following coculture with HUBEC monolayers
. | . | . | Day 7 . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Day 0 . | . | Total . | . | CD34+ . | . | CD34+CD38- . | . | |||||
| Culture condition . | Cell subset . | Cell count, × 105 . | Cell count, × 105 . | Fold change . | Cell count, × 105 . | Fold change . | Cell count, × 105 . | Fold change . | |||||
| HUBECs (contact) + GM36SF | 34+ | 1.0 | 16.2 ± 0.3 | 16 | 3.9 ± 0.1 | 3.9 | 3.7 ± 0.1 | 115 | |||||
| HUBECs (contact) + GM36SF | 34+38+ | 1.0 | 18.3 ± 0.1 | 18 | 2.6 ± 0.1 | 2.6 | 2.4 ± 0.1 | 2.4 | |||||
| HUBECs (contact) + GM36SF | 34+38- | 0.4 | 2.7 ± 0.7 | 6.7 | 1.8 ± 0.5 | 4.5 | 1.8 ± 0.5 | 4.4 | |||||
| HUBECs (noncontact) + GM36SF | 34+38- | 0.4 | 6.7 ± 0.1 | 16.7 | 3.4 ± 0.5 | 8.6 | 2.5 ± 0.1 | 6.4 | |||||
| HUBECs (contact) + TSF | 34+38- | 0.4 | 5.8 ± 0.5 | 14.6 | 5.3 ± 0.4 | 13.4 | 5.2 ± 0.5 | 13.1 | |||||
| GM36SF | 34+38- | 0.4 | 14.4 ± 0.7 | 35.8 | 3.7 ± 0.2 | 9.2 | 0.3 ± 0.1 | — | |||||
| TSF | 34+38- | 0.4 | 1.4 ± 0.2 | 3.5 | 1.0 ± 0.1 | 2.4 | 0.1 ± 0.1 | — | |||||
. | . | . | Day 7 . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Day 0 . | . | Total . | . | CD34+ . | . | CD34+CD38- . | . | |||||
| Culture condition . | Cell subset . | Cell count, × 105 . | Cell count, × 105 . | Fold change . | Cell count, × 105 . | Fold change . | Cell count, × 105 . | Fold change . | |||||
| HUBECs (contact) + GM36SF | 34+ | 1.0 | 16.2 ± 0.3 | 16 | 3.9 ± 0.1 | 3.9 | 3.7 ± 0.1 | 115 | |||||
| HUBECs (contact) + GM36SF | 34+38+ | 1.0 | 18.3 ± 0.1 | 18 | 2.6 ± 0.1 | 2.6 | 2.4 ± 0.1 | 2.4 | |||||
| HUBECs (contact) + GM36SF | 34+38- | 0.4 | 2.7 ± 0.7 | 6.7 | 1.8 ± 0.5 | 4.5 | 1.8 ± 0.5 | 4.4 | |||||
| HUBECs (noncontact) + GM36SF | 34+38- | 0.4 | 6.7 ± 0.1 | 16.7 | 3.4 ± 0.5 | 8.6 | 2.5 ± 0.1 | 6.4 | |||||
| HUBECs (contact) + TSF | 34+38- | 0.4 | 5.8 ± 0.5 | 14.6 | 5.3 ± 0.4 | 13.4 | 5.2 ± 0.5 | 13.1 | |||||
| GM36SF | 34+38- | 0.4 | 14.4 ± 0.7 | 35.8 | 3.7 ± 0.2 | 9.2 | 0.3 ± 0.1 | — | |||||
| TSF | 34+38- | 0.4 | 1.4 ± 0.2 | 3.5 | 1.0 ± 0.1 | 2.4 | 0.1 ± 0.1 | — | |||||
Human BM CD34+ cells (99.1% ± 0.1% CD34+, 3.2% ± 0.1% CD34+CD38-, 96.0% ± 0.1% CD34+CD38+) were cultured with HUBECs supplemented with GM36SF (N = 3). FACS-sorted BM CD34+CD38- cells (N = 8) and BM CD34+CD38+ cells (N = 6) were placed in contact or noncontact cultures with HUBEC monolayers and cytokine supplements as indicated in “Materials and methods.” For comparison, BM CD34+CD38- cells were cultured in liquid suspension with either GM36SF or TSF (N = 3).
— indicates a decline in cells compared to input.
Characterization of CD34+CD38– cells produced during HUBEC coculture
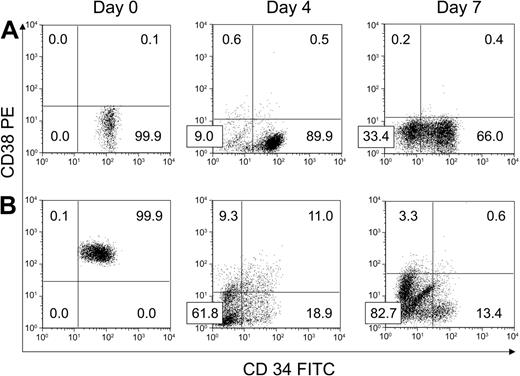
We next performed experiments (N = 8) to characterize the effect of HUBEC culture on more primitive BM CD34+CD38– cells. As shown in Figure 2A, the purity of day-0 FACS-sorted CD34+CD38– populations were greater than 98% in all experiments. At day 4 and 7 of HUBEC culture + GM36SF, 93.0% ± 1.4% and 67.6% ± 10.7% of the cultured cells remained CD34+CD38–, respectively, concomitant with a mean 6.7-fold increase in total cells at day 7. This translated into a mean 4.4-fold increase in CD34+CD38– cells. The majority of the remaining cells at day 7 were CD34–CD38– (32% ± 10.6%). FACS analysis of BM CD34+CD38– cells from a representative experiment at day 0, day 4, and day 7 of HUBEC culture is shown in Figure 2A. Table 1 summarizes the expansion of BM CD34+CD38– cells during HUBEC culture + GM36SF from 8 experiments.
Phenotypic response of purified BM CD34+CD38– and CD34+CD38+ cells to ex vivo culture with HUBECs. (A) A representative experiment showing the phenotypic changes that occurred within FACS-sorted BM CD34+CD38– cells over 7 days of culture with HUBECs + GM36SF. Note that the majority of day-7 HUBEC-cultured cells were either CD34+CD38– or CD34–CD38– (N = 8). (B) A representative experiment showing the phenotypic changes that occurred within FACS-sorted BM CD34+CD38+ cells over 7 days of coculture with HUBECs + GM36SF. At day 7, the majority of the cells are CD34–CD38–, but a minor population of CD34dimCD38– cells remains. Numbers indicate the percentage of cells in each quadrant.
Phenotypic response of purified BM CD34+CD38– and CD34+CD38+ cells to ex vivo culture with HUBECs. (A) A representative experiment showing the phenotypic changes that occurred within FACS-sorted BM CD34+CD38– cells over 7 days of culture with HUBECs + GM36SF. Note that the majority of day-7 HUBEC-cultured cells were either CD34+CD38– or CD34–CD38– (N = 8). (B) A representative experiment showing the phenotypic changes that occurred within FACS-sorted BM CD34+CD38+ cells over 7 days of coculture with HUBECs + GM36SF. At day 7, the majority of the cells are CD34–CD38–, but a minor population of CD34dimCD38– cells remains. Numbers indicate the percentage of cells in each quadrant.
In order to determine whether down-modulation of CD38 antigen expression on CD34+CD38+ cells contributed to the increase in CD34+CD38– cells observed during HUBEC culture of unsorted BM CD34+ cells, we examined the expansion of FACS-sorted BM CD34+CD38+ cells during HUBEC culture + GM36SF (N = 6 experiments). Figure 2B shows a representative phenotype of input BM CD34+CD38+ cells and the progeny of BM CD34+CD38+ cells during 7-day HUBEC culture. By day 4, only 29.7% ± 1.0% of the cells remained CD34+ and 20.6% ± 1.1% were CD34+CD38–. By day 7, more than 85% of the input CD34+CD38+ cells became phenotypically CD34–, but 13.3% ± 1.2% of the day-7 population demonstrated a CD34dimCD38– phenotype, while the total cell number increased by a mean 18-fold. Therefore, from an input of 1 × 105 BM CD34+CD38+ cells, HUBEC culture yielded 2.4 × 105 CD34+CD38– cells or a 240% increase in phenotypic CD34+CD38– cells compared with input.
Consistent with the observation of Dorrell et al19 regarding ex vivo culture of CB, the phenotypic changes that purified BM CD34+CD38+ and CD34+CD38– cells undergo during HUBEC coculture can be extrapolated to the ex vivo expansion of unsorted BM CD34+ cells on HUBEC monolayers. A typical BM CD34+ sample of 1 × 106 cells contains 9.5 × 105 (95%) CD34+ cells, 9 × 105 (90%) CD34+CD38+ cells, and 5 × 104 (5%) CD34+CD38– cells. Given the yields we have observed with purified BM cell subsets, the CD34+CD38+ fraction should produce 2.2 × 106 CD34+CD38– cells and the input CD34+CD38– cells should contribute 2.0 × 105 CD34+CD38– cells. Therefore, 91.7% of the CD34+CD38– cells recovered from CD34+ cells cultured with HUBECs at day 7 would be predicted to derive from committed CD34+CD38+ cells, which contain no repopulating capacity.
Effect of noncontact HUBEC culture and alternative cytokine combinations on BM CD34+CD38– cell expansion
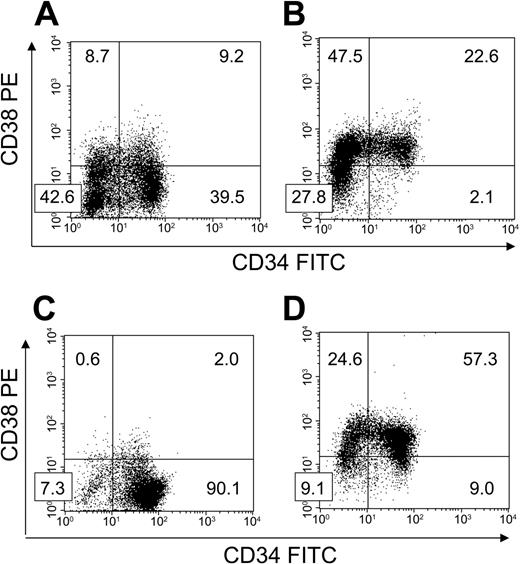
FACS-sorted BM CD34+CD38– cells were cultured with HUBECs separated by transwell inserts to ascertain the importance of cell-to-cell contact on HUBEC-mediated hematopoietic expansion. Interestingly, noncontact HUBEC cultures + GM36SF supported a greater increase in total cells (mean 16.7-fold; N = 3) and CD34+CD38– cells (mean, 6.4-fold) compared with HUBEC contact cultures, with 38.2% ± 1.3% of the day-7 population expressing the CD34+CD38– phenotype. Figure 3A shows a representative phenotype of BM CD34+CD38– cells following 7-day noncontact HUBEC culture + GM36SF. In comparison studies, GM36SF alone supported a marked increase in total cells (mean, 36-fold; N = 3) but only 2.3% ± 0.2% of the population remained CD34+CD38– at day 7, resulting in a loss of CD34+CD38– cells compared with input. A representative phenotype of BM CD34+CD38– cells following 7-day culture with GM36SF alone is shown in Figure 3B. The results of these experiments are summarized in Table 1.
HUBEC noncontact cultures and HUBECs + TSF support the differential maintenance of CD34+CD38– cells. BM CD34+CD38– cells were placed in 7-day cultures to determine phenotype changes over time (N = 3 each). (A) Following 7-day noncontact culture with HUBECs + GM36SF, a high percentage of CD34+CD38– cells persisted over time. (B) Following 7-day culture with GM36SF alone, significant losses of CD34+CD38– cells were observed. (C) Following 7-day culture with HUBECs + TSF, the majority of cells remained CD34+CD38– after culture. (D) Conversely, at day 7 of culture with TSF alone, the majority of the input CD34+CD38– population was lost. Numbers indicate the percentage of cells in each quadrant.
HUBEC noncontact cultures and HUBECs + TSF support the differential maintenance of CD34+CD38– cells. BM CD34+CD38– cells were placed in 7-day cultures to determine phenotype changes over time (N = 3 each). (A) Following 7-day noncontact culture with HUBECs + GM36SF, a high percentage of CD34+CD38– cells persisted over time. (B) Following 7-day culture with GM36SF alone, significant losses of CD34+CD38– cells were observed. (C) Following 7-day culture with HUBECs + TSF, the majority of cells remained CD34+CD38– after culture. (D) Conversely, at day 7 of culture with TSF alone, the majority of the input CD34+CD38– population was lost. Numbers indicate the percentage of cells in each quadrant.
Since the combination of TSF has been shown to optimize the ex vivo maintenance of CB SRCs,11,12 we also examined the expansion of purified BM CD34+CD38– cells during HUBEC culture + TSF. HUBEC culture + TSF supported a mean 14.6-fold (N = 3) increase in total cells with 90.2% ± 0.1% remaining CD34+CD38– at day 7 (Figure 3C). This translated into a 13.1-fold increase in CD34+CD38– cells. Conversely, liquid suspension cultures with TSF alone resulted in a mean 3.5-fold increase in total cells (N = 3) with only 8.8% ± 1.3% of the population remaining CD34+CD38– at day 7 (Figure 3D). As observed with GM36SF alone, TSF alone was associated with a loss of CD34+CD38– cells at day 7 compared with input (Table 1).
Coculture of BM CD34+CD38– cells with HUBECs inhibits progenitor cell maturation
In order to determine the effect of HUBEC culture on the differentiation of primitive BM CD34+CD38– cells, we measured the colony-forming cell (CFC) activity within day-0 BM CD34+CD38– cells and within the progeny of BM CD34+CD38– cells following 7-day culture with HUBECs + GM36SF (N = 6). As expected, day-0 BM CD34+CD38– cells contained nearly undetectable CFC activity, reflecting a more primitive HSC-enriched population (Table 2). Following HUBEC culture + GM36SF, the progeny of BM CD34+CD38– cells contained 12.5-fold increased granulocyte macrophage colony-forming units (CFU-GMs), 4-fold increased erythroid burst-forming units (BFUEs), and 10-fold increased CFU-total compared with input BM CD34+CD38– cells. Conversely, the progeny of BM CD34+CD38– cells cultured with GM36SF alone contained 70-fold increased CFU-GMs, 26-fold increased BFU-Es, and 54-fold increased CFU-total's compared with input BM CD34+CD38– cells. These data indicated that coculture of BM CD34+CD38– cells with HUBECs significantly delayed or inhibited lineage commitment of CD34+CD38– hematopoietic stem/progenitor cells, as evidenced by approximately 6-fold lower total CFC production compared with BM CD34+CD38– cells cultured with GM36SF alone (P = .004, Kruskal-Wallis test).
HUBEC coculture inhibits colony-forming cell differentiation compared with cytokines alone
. | No. of colony-forming cells, × 103 . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Condition . | CFU-GMs . | BFU-Es . | CFUs-Mix . | CFUs-Total . | |||
| BM | |||||||
| CD34+CD38-, day 0 | 6.3 ± 0.2 | 2.4 ± 1.3 | 1.1 ± 0.2 | 9.9 ± 2.5 | |||
| Day-7 HUBECs + GM36SF | 76.2 ± 27.0 | 8.6 ± 4.3 | 11.5 ± 2.5 | 96.4 ± 26.3 | |||
| Day-7 GM36SF | 411.8 ± 26.0 | 62.3 ± 28.6 | 54.8 ± 39.3 | 529.2 ± 92.6 | |||
. | No. of colony-forming cells, × 103 . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Condition . | CFU-GMs . | BFU-Es . | CFUs-Mix . | CFUs-Total . | |||
| BM | |||||||
| CD34+CD38-, day 0 | 6.3 ± 0.2 | 2.4 ± 1.3 | 1.1 ± 0.2 | 9.9 ± 2.5 | |||
| Day-7 HUBECs + GM36SF | 76.2 ± 27.0 | 8.6 ± 4.3 | 11.5 ± 2.5 | 96.4 ± 26.3 | |||
| Day-7 GM36SF | 411.8 ± 26.0 | 62.3 ± 28.6 | 54.8 ± 39.3 | 529.2 ± 92.6 | |||
The colony forming cell (CFC) activity of day-0 FACS-sorted human BM CD34+CD38- cells and their progeny following 7-day culture with HUBECs + GM36SF or GM36SF alone was measured (N = 6). Cells were collected and placed in methylcellulose colony-forming assay cultures as described in “Materials and methods.” At day 14, the number of CFCs were counted in triplicate in each condition. The values presented reflect the mean number of CFCs per culture condition ± standard deviations.
HUBEC culture increases the SRC frequency within BM CD34+CD38– cells
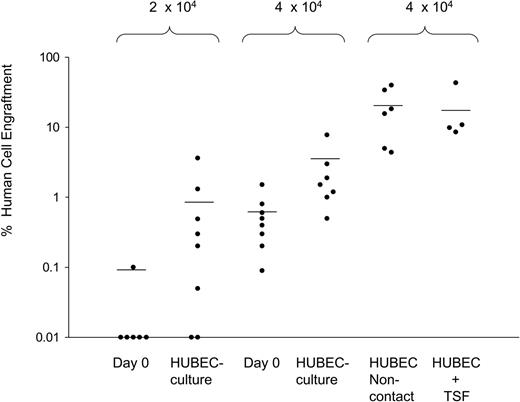
In a series of 5 experiments, NOD/SCID mice received transplants of FACS-sorted BM CD34+CD38– cells (N = 34 mice) or the progeny of HUBEC + GM36SF–cultured BM CD34+CD38– cells (N = 35 mice) over a range of doses designed to achieve nonengraftment in a fraction of mice. As negative controls, 5 mice received transplants of 2 × 105 human CD34– accessory cells, verifying no NOD/SCID engraftment from this population. As shown in Figure 4, transplantation of 1 × 103 to 1 × 104 day-0 BM CD34+CD38– cells resulted in no detectable human cell engraftment in NOD/SCID mice. Similarly, transplantation of the progeny of 1 × 103 to 1 × 104 BM CD34+CD38– cells following HUBEC culture also resulted in no engraftment. At a dose of 2 × 104 day-0 BM CD34+CD38– cells, 1 (16.6%) of 6 mice showed human engraftment. Conversely, the progeny of 2 × 104 BM CD34+CD38– cells following HUBEC coculture engrafted in 7 (88%) of 8 mice. As shown in Figure 4, at a dose of 4 × 104 day-0 BM CD34+CD38– cells, 5 (62.5%) of 8 mice showed engraftment, whereas 7 (100%) of 7 mice of mice that received transplants of the progeny of 4 × 104 BM CD34+CD38– cells showed human cell engraftment with a mean 4-fold higher levels of human CD45+ cells in the marrow compared with mice that received transplants of day-0 BM CD34+CD38– cells at the identical dose. Over the entire range of doses, day-0 BM CD34+CD38– cells engrafted in 6 (17.6%) of 34 mice, whereas the progeny of BM CD34+CD38– cells following HUBEC culture engrafted in 14 (40.0%) of 35 of NOD/SCID mice. Of note, mice that received transplants of the progeny of limiting doses of HUBEC-cultured BM CD34+CD38– cells displayed differentiation of human CD34+ progenitor cells (15.8% ± 6.1%), CD13+ myeloid cells (28.7% ± 8.3%), and CD19+ B-lymphoid cells (56.1% ± 11.3%), which was comparable to that observed in mice engrafted with day-0 BM CD34+CD38– cells, indicating that primitive cells with multilineage differentiation potential were maintained during HUBEC culture.
HUBEC coculture significantly increases the SRC frequency within human BM CD34+CD38– cells. The scatter plot shows the level of human CD45+ cell engraftment in NOD/SCID mice at week 8 following transplantation with FACS-sorted BM CD34+CD38– cells or their progeny as indicated. The progeny of 2 × 104 to 4 × 104 BM CD34+CD38– cells cultured × 7 days with HUBECs + GM36SF showed significantly higher levels of human engraftment compared with day-0 BM CD34+CD38– cells at the same dose. As shown at right, the highest human CD45+ cell engraftment was observed in mice that received transplants of the progeny of 4 × 104 BM CD34+CD38– cells following noncontact HUBEC cultures + GM36SF. Mice that received transplants of the progeny of BM CD34+CD38– cells cultured with HUBECs + TSF displayed comparable engraftment to noncontact HUBEC + GM36SF cultures. Each circle represents an individual mouse that received a transplant of human BM cells. Values on the Y-axis indicate the percentage of human CD45+ cell engraftment within the marrow of individual mice that received NOD/SCID transplants. The cell dosages for each group are shown at top. The mean levels of human CD45+ cell engraftment are indicated by horizontal bars for each group.
HUBEC coculture significantly increases the SRC frequency within human BM CD34+CD38– cells. The scatter plot shows the level of human CD45+ cell engraftment in NOD/SCID mice at week 8 following transplantation with FACS-sorted BM CD34+CD38– cells or their progeny as indicated. The progeny of 2 × 104 to 4 × 104 BM CD34+CD38– cells cultured × 7 days with HUBECs + GM36SF showed significantly higher levels of human engraftment compared with day-0 BM CD34+CD38– cells at the same dose. As shown at right, the highest human CD45+ cell engraftment was observed in mice that received transplants of the progeny of 4 × 104 BM CD34+CD38– cells following noncontact HUBEC cultures + GM36SF. Mice that received transplants of the progeny of BM CD34+CD38– cells cultured with HUBECs + TSF displayed comparable engraftment to noncontact HUBEC + GM36SF cultures. Each circle represents an individual mouse that received a transplant of human BM cells. Values on the Y-axis indicate the percentage of human CD45+ cell engraftment within the marrow of individual mice that received NOD/SCID transplants. The cell dosages for each group are shown at top. The mean levels of human CD45+ cell engraftment are indicated by horizontal bars for each group.
For statistical analysis of SRC frequency within day-0 BM CD34+CD38– cells and HUBEC + GM36SF–cultured BM CD34+CD38– cells, we pooled data from the limiting dilution assays according to methods described previously.26-28 We calculated the frequency of SRCs using the maximum likelihood estimator.27 The SRC frequency within day-0 BM CD34+CD38– cells was 1 in 72 000 (95% confidence interval [CI], 1/35 000 to 1/182 000). The SRC frequency within HUBEC-cultured BM CD34+CD38– cells was 1 in 20 000 (CI, 1/12 000 to 1/38 000). Therefore, coculture of BM CD34+CD38– cells with HUBECs + GM36SF supported a 3.6-fold increase in SRCs compared with input. The difference in the SRC frequencies between day-0 BM CD34+CD38– cells and their HUBEC-cultured progeny was highly significant (P = .009).
Noncontact HUBEC culture and TSF augment BM SRC expansion
In order to determine whether SRC expansion during HUBEC coculture was dependent upon cell-to-cell contact, a group of NOD/SCID mice received transplants of the progeny of 4 × 104 BM CD34+CD38– cells cultured with HUBECs + GM36SF in the absence of contact. Remarkably, mice that received transplants of the progeny of noncontact HUBEC cultures demonstrated 8-fold higher human CD45+ cell repopulation at 8 weeks than mice that received transplants of the progeny of contact HUBEC cultures (mean, 19.5% human CD45+ [huCD45+] cells vs 2.4% huCD45+ cells; P = .01; Figure 4). This result suggested that a significant percentage of primitive BM-repopulating cells may have remained adherent to HUBEC monolayers and unevaluated following HUBEC contact cultures. These data also indicated that cell-to-cell contact was not required for HUBECs to impart SRC expansion.
Since HUBEC culture + TSF supported the largest expansion of BM CD34+CD38– cells, a group of NOD/SCID mice received transplants of the progeny of 4 × 104 BM CD34+CD38– cells following HUBEC culture + TSF. As shown in Figure 4, mice that received transplants of the progeny of HUBEC + TSF–cultured cells also demonstrated high levels of human repopulation at 8 weeks (mean, 18.0% huCD45+ cells), which was comparable to that observed in mice that received transplants of the progeny of noncontact HUBEC cultures + GM36SF and superior to that observed in mice that received transplants of the progeny of HUBEC contact cultures + GM36SF. These data indicated that the substitution of TSF for GM36SF further optimized the expansion of BM SRCs.
Human SRCs are enriched within the CD34+CD38– subset following ex vivo culture
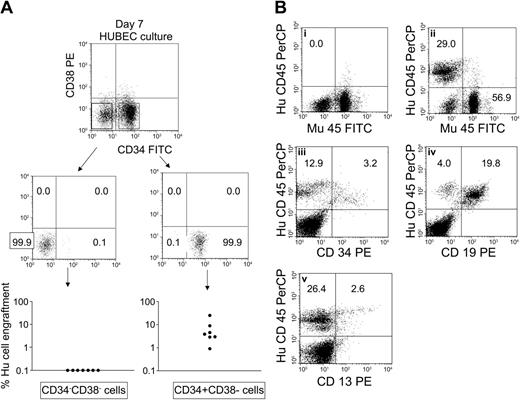
In order to determine the phenotype of primitive SRCs that were amplified during HUBEC culture, we harvested the day-7 progeny of 4 × 104 BM CD34+CD38– cells following culture with HUBECs + GM36SF and then isolated, via FACS sorting, the phenotypic subsets that remained after culture. Specifically, we collected the CD34+CD38– population and the CD34–CD38– subset that encompassed greater than 95% of day-7 HUBEC-cultured cells (Figure 2A). Then, NOD/SCID mice received transplants of the different FACS-sorted subsets to determine which population contained SRC activity. As shown in Figure 5A, 100% of mice (N = 7) that received transplants of day-7 CD34+CD38– cells showed human CD45+ cell repopulation at 8 weeks after transplantation, whereas 0 of 7 mice that received transplants of day-7 FACS-sorted CD34–CD38– cells showed detectable human cell repopulation. These data indicated that SRCs were highly enriched within the CD34+CD38– population compared with the CD34–CD38– subset following HUBEC culture. Of note, human CD45+ cell engraftment in mice that received transplants of day-7 CD34+CD38– sorted cells was 7.0% ± 8.3% compared with 2.4% ± 2.4% in mice that received transplants of the total progeny of HUBEC cultures at day 7. These data demonstrated that CD34– accessory cells did not augment the engraftment of SRCs following HUBEC culture and therefore did not account for the increase in SRC frequency measured following HUBEC culture. Figure 5B illustrates the human multilineage repopulation in a representative NOD/SCID mouse that received a transplant of day-7 FACS-sorted CD34+CD38– cells compared with day-7 FACS-sorted CD34–CD38– cells. Mice that received transplants of HUBEC-cultured day-7 CD34+CD38– cells gave rise to multilineage repopulation in vivo, with 23.9% ± 3.6%, 70.2% ± 4.6%, and 28.0% ± 10.8% of the engrafted human CD45+ cells coexpressing CD34, CD19, and CD13 antigens, respectively. These data confirmed the pluripotent capacity of the CD34+CD38– cell subset following HUBEC culture.
Human SRCs are enriched within the CD34+CD38– subset following expansion divisions. FACS-sorted BM CD34+CD38– cells (4 × 104) were cultured × 7 days with HUBECs + GM36SF. At day 7, the progeny of culture were collected, stained with anti-CD34–FITC and anti-CD38–PE, and flow cytometric analysis and cell sorting was performed. (A) Sterile FACS sorting of day-7 CD34+CD38– and CD34–CD38– cell subsets was performed and each population was collected separately. NOD/SCID mice (N = 7 per group) received transplants of the collected cell subsets and human CD45+ cell engraftment was measured after 8 weeks. SRC activity was detected only within the day-7 CD34+CD38– population, whereas SRC activity was not demonstrable within the day-7 CD34–CD38– subset. (B) Lineage distribution of engrafted human cells is shown within a representative mouse 8 weeks after transplantation with day-7 FACS-sorted CD34+CD38– cells. (Bi) No huCD45+ cell engraftment is demonstrable within a representative mouse that received a transplant of day-7 CD34–CD38– cells. (Bii) huCD45+ cell engraftment is evident in a mouse that received a transplant of day-7 FACS-sorted CD34+CD38– cells. CD34+ progenitor cell engraftment (Biii), CD19+ B-cell differentiation (Biv), and CD13+ myeloid differentiation (Bv) are also shown. PerCP indicates peridinin chlorophyll A protein; and Mu45, murine CD45.
Human SRCs are enriched within the CD34+CD38– subset following expansion divisions. FACS-sorted BM CD34+CD38– cells (4 × 104) were cultured × 7 days with HUBECs + GM36SF. At day 7, the progeny of culture were collected, stained with anti-CD34–FITC and anti-CD38–PE, and flow cytometric analysis and cell sorting was performed. (A) Sterile FACS sorting of day-7 CD34+CD38– and CD34–CD38– cell subsets was performed and each population was collected separately. NOD/SCID mice (N = 7 per group) received transplants of the collected cell subsets and human CD45+ cell engraftment was measured after 8 weeks. SRC activity was detected only within the day-7 CD34+CD38– population, whereas SRC activity was not demonstrable within the day-7 CD34–CD38– subset. (B) Lineage distribution of engrafted human cells is shown within a representative mouse 8 weeks after transplantation with day-7 FACS-sorted CD34+CD38– cells. (Bi) No huCD45+ cell engraftment is demonstrable within a representative mouse that received a transplant of day-7 CD34–CD38– cells. (Bii) huCD45+ cell engraftment is evident in a mouse that received a transplant of day-7 FACS-sorted CD34+CD38– cells. CD34+ progenitor cell engraftment (Biii), CD19+ B-cell differentiation (Biv), and CD13+ myeloid differentiation (Bv) are also shown. PerCP indicates peridinin chlorophyll A protein; and Mu45, murine CD45.
Discussion
The application of monoclonal antibodies that recognize human CD34 and CD38 surface antigens allows convenient analysis and isolation of steady-state hematopoietic cells that are enriched for stem (CD34+CD38–) and progenitor cell content.4,5,19,29,30 CD34+ cell content correlates with clinical hematopoietic recovery following autologous and allogeneic stem cell transplantation31,32 and the CD34+CD38– phenotype identifies hematopoietic cells enriched for long-term repopulating capacity as demonstrated in the NOD/SCID experimental model.4,5,19 A phenotypic indicator of HSC content would also be valuable following ex vivo culture of CD34+ and CD34+CD38– subsets, since ex vivo expansion has a potential role in adult CB transplantation,33 development of tolerizing hematopoietic grafts,34 gene therapy,35 and tissue generation/repair.36 However, recent studies have suggested that the CD34+CD38– phenotype is not a reliable indicator of HSC content following ex vivo culture of CD34+ cells due to the down-modulation of CD38 surface antigen, which occurs on committed CD34+CD38+ cells contained in culture.19 In this study, we have demonstrated both directly and indirectly that the most primitive assayable human hematopoietic cell, the SRC, can be monitored via expression of the CD34+CD38– phenotype following ex vivo expansion. When HUBEC cultures were initiated with purified BM CD34+CD38– cells, the subsequent expansion of CD34+CD38– cells (4.4-fold) correlated well with SRC expansion (3.6-fold) over the same time period. Furthermore, flow cytometric sorting and transplantation of purified CD34+CD38– and CD34–CD38– subsets following HUBEC culture demonstrated that SRCs were significantly enriched after culture within the CD34+CD38– population. Therefore, when ex vivo culture studies are initiated with HSC-enriched CD34+CD38– cells, persistence of the CD34+CD38– population during culture is a reliable indicator of HSC content. The correlation between CD34+CD38– phenotype and HSC content following culture can be exploited to allow the collection and more precise analysis of HSCs that have undergone expansion.
Limiting dilution analysis in this study demonstrated that contact culture of BM CD34+CD38– cells with HUBECs supplemented with GM36SF induced a 3.6-fold increase in SRCs compared with input BM CD34+CD38– cells. Surprisingly, culture of BM CD34+CD38– cells with HUBECs + GM36SF in the absence of cell-to-cell contact resulted in 8-fold higher huCD45+ cell SCID repopulation compared with mice that received transplants of contact HUBEC–cultured cells. Our recent studies indicated that CB SRCs could be maintained equally under contact and noncontact HUBEC cultures,37 but the augmented expansion of adult BM SRCs under noncontact conditions presented here suggests a unique interaction between HUBEC-soluble factors and primitive CD34+CD38– cells. Since 10% to 20% of plated hematopoietic cells become tightly adherent to HUBECs during culture, noncontact cultures may simply provide a higher yield of primitive SRCs compared with contact cultures. It is also possible, as suggested by studies of stromal culture,38 that cell-to-cell contact between HSCs and HUBECs may provide relative inhibition of stem cell proliferation, resulting in lower SRC amplification compared with noncontact cultures. Interestingly, in contrast to the cell contact–dependent hematopoietic activities of several reported murine and human stromal cell lines,39-41 HUBECs appear to elaborate soluble factors that potently expand primitive human HSCs. We have undertaken subtractive gene expression analysis of HUBECs compared with nonbrain ECs to identify candidate genes and secreted gene products that account for this unique hematopoietic activity.
In addition to the benefit of noncontact HUBEC culture conditions, we observed that the addition of TSF to HUBEC cultures augmented SRC expansion beyond what we had observed with HUBECs + GM36SF. In prior studies, TSF, with and without IL-6/IL-6 receptor, has optimized the maintenance of CB SRCs during short-term and extended cultures.11,12,28 In this study, the combination of HUBECs + TSF resulted in 7.5-fold higher SCID repopulation compared with HUBECs + GM36SF. Of note, both HUBECs + TSF cultures and noncontact HUBECs + GM36SF cultures augmented CD34+CD38– cell expansion compared with contact HUBECs + GM36SF cultures (13.1-fold and 6.4-fold vs 4.4-fold, respectively) and this appeared to correlate, although not linearly, with increasing SCID-repopulating cell capacity in these groups. The additive effect of TSF on HUBEC cultures may be a direct result of thrombopoietin stimulation of homeobox 4 (HOXB4) expression in HSCs41 or may reflect the activity of these cytokines, individually and in combination, on HSC maintenance in vitro.42-44 Alternatively, endothelial cells express C-myeloproliferative leukemia virus ligand (C-mpl) and c-kit receptors45-47 and it is plausible that TPO or SCF may signal HUBECs to secrete other factors that promote HSC expansion during culture. Via limiting dilution analysis, we will subsequently attempt to quantify the individual and combined effects of noncontact HUBEC culture and the addition of TSF on human SRC expansion. To date, noncontact HUBEC culture conditions and TSF appear to optimize the ex vivo expansion of adult human HSCs.
Previous studies have suggested that CD34– and/or CD34+CD38+ accessory cells contained within transplant grafts facilitate the engraftment of limiting doses of primitive HSCs.24,48 Bonnet et al24 showed that engraftment of limiting doses of CB CD34+ cells in NOD/SCID mice was significantly improved when CD34– or CD34+CD38+ cells were cotransplanted. In our previous studies, we postulated that the generation of CD34– or CD34+CD38+ accessory cells during HUBEC culture may have accounted for the increased SCID-repopulating capacity we observed in HUBEC-cultured progeny.15 In this study, FACS-sorted day-7 CD34+CD38– cells demonstrated equal or greater SCID-repopulating capacity (mean, 7.2% huCD45+ cell repopulation) than the total cultured day-7 progeny (mean, 2.4% huCD45+), which contained CD34– cells. Therefore, accessory cells contained within HUBEC-cultured grafts clearly do not account for the increased SRC capacity of HUBEC-cultured progeny. Rather, these data indicate that the SRC expansion observed during HUBEC culture reflects the amplification of stem/repopulating cells. Our results also raise the question whether human HSC expansion might be improved via initiation of expansion cultures with purified CD34+CD38– cells rather than heterogeneous CD34+ cells. Previous studies have shown that unselected human CD34+ populations elaborate many cytokines in culture, including tumor necrosis factor alpha (TNF-alpha), interferon alpha (IFN-alpha), and IL-1 beta, which could have deleterious effects on the ex vivo maintenance of HSCs.49 We are undertaking experiments to answer this question by comparing the SRC expansion of human CD34+CD38– cells cultured with and without CD34+CD38+ committed progenitors. If these studies confirm that human SRC expansion is significantly improved via exclusion of CD34+CD38+ cells from culture, these results could have implications for strategies to clinically expand HSCs for transplantation. In addition, our studies have not yet addressed the potential for HUBEC culture to expand SRCs contained in steady-state human CD34– cells.50,51 Although this study suggests little, if any, SRC activity within the CD34–CD38– population that was derived from input CD34+CD38– cells, long-term culture of input CD34– cells with HUBECs may yield additional increases in SRC content.50
The application of flow cytometry and lineage depletion to isolate steady-state murine and human HSCs has allowed remarkable molecular characterization of these rare cells.52-55 Similarly, flow cytometric isolation of human HSCs that have undergone self-renewal divisions has the potential to allow definitive insights into the signals involved in the HSC self-renewal process. However, the lack of determination of the surface phenotype of self-renewing HSCs has impeded such progress. The model we have described demonstrates that primitive human repopulating cells are enriched within the CD34+CD38– subset following culture conditions in which SRC amplification has occurred. When purified BM CD34+CD38– cells are used to initiate culture, the persistence of the CD34+CD38– population is a reliable indicator of HSC content and the expansion of this population correlates well with an increase in long-term repopulating cells. We anticipate that the results of this study will allow more definitive characterization of human HSCs as they undergo expansion as well as the signals that mediate this process.
Prepublished online as Blood First Edition Paper, September 2, 2004; DOI 10.1182/blood-2004-04-1467.
Supported in part by a cooperative research and development agreement (CRADA) between the US Navy and Large Scale Biology Corporation (NCRADA–Naval Medical Research and Development Command/Naval Medical Research Institute [NMRDC/NMRI]/Biosource-97-588).
One of the authors (J.P.C.) has a declared financial interest in Large Scale Biology Corporation, whose potential product was studied in the present work. One of the authors (G.G.M.) is employed by Large Scale Biology Corporation, whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr David Venzon for his critical assistance with the statistical analysis. The human brain endothelial cells (HUBECs) were kindly provided by the Naval Medical Research Center (Silver Spring, MD).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal