Abstract
Interleukin-15 (IL-15) is a pleiotropic proinflammatory cytokine with inefficient posttranscriptional processing. We hypothesized that endogenous IL-15 could affect disease progression in the well-described C57Bl/6 (B6) → (C57Bl/6 × DBA/2) F1 hybrid (B6D2F1) murine model of acute allogeneic graft-versus-host disease (GVHD). B6D2F1 allogeneic recipients received transplants of IL-15-/- B6 bone marrow cells or B6 bone marrow cells expressing a murine IL-15 transgene (IL-15 tg) modified for efficient translation and secretion. Mice that received transplants of IL-15-/- B6 bone marrow cells displayed a significantly longer median survival time (MST) compared with mice that received transplants of wild-type (wt) B6 bone marrow; in contrast, mice that received transplants of IL-15 tg B6 bone marrow cells had a dramatically decreased MST. This decrease in survival was associated with a substantial activation and expansion of effector-memory (CD44highCD62Llow) CD8+ T lymphocytes. Finally, in vivo depletion of either CD4+ or CD8+ T lymphocyte subsets significantly prolonged survival in mice receiving IL-15 tg B6 marrow, while depletion of both CD4+ and CD8+ T cells provided complete protection from acute GVHD. We thus show that acute GVHD is attenuated in the absence of donor bone marrow–derived IL-15 and conclude that donor-derived IL-15 is a critical mediator of T-cell function in acute GVHD.
Introduction
Bone marrow transplantation (BMT) is a potentially curative therapy for patients with heritable immunodeficiencies and malignant diseases including leukemia, lymphoma, and myeloma.1 While the conditioning regimen for BMT leads to direct tumor destruction, donor-derived allogeneic T cells can exert an important graft-versus-tumor (GVT) effect: recipients of allogeneic transplants have a decreased probability of relapse compared with recipients of autologous, syngeneic, or T-cell–depleted bone marrow transplants.2,3 However, allogeneic BMT also carries a significant risk for graft-versus-host disease (GVHD), an immunologic attack of allogeneic donor T lymphocytes against normal recipient tissues, the magnitude of which depends in large part on the degree of HLA incompatibility between donor and recipient. Even with appropriate prophylaxis, the incidence of acute GVHD ranges from 30% in HLA-identical donor-recipient pairs to 70% for donor-recipient pairs HLA-incompatible at 2 loci.1,4 Thus, GVHD remains the most significant obstacle to the wider application of BMT for the treatment of malignancy.
GVHD is characterized by a cycle of tissue destruction and inflammation initiated by the preparative regimen for transplantation and propagated by alloreactive donor T cells.5 Transplant recipients are prepared for BMT with a regimen that obliterates their current bone marrow stores and potentially their residual leukemia. However, this regimen is also highly toxic to the gastrointestinal system and allows release of gram-negative bacterial lipopolysaccharide (LPS) across the intestinal mucosa.6 LPS is a potent stimulator for the production of tumor necrosis factor α (TNF-α), interleukin-1 (IL-1), and IL-12.6,7 IL-12 polarizes donor-derived T cells toward a proinflammatory Th1/Tc1 phenotype, producing interferon γ (IFN-γ) and TNF-α;8,9 IFN-γ and TNF-α induce major histocompatibility complex (MHC) expression by host-derived antigen-presenting cells (APCs), which results in efficient presentation of alloantigen to donor-derived T cells.10,11 These donor-derived Th1/Tc1-polarized T cells in turn produce IL-2, expand, and infiltrate host epithelial tissues. Thus, the inflammation of acute GVHD is influenced in large part by the cytokine cascade within the host after transplantation.
The role of IL-15, a T- and natural killer (NK) cell growth factor, in allogeneic GVHD has not been addressed. Originally isolated because of its ability to restore T-cell growth in the presence of IL-2–neutralizing antibodies,12,13 IL-15 mRNA is widely and abundantly expressed in multiple tissues. However, IL-15 is inefficiently translated and secreted with multiple posttranscriptional checkpoints.14-16 IL-15 protein is produced predominantly by cells of the monocyte/macrophage,17 dendritic,18 and stromal cell lineages.19 Signaling through the shared IL-2/15Rβγc receptor complex, IL-15 promotes the development and survival of NK cells,19-23 induces immunoglobulin expression by B cells,24 and is required for the survival23,25,26 and homeostatic proliferation27-29 of memory CD8+ T cells. IL-15 has been shown to increase CD8+ T-cell function in vivo against tumor cell lines30,31 and active viral32 and microbial infections,33 and to augment the primary34 and memory35 CD8+ T-cell responses after vaccination. Moreover, blockade of IL-15 signaling has been shown to extend the life of cardiac and pancreatic islet allografts in murine models of graft rejection.36,37
We therefore hypothesized that alteration of endogenous IL-15 production after allogeneic transplantation would affect the donor antihost immune responsiveness that is characteristic of acute allogeneic GVHD. To address this hypothesis, we used the C57Bl/6 (B6) → (C57Bl/6 × DBA/2) F1 hybrid (B6D2F1) murine model of acute allogeneic GVHD using bone marrow (BM) cells obtained from IL-15-/- B6 mice or B6 mice with a ubiquitously expressed IL-15 transgene (IL-15 tg B6) that allows for efficient IL-15 protein production and secretion.23,38-40 Our results indicate that eliminating endogenous IL-15 gene expression in donor BM cells prevents GVHD in host B6D2F1 wild-type (wt) mice, and that deregulation of IL-15 gene expression in donor BM cells promotes acute GVHD in host B6D2F1 wt mice in a dose-dependent fashion. IL-15–mediated GVHD was associated with a dramatic expansion and activation of wt effector-memory CD8+ T cells with up-regulated Bcl-2 protein, whose depletion significantly improved survival. Collectively, these data identify donor bone marrow–derived cells as the predominant source of IL-15 critical for T-cell function in acute GVHD.
Materials and methods
Reagents
The following rat antimouse monoclonal antibodies (mAbs) were purchased from BD Pharmingen (San Diego, CA) as fluorescein isothiocyanate (FITC), R-phycoerythrin (PE), peridinin chlorophyll (PerCP), or allophycocyanin conjugates: CD3ϵ, CD4, CD8α, CD43, CD44, CD62L, and T-cell receptor β (TCRβ). The following mAbs were used for in vivo depletion of lymphocytes: antimouse CD4 (GK1.5), anti-CD8 (2.43) (National Cell Culture Center, Minneapolis, MN), and rat immunoglobulin G (IgG) isotype control (Sigma Chemical, St Louis, MO). Bcl-2 protein was detected using a hamster antimouse Bcl-2 antibody (BD Pharmingen). Bromodeoxyuridine (BrdU; Zymed, South San Francisco, CA) and carboxy-fluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) were used according to the manufacturers' instructions. Anti-PE microbeads were purchased from Miltenyi Biotec (Auburn, CA). IL-15 transgene mRNA was reverse transcribed using standard techniques, and cDNA was amplified using the following primers: forward, 5′-CGCCGCGGACGCTGGTTAT-3′; reverse, 5′-GTAATCTTGCAACTGGGATGAA-3′. Annealing temperature was 55°C.
Flow cytometry
For surface staining, 5.0 × 105 cells were incubated with mAb for 25 minutes at 4°C, washed once with phosphate-buffered saline (PBS) containing 1% fetal bovine serum, and fixed in PBS containing 1% formalin. Intracellular staining was performed with the Intracellular Flow Cytometry Kit according to the manufacturer's instructions (BD Pharmingen). Cells were analyzed on a FACSCalibur flow cytometer using CellQuest software (Becton Dickinson, San Jose, CA).
Mice
Female B6 (H-2b) and B6D2F1 (H-2b/d) mice (6- to 7-week-old) were purchased from Jackson Laboratories (Bar Harbor, ME). Female IL-15-/- B6 mice (6- to 7-week-old) were purchased from Taconic Farms (Germantown, NY). Murine IL-15 transgenic mice (IL-15 tg B6, C57Bl/6 background, H-2b) were created as described for FVB mice40 and bred under specific pathogen-free conditions at The Ohio State University. Although measurements of serum murine IL-15 in the C57Bl/6 strain of IL-15 tg mice are below our enzyme-linked immunosorbent assay (ELISA) detection limits of 50 pg/mL, these mice consistently exhibit moderate expansions of NK and memory CD8+ T cells early in life that are similar to other lines of IL-15 tg mice with measurable IL-15. Unlike the FVB strain,40 the IL-15 tg B6 mice do not exhibit evidence of malignant lymphoproliferation or leukemic transformation within the first year of life. All mice were between 8 and 12 weeks old at the beginning of each experiment. Mice that underwent transplantation were maintained in sterilized microisolators, and received irradiated rodent chow and acidified water plus oral antibiotic (Baytril, 0.2 mg/mL) for 21 days following transplantation. All animal research was reviewed and approved by the Institutional Laboratory Animal Care and Use Committee (ILACUC) at The Ohio State University.
Bone marrow transplantation
The standard B6 → B6D2F1 model of allogeneic BMT was used for these experiments.38 Briefly, the F1 progeny of wt B6 and wt DBA/2 mice (B6D2F1, H-2b/d) were used as allogeneic recipients for a combined bone marrow and splenic T-cell graft from the parental (B6) strain of mice. The signs and symptoms of acute GVHD in these mice are well described and include tissue damage to the liver, intestine, and skin; weight loss; and death.38 In this model, wt B6 mice are used as syngeneic control recipients and display little tissue damage or weight loss and no mortality due to GVHD. Donor mice were killed by cervical dislocation, and bone marrow and spleen were harvested by standard techniques. IL-15 tg B6 splenic T cells were not used to induce GVHD in any experiments because these mice display altered T-cell populations and phenotypes early in life.40 Bone marrow cells were labeled with PE-conjugated rat antimouse monoclonal antibodies directed against CD3ϵ, CD4, and CD8α followed by anti-PE microbeads. Bone marrow T cells were then depleted by passage through a magnetic-activated cell sorter (MACS) LD separation column according to the manufacturer's instructions (Miltenyi Biotec). Depletion efficiency was determined by flow cytometric analysis and was more than 99%. After red blood cell lysis, 5 × 105 splenocytes were stained with anti-CD8α FITC and anti-CD4 PerCP and analyzed by flow cytometry. For independent experiments, T-cell content of the transplanted splenocytes was standardized by flow cytometric analysis. T-cell–depleted BM cells and splenocytes were mixed in serum-free RPMI 1640 at the appropriate concentrations to transfer 1 × 107 BM cells and 5 × 106 splenic T cells in 0.5 mL. In some experiments, IL-15 tg B6 BM cells and wt B6 BM cells were mixed such that the BM portion of the graft contained 50% or 25% IL-15 tg B6 BM cells. To prepare the recipients for transplantation, mice were given 1300 cGy gamma irradiation (Gammacell 40, Atomic Energy of Canada Limited, Ottawa, ON, Canada; 93.5 cGy/min) split into 2 fractions. Mice were then injected with 0.5 mL of the combined BM cell/splenic T-cell graft via the lateral tail vein. Recipients were hydrated with 0.5 mL sterile RPMI 1640 medium, given intraperitoneally, for the first 5 days after transplantation.
In vivo lymphocyte depletions
To deplete CD4+ or CD8+ T-cell populations in vivo, mice were injected intraperitoneally with 1 mg rat IgG, GK1.5, 2.43, or both GK1.5 and 2.43 mAbs, on the day of transplantation, followed by intraperitoneal injections of 0.5 mg every other day thereafter. Depletion efficiency was verified by flow cytometry and was more than 99%.
Assessment of GVHD
Mice were observed in a blinded fashion every 3 days after transplantation for the clinical symptoms of GVHD as described.41 Briefly, animals were scored from 0 to 2 for weight loss, inactivity, skin lesions, roughened coat, and hunching, and the scores were combined to provide a clinical score from 0 to 10 at each time point. Mice that were unresponsive or unable to obtain food and water were determined to be moribund and killed in accordance with The Ohio State University ILACUC guidelines.
Histology
Tissues were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin by standard techniques. Blinded samples were submitted for semiquantitative histopathologic analysis, performed by D.F.K., a board-certified veterinary pathologist who directs the Mouse Phenotyping Shared Resource of The Ohio State University Comprehensive Cancer Center. Acute GVHD lesions were graded as absent (0), minimal (1), mild (2), moderate (3), marked (4), or very marked (5) depending on their extent and severity.
Mixed lymphocyte reactions
Wt B6 splenic T cells (1 × 108) were primed in vivo in wt B6D2F1 allogeneic or wt B6 syngeneic hosts. After 6 days, spleens were harvested and splenocytes were isolated by standard techniques. Of these responder cells, 1 × 105 were restimulated in vitro with irradiated (30 Gy) naive wt B6 or wt B6D2F1 stimulator cells at a ratio of 1:4 in the presence of recombinant murine IL-15 (R&D Systems, Minneapolis, MN) at 10 ng/mL or 100 ng/mL or PBS control. Supernatants were harvested 24 or 72 hours after initiation of the culture and stored at -80°C until analysis.
Cytokine analysis
The Mouse Th1/Th2 Cytokine Bead Array (BD Pharmingen) was used to measure IL-2, IL-4, IL-5, IFN-γ, and TNF-α in mouse sera and culture supernatants. Samples were analyzed neat or diluted 1:2 or 1:4 with PBS. The FL-3 and FL-2 channels of a BD FACSCalibur flow cytometer were used to individually quantitate cytokine binding to multiple beads of known fluorescence. Samples were then analyzed using Cytokine Bead Array software (BD Biosciences.) In some experiments, IFN-γ was measured using the Quantikine Murine IFN-γ ELISA kit (R&D Systems). All assays were performed according to the manufacturers' instructions.
Statistics
Survival data were analyzed using the log-rank test. All other data were compared using Student 2-tailed t test. P less than .05 was considered statistically significant.
Results
The absence of IL-15 in donor bone marrow cells ameliorates acute GVHD mortality
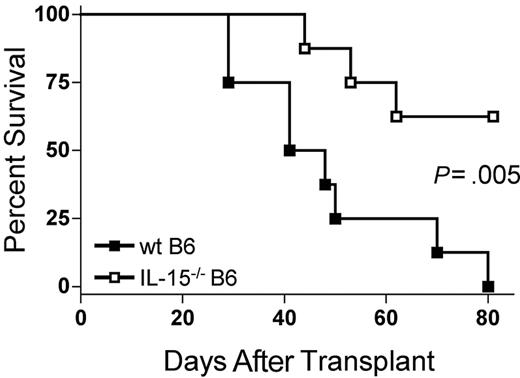
Because IL-15 is a proinflammatory cytokine, we hypothesized that decreasing endogenous IL-15 expression by reconstitution of allogeneic B6D2F1 recipients with IL-15-/- B6 BM cells would reduce mortality from acute GVHD. B6D2F1 mice receiving IL-15-/- B6 BM cells and IL-15-/- B6 T cells had a significantly longer median survival time (MST) after transplantation compared with identical mice receiving wt B6 BM cells and IL-15-/- B6 T cells (44.5 days versus 62.5% survival 80 days after transplantation; P = .005; Figure 1).
The absence of donor bone marrow cell–derived IL-15 ameliorates acute GVHD mortality. Wild-type B6D2F1 mice received transplants of 5 × 106 IL-15-/- B6 splenic T cells and 1 × 107 wt B6 BM cells (▪, n = 8) or IL-15-/- B6 BM cells (□, n = 8) and were observed for acute GVHD mortality. Survival times were compared using the log-rank test: P = .005.
The absence of donor bone marrow cell–derived IL-15 ameliorates acute GVHD mortality. Wild-type B6D2F1 mice received transplants of 5 × 106 IL-15-/- B6 splenic T cells and 1 × 107 wt B6 BM cells (▪, n = 8) or IL-15-/- B6 BM cells (□, n = 8) and were observed for acute GVHD mortality. Survival times were compared using the log-rank test: P = .005.
Deregulation of endogenous IL-15 in donor BM cells increases the mortality and morbidity of acute allogeneic GVHD and is dose dependent
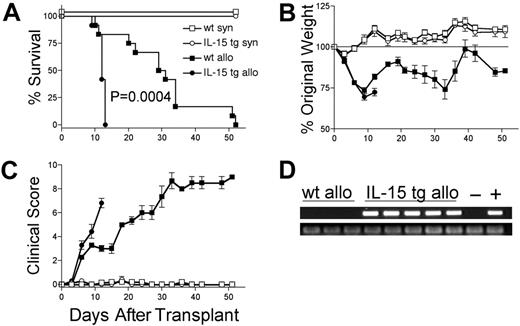
We hypothesized that deregulation of endogenous IL-15 expression by transplantation of IL-15 tg B6 BM cells would promote acute GVHD. B6D2F1 mice receiving wt B6 T cells and IL-15 tg B6 BM cells had dramatically decreased MST compared with identical recipients receiving wt B6 T cells and wt B6 BM cells (MST, 12 days versus 30 days; P = .0004; Figure 2A). Wt B6D2F1 mice receiving IL-15 tg B6 BM cells developed more weight loss (72.4 ± 2.3% original weight versus 81.8 ± 1.6% beginning on day 12 after transplantation; P = .008; Figure 2B) and worse clinical scores (3.3 ± 0.4 versus 2.3 ± 0.2 beginning on day 6 after transplantation; P = .029; Figure 2C) compared with B6D2F1 mice receiving wt B6 BM cells. Although in vivo detection of endogenous murine IL-15 protein was not possible, IL-15 transgene mRNA was detected in the bone marrow harvested from recipients of IL-15 tg B6 BM cells but not wt B6 BM cells (Figure 2D). Further, bone marrow mixing experiments indicated that IL-15–mediated mortality is significantly reduced when the fraction of IL-15 tg BM cells contained in the graft is decreased below 50% (Table 1). B6 mice receiving either syngeneic wt B6 BM cells or syngeneic IL-15 tg B6 BM cells had 100% survival and did not develop clinical evidence of GVHD (Figure 2A-C).
Deregulation of endogenous IL-15 increases the mortality and morbidity from acute GVHD. B6 or B6D2F1 mice received transplants of 5 × 106 wt B6 splenic T cells and 1 × 107 BM cells from either wt B6 or IL-15 tg B6 mice. (A) Survival of wt B6D2F1 mice receiving IL-15 tg B6 allogeneic (IL-15 tg allo, •,n = 12) or wt B6 allogeneic BM cells (wt allo, ▪, n = 15); P = .0004. Survival of wt B6 mice receiving IL-15 tg B6 syngeneic (IL-15 tg syn, ○, n = 7) or wt B6 syngeneic (wt syn, □, n = 7) BM cells. Data represent combined results from 2 independent experiments. (B-C) Mice from each group described in panel A were weighed and scored for clinical GVHD as described in “Materials and methods.” Data are representative of results (mean ± SE) from 2 similar experiments. (D) Upper panel: IL-15 transgene expression in bone marrow of recipients of IL-15 tg B6 allogeneic BM cells. Positive control (+) cDNA was prepared from a known IL-15 tg B6 mouse, and negative control cDNA (-) was prepared from a wt B6 mouse. Lower panel: sample integrity was established by polymerase chain reaction (PCR) amplification of 18s cDNA.
Deregulation of endogenous IL-15 increases the mortality and morbidity from acute GVHD. B6 or B6D2F1 mice received transplants of 5 × 106 wt B6 splenic T cells and 1 × 107 BM cells from either wt B6 or IL-15 tg B6 mice. (A) Survival of wt B6D2F1 mice receiving IL-15 tg B6 allogeneic (IL-15 tg allo, •,n = 12) or wt B6 allogeneic BM cells (wt allo, ▪, n = 15); P = .0004. Survival of wt B6 mice receiving IL-15 tg B6 syngeneic (IL-15 tg syn, ○, n = 7) or wt B6 syngeneic (wt syn, □, n = 7) BM cells. Data represent combined results from 2 independent experiments. (B-C) Mice from each group described in panel A were weighed and scored for clinical GVHD as described in “Materials and methods.” Data are representative of results (mean ± SE) from 2 similar experiments. (D) Upper panel: IL-15 transgene expression in bone marrow of recipients of IL-15 tg B6 allogeneic BM cells. Positive control (+) cDNA was prepared from a known IL-15 tg B6 mouse, and negative control cDNA (-) was prepared from a wt B6 mouse. Lower panel: sample integrity was established by polymerase chain reaction (PCR) amplification of 18s cDNA.
Assessment of survival following BMT with alterations in the fraction of IL-15 tg B6 donor BM cells transplanted into B6D2F1 recipients
Recipient . | Percent IL-15 tg B6 BM cells . | Median survival time, d . | P, log-rank test . |
|---|---|---|---|
| B6D2F1 | 100 | 14.5 | NA |
| B6D2F1 | 50 | 14 | NS |
| B6D2F1 | 25 | 22 | .004 |
| B6D2F1 | 0 | 30 | .01 |
Recipient . | Percent IL-15 tg B6 BM cells . | Median survival time, d . | P, log-rank test . |
|---|---|---|---|
| B6D2F1 | 100 | 14.5 | NA |
| B6D2F1 | 50 | 14 | NS |
| B6D2F1 | 25 | 22 | .004 |
| B6D2F1 | 0 | 30 | .01 |
For all recipients, the total number of wt B6 splenic T cells was 5 × 106, and the total number of BM cells was 1 × 107. Median survival times for mice that received transplants of a given percentage of IL-15 tg BM cells were compared with the MSTs for mice that received transplants with an immediately higher percentage of IL-15 tg BM cells, as shown in the table. NA indicates not applicable; NS, not significant.
Deregulation of endogenous IL-15 increases cholangiohepatitis and enteritis in recipients of allogeneic bone marrow transplants
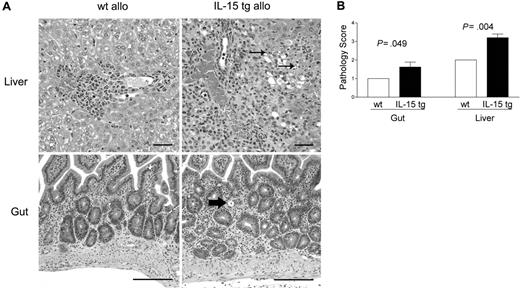
B6D2F1 mice receiving IL-15 tg B6 BM cells or wt B6 BM cells were killed 13 days after transplantation and analyzed in a blinded fashion for histopathology characteristic of acute GVHD in the gut and liver.42,43 B6D2F1 mice receiving IL-15 tg B6 BM cells had significantly more severe cholangiohepatitis (grade 3 versus grade 2, Figure 3A, upper panels; P = .004, Figure 3B) and enteritis (grade 2 versus grade 1, Figure 3A, bottom panels; P = .049, Figure 3B) compared with mice receiving wt B6 BM cells. No difference in pulmonary inflammation was noted between recipients of IL-15 tg B6 and wt B6 allogeneic BM cells (data not shown). B6 mice receiving syngeneic wt B6 or syngeneic IL-15 tg B6 BM cells had no evidence of cholangiohepatitis or enteritis (data not shown).
Deregulation of endogenous IL-15 increases cholangiohepatitis and enteritis in recipients of allogeneic bone marrow transplants. (A) Upper panel: Cholangiohepatitis in animals with acute GVHD. Mononuclear inflammatory cells surround bile ducts (asterisks). Lesions shown in a liver harvested from a recipient of IL-15 tg B6 allogeneic BM cells (IL-15 tg allo) are more severe than those shown in a liver from a recipient of wt B6 allogeneic BM cells (wt allo) and include areas of hepatocyte necrosis (arrows). Bar = 50 μm. Lower panel: enteritis in animals with acute GVHD. There is a mononuclear inflammatory cell infiltrate in the lamina propria; crypts are hyperplastic and contain numerous mitotic figures. The intestine harvested from a recipient of IL-15 tg B6 allogeneic BM cells has more severe lesions than that harvested from a recipient of wt B6 allogeneic BM cells, including an area of crypt necrosis (arrow). Bar = 100 μm. Images were visualized using an Olympus BX41 microscope equipped with an Olympus 3040 camera and a Plan 20 × objective with an aperture of 0.40 (Olympus America, Melville, NY). The image medium was air, and the acquisition software was Adobe Photoshop (Adobe Systems, San Jose, CA). (B) Coded slides were scored by a veterinary pathologist in a blinded fashion as described in “Materials and methods.” Mice receiving IL-15 tg B6 allogeneic BM cells (solid bars) had significantly worse GVHD histopathology in the gut (P = .049) and liver (P = .004) compared with recipients of wt B6 allogeneic BM cells. Results are representative of 2 independent experiments involving a total of 12 mice.
Deregulation of endogenous IL-15 increases cholangiohepatitis and enteritis in recipients of allogeneic bone marrow transplants. (A) Upper panel: Cholangiohepatitis in animals with acute GVHD. Mononuclear inflammatory cells surround bile ducts (asterisks). Lesions shown in a liver harvested from a recipient of IL-15 tg B6 allogeneic BM cells (IL-15 tg allo) are more severe than those shown in a liver from a recipient of wt B6 allogeneic BM cells (wt allo) and include areas of hepatocyte necrosis (arrows). Bar = 50 μm. Lower panel: enteritis in animals with acute GVHD. There is a mononuclear inflammatory cell infiltrate in the lamina propria; crypts are hyperplastic and contain numerous mitotic figures. The intestine harvested from a recipient of IL-15 tg B6 allogeneic BM cells has more severe lesions than that harvested from a recipient of wt B6 allogeneic BM cells, including an area of crypt necrosis (arrow). Bar = 100 μm. Images were visualized using an Olympus BX41 microscope equipped with an Olympus 3040 camera and a Plan 20 × objective with an aperture of 0.40 (Olympus America, Melville, NY). The image medium was air, and the acquisition software was Adobe Photoshop (Adobe Systems, San Jose, CA). (B) Coded slides were scored by a veterinary pathologist in a blinded fashion as described in “Materials and methods.” Mice receiving IL-15 tg B6 allogeneic BM cells (solid bars) had significantly worse GVHD histopathology in the gut (P = .049) and liver (P = .004) compared with recipients of wt B6 allogeneic BM cells. Results are representative of 2 independent experiments involving a total of 12 mice.
Deregulation of endogenous IL-15 expands activated effector-memory CD8+ T cells after allogeneic BMT
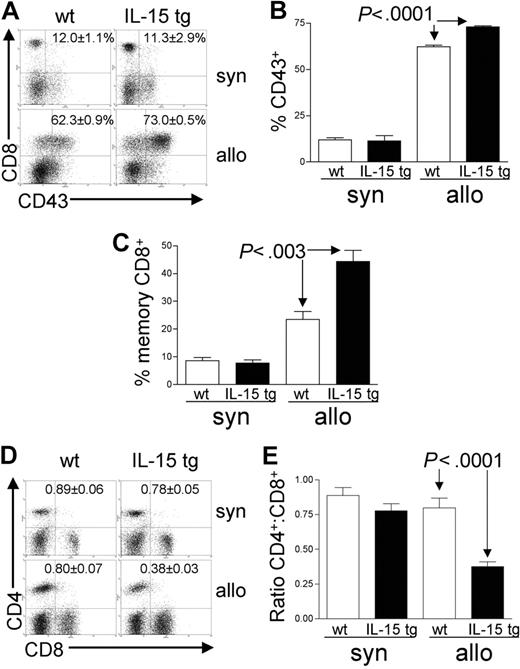
Analysis of recipient splenocytes at day 12 or 13 after BMT demonstrated an increase in alloreactive CD8+ T cells coexpressing the GVHD activation marker CD43 in recipients of IL-15 tg B6 allogeneic BM cells compared with recipients of wt B6 allogeneic BM cells (73.0 ± 0.5% versus 62.3 ± 0.9%; P < .0001; Figure 4A-B). There was no difference in CD43 expression by CD8+ T cells from recipients of IL-15 tg B6 and wt B6 syngeneic BM cells (Figure 4A-B); nor was there a difference in the proportion of alloreactive CD43+CD4+ T cells between recipients of IL-15 tg B6 and wt B6 allogeneic BM cells (data not shown). Further, splenocytes from recipients of IL-15 tg B6 allogeneic BM cells displayed a significant increase in the proportion of CD44highCD62Llow effector-memory CD8+ T cells (44.4 ± 4.1% versus 23.5 ± 2.9%; P < .003; Figure 4C) and a significant decrease in the CD4+/CD8+ T-cell ratio (0.38 ± 0.03 versus 0.80 ± 0.07; P < .0001; Figure 4D-E) compared with recipients of wt B6 allogeneic BM cells.
Deregulation of endogenous IL-15 alters the T-cell phenotype after allogeneic BMT. Spleens were harvested from syngeneic or allogeneic bone marrow transplant recipients 12 or 13 days after transplantation. (A-B) The percentage of gated CD8+ T lymphocytes that were also positive for the activation antigen CD43 was determined by 2-color flow cytometric analysis. Values represent mean ± SE for all groups and are representative of 2 independent experiments involving a total of 17 mice. (C) The percentage of gated lymphocytes that was CD8+CD44highCD62Llow was determined by 3-color flow cytometric analysis. Values represent combined mean ± SE from 3 independent experiments involving a total of 24 mice. (D-E) The ratio of CD4+/CD8+ T lymphocytes was determined by 2-color flow cytometric analysis. Values represent combined mean ± SE from 3 independent experiments involving a total of 24 mice.
Deregulation of endogenous IL-15 alters the T-cell phenotype after allogeneic BMT. Spleens were harvested from syngeneic or allogeneic bone marrow transplant recipients 12 or 13 days after transplantation. (A-B) The percentage of gated CD8+ T lymphocytes that were also positive for the activation antigen CD43 was determined by 2-color flow cytometric analysis. Values represent mean ± SE for all groups and are representative of 2 independent experiments involving a total of 17 mice. (C) The percentage of gated lymphocytes that was CD8+CD44highCD62Llow was determined by 3-color flow cytometric analysis. Values represent combined mean ± SE from 3 independent experiments involving a total of 24 mice. (D-E) The ratio of CD4+/CD8+ T lymphocytes was determined by 2-color flow cytometric analysis. Values represent combined mean ± SE from 3 independent experiments involving a total of 24 mice.
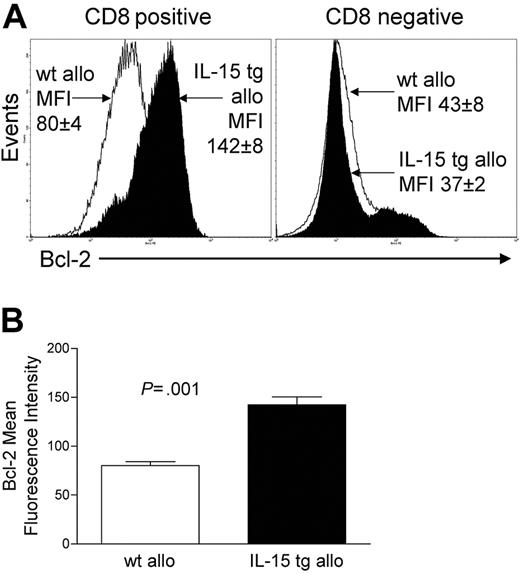
Deregulation of endogenous IL-15 after allogeneic BMT increases CD8+ T-cell survival
We assessed the expression of Bcl-2 protein in donor-infused wt B6 allogeneic T cells of B6D2F1 recipient mice that had received either IL-15 tg B6 BM cells or wt B6 BM cells. CD8+ T cells from the former had a mean fluorescence intensity (MFI) of 142 ± 8, while CD8+ splenocytes from the latter had an MFI of 80 ± 4 (P = .001; Figure 5A-B). There was no difference in Bcl-2 expression for CD8- splenocytes (Figure 5A). Further, T-cell proliferation was assessed at days 4 and 6 after transplantation by CFSE staining and from days 7 to 12 by BrdU incorporation. No difference in T-cell proliferation was detected between allogeneic B6D2F1 recipients of IL-15 tg B6 and wt B6 BM cells by these methods.
Deregulation of endogenous IL-15 increases Bcl-2 expression in CD8+ T lymphocytes after allogeneic BMT. Spleens were harvested from recipients of IL-15 tg B6 or wt B6 allogeneic BM cells 12 or 13 days after transplantation. (A) Flow cytometry histogram showing Bcl-2 protein expression in gated CD8+ or CD8- splenocytes taken from recipients of wt B6 allogeneic (open histogram) or IL-15 tg B6 allogeneic (solid histogram) BM cells. Mean fluorescence intensity (MFI) values for each group (mean ± SE) are indicated. (B) MFI values for Bcl-2 were obtained from gated CD8+ lymphocyte populations. Mean Bcl-2 MFI values ± SE for recipients of wt B6 or IL-15 tg B6 allogeneic BM cells are shown. Data are representative of 2 independent experiments involving a total of 13 mice.
Deregulation of endogenous IL-15 increases Bcl-2 expression in CD8+ T lymphocytes after allogeneic BMT. Spleens were harvested from recipients of IL-15 tg B6 or wt B6 allogeneic BM cells 12 or 13 days after transplantation. (A) Flow cytometry histogram showing Bcl-2 protein expression in gated CD8+ or CD8- splenocytes taken from recipients of wt B6 allogeneic (open histogram) or IL-15 tg B6 allogeneic (solid histogram) BM cells. Mean fluorescence intensity (MFI) values for each group (mean ± SE) are indicated. (B) MFI values for Bcl-2 were obtained from gated CD8+ lymphocyte populations. Mean Bcl-2 MFI values ± SE for recipients of wt B6 or IL-15 tg B6 allogeneic BM cells are shown. Data are representative of 2 independent experiments involving a total of 13 mice.
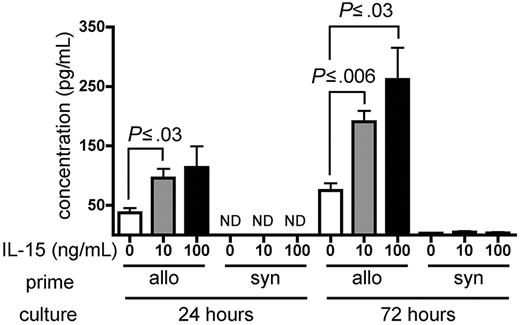
IL-15 increases IFN-γ production by alloreactive T cells in vitro
Wild-type B6 splenocytes were primed for 6 days in wt B6D2F1 allogeneic hosts and restimulated in vitro with naive irradiated B6D2F1 splenocytes in the presence of recombinant murine (rm) IL-15 or PBS as a control. Primed B6 splenocytes cultured in the presence of either 10 ng/mL or 100 ng/mL rm IL-15 produced higher amounts of IFN-γ compared with identical splenocytes cultured in the absence of exogenous IL-15 at 24 hours (P ≤ .03) and at 72 hours after initiation of the culture (P ≤ .03; Figure 6). Splenocytes primed in wt B6 syngeneic hosts produced little IFN-γ upon stimulation in the presence or absence of rm IL-15 (Figure 6).
IL-15 increases IFN-γ production by alloreactive T cells in vitro. Wild-type B6 splenocytes (1 × 108) were primed in vivo in allogeneic (wt B6D2F1, n = 4) or syngeneic (wt B6, n = 1) hosts for 6 days. Splenocytes were then harvested and restimulated in vitro with irradiated naive B6D2F1 splenocytes for 24 or 72 hours. IFN-γ production by allogeneic- or syngeneic-primed splenocytes in the presence of PBS, or 10 ng/mL or 100 ng/mL rm IL-15 is expressed as the mean ± SE of triplicate cultures. ND indicates not detectable.
IL-15 increases IFN-γ production by alloreactive T cells in vitro. Wild-type B6 splenocytes (1 × 108) were primed in vivo in allogeneic (wt B6D2F1, n = 4) or syngeneic (wt B6, n = 1) hosts for 6 days. Splenocytes were then harvested and restimulated in vitro with irradiated naive B6D2F1 splenocytes for 24 or 72 hours. IFN-γ production by allogeneic- or syngeneic-primed splenocytes in the presence of PBS, or 10 ng/mL or 100 ng/mL rm IL-15 is expressed as the mean ± SE of triplicate cultures. ND indicates not detectable.
Deregulation of endogenous IL-15 affects the Th2/Tc2 cytokine profile in acute GVHD
Analysis of serum collected from recipients of IL-15 tg and wt allogeneic BM cells at 12 or 13 days after transplantation showed no significant difference in the amounts of the Th1/Tc1 cytokines IFN-γ (96.1 ± 26.6 pg/mL and 40.9 ± 2.8 pg/mL, respectively), TNF-α (166.5 ± 11.4 pg/mL and 164.9 ± 13.0 pg/mL, respectively), and IL-2 (1.5 ± 0.7 pg/mL and 3.5 ± 0.2 pg/mL, respectively). However, recipients of IL-15 tg allogeneic BM cells displayed lower serum concentrations of the Th2/Tc2 cytokine IL-5 (10.4 ± 3.8 pg/mL versus 34.4 ± 2.3 pg/mL, respectively; P = .001; not shown). No IL-4 was detected in any of our assays.
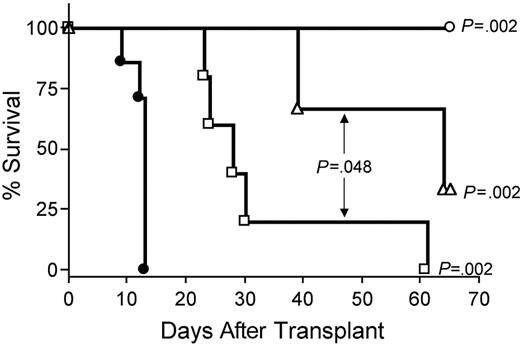
IL-15–mediated exacerbation of GVHD requires CD8+ T cells or CD4+ T cells
Depletion of CD8+ T cells in vivo with anti-CD8 mAb extended survival of recipients of IL-15 tg B6 allogeneic BM cells compared with control treatment with rat IgG (MST, 64 days versus 13 days; P = .002; Figure 7). Depletion with anti-CD4 mAb also provided protection from IL-15–mediated GVHD (MST, 28 days versus 13 days; P = .002; Figure 7), although the benefit was significantly less than that provided by CD8 depletion (MST, 64 days versus 28 days; P = .048; Figure 7). Finally, in vivo depletion of both CD4+ and CD8+ T cells provided complete protection from IL-15–mediated acute GVHD (100% survival compared with control injected recipients of IL-15 tg allogeneic BM cells; P = .002; Figure 7).
IL-15–mediated exacerbation of acute GVHD is dependent upon CD8+ or CD4+ T lymphocytes. B6D2F1 mice received transplants of 5 × 106 wt B6 splenic T cells and 1 × 107 BM cells from either wt B6 or IL-15 tg B6 mice. Survival of recipients of IL-15 tg B6 allogeneic BM cells treated with control rat IgG (•, MST = 13 days, n = 7) or depleting doses of rat antimouse CD4 (□, MST = 28 days, n = 5), CD8 (▵, MST = 64 days, n = 5), or both (○, 100% survival, n = 5). Median survival time for B6D2F1 mice that received transplants of 5 × 106 wt B6 splenic T cells, and 1 × 107 BM cells from wt B6 mice treated with control rat IgG was 34 days (not shown). □ versus •, P = .002; ▵ versus •, P = .002; ○ versus •, P = .002; and □ versus ▵, P = .048.
IL-15–mediated exacerbation of acute GVHD is dependent upon CD8+ or CD4+ T lymphocytes. B6D2F1 mice received transplants of 5 × 106 wt B6 splenic T cells and 1 × 107 BM cells from either wt B6 or IL-15 tg B6 mice. Survival of recipients of IL-15 tg B6 allogeneic BM cells treated with control rat IgG (•, MST = 13 days, n = 7) or depleting doses of rat antimouse CD4 (□, MST = 28 days, n = 5), CD8 (▵, MST = 64 days, n = 5), or both (○, 100% survival, n = 5). Median survival time for B6D2F1 mice that received transplants of 5 × 106 wt B6 splenic T cells, and 1 × 107 BM cells from wt B6 mice treated with control rat IgG was 34 days (not shown). □ versus •, P = .002; ▵ versus •, P = .002; ○ versus •, P = .002; and □ versus ▵, P = .048.
Discussion
Our results indicate that endogenous IL-15 provided by donor wt BM cells is a critical component for the manifestations of acute allogeneic GVHD. Deregulation of IL-15 expression by transplanted BM cells resulted in an expansion of wt alloreactive (CD43+) effector-memory (CD44highCD62Llow) CD8+ T cells in the spleen. Our demonstration of a skewed CD4+/CD8+ T-cell ratio and unaltered frequency of alloreactive (CD43+) CD4+ T cells in recipients of IL-15 tg B6 allogeneic BM cells suggest that the effect of IL-15 in these experiments was specific to effector-memory CD8+ T cells. Further, wt B6 splenocytes primed in vivo and restimulated for only 24 hours in vitro with naive allogeneic splenocytes produced significantly more IFN-γ in the presence of exogenous recombinant IL-15 than in the absence of exogenous IL-15. It is likely, then, that IL-15 from donor-derived BM cells promotes both the activation and expansion of alloreactive T cells after allogeneic BMT. These alterations in T-cell phenotype and function were associated with a significant increase in tissue inflammation in the gut and liver and a striking increase in acute GVHD morbidity and mortality. IL-15–mediated acute GVHD was completely abrogated by T-cell depletion, indicating that this process was T-cell dependent. There are additional data in support of this notion that donor-derived IL-15 is not mediating its effect directly but rather via a T-cell–dependent process. First, IL-15 tg B6 marrow alone was able to fully reconstitute hematopoiesis in wt B6D2F1 allogeneic hosts without morbidity and mortality (data not shown), and, second, IL-15 tg B6 marrow had no deleterious effect when infused with wt B6 splenic T cells into irradiated wt B6 syngeneic hosts. Most surprisingly, however, transplantation of IL-15-/- B6 BM cells prolonged survival of wt allogeneic hosts compared with transplantation of wt B6 BM cells. For the first time, these data identify donor bone marrow–derived cells as a population wherein IL-15 expression predicts outcome after allogeneic BMT.
In acute GVHD, CD4+ Th2-polarized cells can prevent disease by reducing IFN-γ and TNF-α production and by inhibiting donor CD8+ T-cell expansion.44,45 Moreover, CD8+ Tc2-polarized cells demonstrate a reduced capacity for causing GVHD while maintaining a GVT effect.46 The data presented here demonstrate that deregulation of IL-15 significantly decreases serum concentrations of the Th2/Tc2 cytokine IL-5 after allogeneic BMT and significantly increases the production of the classic Th1/Tc1 cytokine IFN-γ by alloreactive T cells in vitro. These findings are in agreement with those of Ishimitsu et al47 that IL-15 tg mice show reduced serum concentrations of IL-5 due to expanded CD8+ Tc1 cells in a murine model of asthma, and extend work demonstrating the costimulation of IFN-γ production by IL-15 and IL-12 in NK and T cells.33,48,49 Although we observed only sporadic increases of IFN-γ in the serum of recipients of IL-15 tg B6 allogeneic BM cells, collectively these data indicate that deregulation of IL-15 after allogeneic BMT can cause a polarization away from the Th2/Tc2 cytokine response and toward the Th1/Tc1 type response. Importantly, TGF-β or other Th2/Tc2 cytokines such as IL-10 inhibit T-cell responses to alloantigen50 and may also have been down-regulated in recipients of IL-15 tg B6 allogeneic BM cells. Nevertheless, these findings represent, to our knowledge, the first in vivo evidence of a role for IL-15 in polarizing T-cell responses in GVHD.
The T-cell response to IL-15 was originally demonstrated to require expression of the IL-2Rβ and γc chains12,13,51 ; a private receptor alpha chain (IL-15Rα) was subsequently identified that binds IL-15 with high affinity (dissociation constant [Kd], 10-50 pM) and signals through the IL-2/15Rβγc complex.52 Dubois et al53 reported that IL-15 functions primarily as a surface-bound cytokine, presented by IL-15Rα in “trans” to target cells expressing the IL-2/15Rβγc. Emerging evidence suggests that while IL-15Rα expression by bone marrow–derived cells is necessary for the homeostatic proliferation of splenic memory CD8+ T cells,54 expression of IL-15Rα by CD8+ T cells, themselves, is not.55 In our experiments, IL-15 tg B6 bone marrow cells efficiently produced and secreted IL-15 protein that was presumably captured by IL-15Rα on host APCs in lymphoid tissue and presented in trans to donor-derived CD8+ T cells. In contrast, IL-15-/- bone marrow cells were unable to produce the cytokine, and as a result host APCs stimulated donor-derived CD8+ T cells less efficiently. This hypothetical scenario is supported by the elegant work of Teshima et al56 and Duffner et al57 who proved that the alloantigen stimulus for donor-derived CD4+ and CD8+ T cells is delivered by host-derived APCs. Thus our data identify a unique interaction between donor and host hematopoietic cells mediated by IL-15 and suggest that this molecule may be an important “third signal” for lymphocyte activation in acute GVHD.
While depletion of either CD4+ or CD8+ T cells improved MST in our model, CD8+ T-cell depletion had the more profound impact on survival, consistent with an expansion of effector-memory CD8+ T cells in recipients of IL-15 tg B6 allogeneic BM cells. Thus, we hypothesized that IL-15 may promote the homeostatic proliferation or survival of effector-memory CD8+ T cells after allogeneic BMT. Studies have demonstrated that IL-15 promotes phosphorylation of signal transducer and activator of transcription 5 (STAT5) and induces binding of STAT5 and c-myb to promoter elements of the antiapoptotic molecule Bcl-2.58,59 In vitro and in vivo evidence has demonstrated that IL-15 up-regulates Bcl-2 protein expression and maintains memory CD8+ T cells by serving as a survival factor.26,60-62 Consistent with this, recipients of IL-15 tg B6 allogeneic BM cells had an increase in wt effector-memory CD8+ donor T cells that expressed more Bcl-2 protein than wt CD8+ donor T cells from recipients of wt B6 allogeneic BM cells. Somewhat surprisingly, we did not observe any evidence for enhanced CD8+ T-cell proliferation in recipients of IL-15 tg B6 allogeneic BM cells by CFSE dilution on day 4 or 6 or by BrdU incorporation from days 7 to 12 after transplantation. Therefore in our experiments, it is likely that the expansion of wt effector-memory CD8+ donor T cells was predominantly the result of IL-15–induced survival.
Thus far, 2 studies have shown that in patients undergoing allogeneic BMT for various hematologic malignancies, those who developed GVHD had higher serum concentrations of IL-15 compared with those who did not develop GVHD.63,64 Although the etiology of increased serum IL-15 is not addressed in these reports, there may be polymorphisms that result in deregulated expression of IL-15 protein. Recently, Lin et al65 (reviewed by Cooke and Ferrara66 ) reported that polymorphisms in the IL-10 promoter region that result in high protein expression are associated with decreased incidence of severe acute GVHD in recipients of allogeneic bone marrow transplants. Because IL-15 expression is regulated primarily at the posttranscriptional level by multiple start codons present in the 5′–untranslated region (UTR) and an inefficient signal peptide, polymorphisms in these regions may result in efficient IL-15 protein production.14,15 The data presented here suggest that potential bone marrow donors with such polymorphisms may confer increased risk of acute GVHD to recipients. Thus, a detailed analysis of donor IL-15 gene polymorphisms and posttransplantation IL-15 expression in patients with and without acute GVHD may allow for further risk stratification of potential allogeneic BMT donor-recipient pairs.
Because of its apparent role in the priming and survival of allogeneic CD8+ T cells, blockade of IL-15 signaling may be an effective treatment or prophylaxis for acute GVHD. Clinical experience with anti–IL-2Rα antibodies has demonstrated the feasibility of modulating cytokine signaling for the treatment of this disease. Basiliximab and daclizumab, chimeric and humanized anti–IL-2Rα monoclonal antibodies, respectively, achieved response rates of 71% and 47% in steroid-resistant acute GVHD,67,68 although it is not clear whether therapeutic effect in these trials was achieved through interrupting IL-2 signaling or depletion of activated IL-2Rα+ T cells. However, many patients with GVHD failed to respond to IL-2Rα blockade,69 raising the possibility that trans presentation of IL-15 may provide redundant signaling through the IL-2/15Rβγc complex. A fully human anti–IL-15 antibody, HuMax-IL15, has shown efficacy in a xenograft model of human psoriasis70 and is currently in clinical trials for the treatment of rheumatoid arthritis. Therefore, IL-15 may be an attractive target for neutralization, particularly in patients refractory to anti–IL-2Rα targeted therapy.
While blocking IL-15 signaling may be a promising therapy for acute GVHD, it is not clear how such treatment may alter the GVT effect. IL-15 has been shown to enhance the in vivo antitumor activity of polyclonal,31 antigen-specific30,71 and genetically targeted CD8+ T cells72 after adoptive transfer. Additionally, Katsanis et al73 demonstrated a survival benefit for IL-15–treated lymphoma-bearing mice after transplantation with syngeneic bone marrow and activated T cells. Consequently, it is possible that blocking IL-15 signaling may result in a loss of tumor-specific memory CD8+ T cells and diminish the GVT effect in vivo. To date, no studies have addressed the in vivo role of IL-15 in promoting or inhibiting CD8+ T-cell activity against allogeneic tumor targets.
Despite years of intense investigation, GVHD remains the most significant obstacle to the wider application of allogeneic BMT. The results presented herein suggest a role for IL-15 in promoting acute GVHD by activating and supporting the survival of alloreactive effector-memory CD8+ T cells. Moreover, these data identify donor bone marrow–derived cells as the predominant source of IL-15 critical for acute GVHD. Further investigation is warranted to determine if IL-15 should be pursued clinically as a target for neutralization in GVHD prophylaxis and treatment.
Prepublished online as Blood First Edition Paper, September 16, 2004; DOI 10.1182/blood-2004-05-1687.
Supported by CA068458 (M.A.C.) and Medical Scientist Program Fellowships (B.W.B., S.R.).
B.W.B. and S.R. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Donna Bucci, Tamra Brooks, and Karen Feasel for administrative assistance and preparation/submission of this manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal