Abstract
We have previously identified a common polymorphism at the tissue-type plasminogen activator (t-PA) locus (–7351C>T), located within a GC-box in the retinoic acid (RA) and steroid hormone responsive t-PA enhancer. The aim of the present study was to functionally characterize this t-PA variant. Electrophoretic mobility shift assays (EMSAs) using crude nuclear extracts from human endothelial, HeLa, and NT2 neuronal cells revealed a 10-fold greater protein binding affinity to the wild-type C allele compared with the mutant T allele variant. Sp1 and Sp3 were identified as the GC-box binding proteins. Luciferase reporter assays showed that the C allele generated higher transcriptional activity after induction by RA, compared with the T allele variant. Further EMSAs showed that RA treatment enhanced Sp1/Sp3 binding to the GC-box. Formation of the Sp1/Sp3 containing complex was inhibited by anti–RA receptor (RAR) antibodies, suggesting that Sp1/Sp3 and RAR interact. The t-PA –7351C>T polymorphism is therefore functional at the level of transcription. The reduced binding affinity of Sp1/Sp3 to the T allele could explain our earlier observations of a reduced t-PA release and an increased risk of myocardial infarction in individuals carrying this allele.
Introduction
Tissue-type plasminogen activator (t-PA) is an endogenous plasminogen activator that converts plasminogen into plasmin, which leads to subsequent breakdown of fibrin and lysis of blood clots. Secretion of t-PA from endothelial cells shows a direct correlation to synthesis rate,1 which in turn is mainly regulated at the level of transcription.2 We have observed an association between t-PA release and an Alu polymorphism at the t-PA locus in healthy subjects.3 Since the Alu polymorphism is neutral (ie, unlikely to be the causal variant by itself) we screened the t-PA gene for putative functional variants. By resequencing the promoter, the 3′ untranslated region (3′-UTR), and all coding regions of the t-PA gene, 8 novel single-nucleotide polymorphisms (SNPs) were identified.4 Three of these were associated with t-PA release: –7351C>T, 20 099T>C, and 27 445T>A. The coding 20 099T>C SNP is synonymous and the intronic 27 445T>A SNP is not located in a splice site. However, the t-PA –7351C>T variant is a functional candidate, since it is located within an enhancer region. We have also found that t-PA –7351T allele carriers have an increased risk of myocardial infarction,5 and more recently an increased risk of ischemic stroke was reported for the t-PA TT genotype.6 This is in agreement with our physiologic observation of a reduced t-PA release rate in individuals carrying this allele.4
The t-PA enhancer mediates induction of t-PA gene transcription in response to vitamin A (retinoic acid, RA) as well as to all steroid hormones except estrogens.7 The RA response is mediated by an RA-responsive element (RARE) comprising a DR5 element located in the vicinity of the polymorphic site (ie, at –7319). The steroid hormone response is mediated through a hormone responsive unit consisting of 4 glucocorticoid responsive elements (GREs) located upstream of the polymorphic site (ie, at –7960, –7942, –7703, and –7501).
To induce gene transcription, the distal enhancer needs to physically associate with the transcription start site. The t-PA promoter contains 2 major transcription start sites, the TATA-independent site being the predominant one.8 There are 2 Sp1 binding motifs just upstream of this TATA-less initiation site,9,10 both of which have been shown to be involved in enhancer-mediated induction of t-PA gene transcription.11 The C to T transition at –7351 in the t-PA enhancer alters a core nucleotide in a Sp1 binding motif.4 Since Sp1 can promote long distant interactions by self-associating,12-14 we have tested the hypothesis that this sequence variation has a direct effect on t-PA promoter-directed transcription. Here, we provide evidence that the t-PA –7351C>T SNP is functional at the level of transcription. Interestingly, our results also suggest that Sp1 and RA receptor (RAR) interact in mediating induction by RA through the enhancer.
Materials and methods
Cell culture
Fresh umbilical cords were obtained from the maternity ward. The procedure was approved by the Ethics Committee of Göteborg University, and informed consent was provided according to the Declaration of Helsinki. Human umbilical vein endothelial cells (HUVECs) were prepared by collagenase digestion according to Jaffe et al.15 Cells were plated into gelatin-coated (0.2%) tissue culture dishes and maintained in endothelial cell growth factor containing medium-2 (EGM-2; Clonetics/BioWhittaker, Walkersville, MD) with 2% fetal bovine serum at 37°C in a 5% CO2 atmosphere. The medium was replaced every 2 to 3 days. Subcultures were obtained by trypsin/EDTA (ethylenediaminetetraacetic acid) treatment of confluent monolayers at a split ratio of 1:3. HUVECs were cultured for maximally 3 passages. C11STH, spontaneously transformed HUVECs,16 were cultured in gelatin-coated (0.2%) tissue culture dishes and maintained in Medium 199 supplemented with 20 μg/mL endothelial growth factor, 20 μg/mL heparin, 2 mM glutamine, nonessential amino acids (Sigma, St Louis, MO), 1 mM sodium pyruvate, 0.25% sodium bicarbonate, 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), penicillin (100 U/mL), streptomycin (100 μg/mL), and 20% heat inactivated fetal calf serum (Gibco BRL, Grand Island, NY). HeLa cells (European Collection of Cell Cultures, Salisbury, United Kingdom) and NT2 neuronal-like cells17 (kindly supplied by Paul Ekert, WEHI, Melbourne, Australia) were grown in Dulbecco modified Eagle medium (DMEM; Gibco BRL) supplemented with 10% heat inactivated fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 μg/mL).
Cell treatment and preparation of nuclear extracts
The preparation of nuclear extracts was performed as previously described,18 and protein concentration was quantified with a fluorometer (FLUOstar Optima; BMG LabTechnologies, Offenburg, Germany) using BioRad reagents. Stock solutions of all-trans-RA (Sigma) were dissolved in dimethyl sulfoxide (DMSO; Sigma) at a concentration of 10 mM and stored at –80°C. The experiments involving RA were performed in subdued light, and stock solutions were diluted in culture medium just prior to the start of an experiment. For treatment with RA, HUVECs in the second or third passage were cultured to 80% to 90% confluency as described in “Cell culture” and incubated with culture medium containing RA for the indicated time period before being harvested. An RA concentration of 10–6 M was chosen, as this concentration resulted in maximal t-PA response in HUVECs (A.T.W., R.L.M., C.J., unpublished observation, 2003).
Preparation and labeling of oligonucleotides
Sequences of the oligonucleotides used as probes and competitors in the electrophoretic mobility shift assay (EMSA) are listed in Table 1. Labeling of high-performance liquid chromatography (HPLC)–purified oligomers (100 ng) was performed as described,19 using T4 polynucleotide kinase and [γ-32P]ATP (adenosine triphosphate). Annealing, oligomer processing, and preparation for EMSAs was performed as described.20
Sequences of oligonucleotides used in the electrophoretic mobility shift assay (only upper strand shown)
Name . | Location . | Sequence . |
|---|---|---|
| Wild-type GC-box | -7363/C/-7339 | CCCAAAGGAGCCCGCCCCAGACACA |
| Mutant GC-box | -7363/T/-7339 | CCCAAAGGAGCCTGCCCCAGACACA |
| Scrambled GC-box | -7363/scrambled/-7339 | CGCAGACACCAGCCACGACGACCAC |
| DR5 | -7323/-7299 | TCTGGGGTCACCCTGGGGTCAGAAG |
| Scrambled DR5 | -7323/scrambled/-7299 | GCAGCTGAGTCGTAGCGTCGAGCTG |
| GC-box/DR5 | -7363/C/-7289 | CCCAAAGGAGCCCGCCCCAGACACA |
| GCCATGGCCTGGGACTCTGGGGTCA | ||
| CCCTGGGGTCAGAAGGAATTATCTG |
Name . | Location . | Sequence . |
|---|---|---|
| Wild-type GC-box | -7363/C/-7339 | CCCAAAGGAGCCCGCCCCAGACACA |
| Mutant GC-box | -7363/T/-7339 | CCCAAAGGAGCCTGCCCCAGACACA |
| Scrambled GC-box | -7363/scrambled/-7339 | CGCAGACACCAGCCACGACGACCAC |
| DR5 | -7323/-7299 | TCTGGGGTCACCCTGGGGTCAGAAG |
| Scrambled DR5 | -7323/scrambled/-7299 | GCAGCTGAGTCGTAGCGTCGAGCTG |
| GC-box/DR5 | -7363/C/-7289 | CCCAAAGGAGCCCGCCCCAGACACA |
| GCCATGGCCTGGGACTCTGGGGTCA | ||
| CCCTGGGGTCAGAAGGAATTATCTG |
The polymorphic nucleotide is indicated in bold type, and the GC-box and DR5 (RA receptor responsive element [RARE], spaced by a 5 base sequence) sequences are underlined.
EMSA
Nuclear protein extract (5 μg; unless otherwise stated) in 4 μL Osborne buffer D (10 mM HEPES, pH 7.9, 50 mM KCl, 2 nM MgCl2, 5 mM dithiothreitol, 5 mM PhMeSO2F, 0.2 mM EDTA, 20% glycerol),18 was preincubated at 4°C for 15 minutes with 1 μL poly[d(I-C)] [polydeoxy(inosinate-cytidylate)] (1 μg; used as a nonspecific competitor; Roche, Indianapolis, IN) and 3 μL SMK buffer (12 mM spermidine, 12 mM MgCl2, and 200 mM KCl).21 32P-labeled probe (4 μL; 100 counts per second (cps) diluted in buffer D) was then added and incubated for another 15 minutes on ice before being applied to a native 5% polyacrylamide gel as previously described.20 For competition and cross-competition titration experiments, extracts were incubated with unlabeled double-stranded wild-type or mutant GC-box oligomers (0.5-, 5-, 50- or 500-fold molar excess) for 15 minutes on ice prior to the addition of the probe. As a nonspecific competitor, an unlabeled scrambled oligonucleotide with the same base composition as that of the probes was used at 500-fold molar excess. Samples were loaded on a 5% native polyacrylamide gel (Acrylamide/Bis 29:1) containing 0.25 × Tris (tris(hydroxymethyl)aminomethane)/borate/EDTA buffer (1 times TBE, 100 mM Tris, 90 mM boric acid, 1 mM EDTA, pH 8.4). Electrophoresis was carried out at room temperature at 320 V. Gels were vacuum heat-dried and subjected to autoradiography.
To identify specific proteins involved in DNA binding, supershift experiments were performed using antibodies against members of the Sp family: Sp1 (no. sc-420), Sp2 (no. sc-643), Sp3 (no. sc-644), Sp4 (no. sc-645); and RARs: RARα (no. sc-551), RARβ (no. sc-552), and RARγ (no. sc-550) (Santa Cruz Biotech, Santa Cruz, CA). For experiments involving the –7351C>T site, probes corresponding to the wild-type C allele variant were used. Supershifting was performed according to the same procedure as that described for standard EMSAs, except that 1 μL specific antibody (2 μg total) was added to the nuclear extracts for 1 hour after addition of the labeled oligomer.
Real-time quantitative RT-PCR
HUVECs were treated with RA as described in “Cell treatment and preparation of nuclear extracts,” except that confluent cultures were used. Total RNA was extracted at baseline and after 2, 4, 6, and 8 hours of treatment with RA using RNeasy Mini kit (QIAGEN, Hilden, Germany), as recommended by the supplier. mRNA was converted to cDNA with GeneAmp RNA PCR kit (Applied Biosystems, Foster City, CA). Quantitative reverse transcription–polymerase chain reaction (RT-PCR) assays were performed for t-PA, RARα, RARβ, RARγ, and Sp1 using the ABI PRISM 7700 TaqMan sequence detector and Sequence Detector v1.9.1 software (Applied Biosystems). Target mRNA was normalized relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA. Oligonucleotide primers and the fluorogenic probe sequences for t-PA and GAPDH were as described.22 For amplification of RARα, RARβ, RARγ, and Sp1, primers and probes were purchased directly from Applied Biosystems (Assay-on-demand Hs00230907_m1, Hs00233407_m1, Hs00171273_m1, and Hs00412720_m1, respectively). Thermocycling conditions were 2 minutes at 50°C and 10 minutes of initial denaturation at 95°C, which was followed by 40 cycles of 2-step PCR that consisted of 15 seconds at 95°C and 1 minute at 60°C. Relative quantification of gene expression was analyzed as a treatment-to-control expression ratio using the comparative CT method (User Bulletin 2; Applied Biosystems). Each sample was analyzed in triplicate for both target and GAPDH. The statistical significance of the response was assessed by one-way analysis of variance (ANOVA) for repeated measures, followed by contrast analysis when ANOVA indicated a significant overall effect.
Luciferase reporter gene construction
The genomic sequences analyzed in this study are numbered relative to the major transcription initiation site as determined by Henderson and Sleigh.8 The minimal t-PA enhancer as defined by Bulens et al7 was amplified by PCR from 1 individual homozygous for the –7351C allele and 1 homozygous for the T allele. These 950–base pair (bp) genomic fragments, spanning between –7137 and –8087, were then subcloned into pCR2.1-TOPO (Invitrogen, Groningen, the Netherlands) and propagated in competent Escherichia coli (Top10; Invitrogen). The pCR2.1-TOPO plasmids were digested with XhoI and SacI, and the purified enhancer C and T fragments were cloned into the XhoI/SacI sites of the firefly luciferase expressing pGL3-promoter vector (Promega, Madison, WI). The t-PA950(–7351C)-Luc and t-PA950(–7351T)-Luc plasmids were amplified in DH5α (Gibco BRL Life Technologies, Paisley, United Kingdom) and purified (Endofree Plasmid Purification Maxi kit; Qiagen). Both constructs were sequenced on an ABI Prism 310 Genetic Analyzer (Applied Biosystems) as described4 to verify that the only ambiguity was the polymorphic site.
Transfection experiments
HeLa cells were seeded in 12-well tissue-culture plates and cultured in DMEM supplemented with 10% heat inactivated fetal bovine serum to 60% confluence on the day of transfection. The cell cultures were then washed with phosphate-buffered saline (PBS), fed with fresh culture medium, and transiently transfected with the t-PA950(–7351C)-Luc, t-PA950(–7351T)-Luc construct, or empty vector (ie, pGL3-promoter vector lacking the t-PA enhancer) (1 μg/well) using FuGene Transfection Reagent (Roche Molecular Biochemicals, Indianapolis, IN) according to the manufacturer's instructions. Cotransfection with pRL-TK (Renilla luciferase, 20 ng/well; Promega) was performed to control for variations in transfection efficiency. In addition to the luciferase constructs, a green fluorescent protein (GFP)–expressing vector (pSI-hGFPs65t; a kind gift from Dr P.A. Svensson, RCEM, Göteborg University) was used to optimize transfection conditions.
Twenty-four hours after transfection, cells were washed twice with PBS, and then cultured in serum-and phenol red–free DMEM containing RA (10–5, 10–6, 10–7, 10–8, 10–9 M), dexamethasone (DEX; 10–6, 10–7, 10–8, 10–9 M) or vehicle. Cells were then grown for a further 24 hours before being harvested. Stock solutions of DEX (Sigma) were dissolved in ethanol at a concentration of 2.5 mM and stored at –80°C. Before use RA (dissolved as described in “Cell treatment and preparation of nuclear extracts”) and DEX were diluted in serum- and phenol-free DMEM to the indicated concentrations. Control cells were exposed to the corresponding maximum concentration of DMSO or ethanol vehicle (0.1% and 0.04%, respectively).
Firefly and Renilla luciferase activities in cell extracts were quantified with a Dual Luciferase Assay (Promega) on a MLX luminometer (Dynex, West Sussex, United Kingdom). Mean intra-assay coefficient of variation was 2.5%. Stimulations of t-PA950(–7351C)-Luc, t-PA950(–7351T)-Luc construct, or empty vector transfectants were performed in 9 separate wells (3 wells on 3 separate occasions) for each construct and concentration of RA, DEX, or vehicle. Two different plasmid preparations were used. Data are presented as the fold increase in normalized activity relative to empty vector. Transcriptional activity between constructs was compared by 2-way ANOVA for repeated measures. Post hoc was performed by Student t test. Statistical tests were considered significant at P less than .05 (2-sided test).
Results
The t-PA –7351C>T enhancer polymorphism affects protein binding affinity
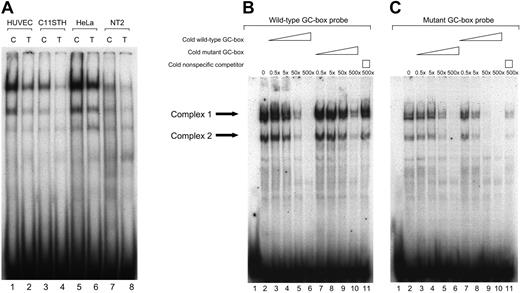
EMSAs were performed to determine whether nuclear proteins would bind to the polymorphic GC-box and whether the binding affinity differed between the allelic variants. As shown in Figure 1A, the same migration patterns were obtained using both the wild-type (C allele) and mutant (T allele) GC-box probes. However, for all cell types tested, the mutant T allele probe displayed substantially weaker shifts than the probe containing the wild-type C allele. Furthermore, it appears that the mobility and relative proportion of the DNA-protein complexes were similar between the different cell types tested. However, nuclear extracts from HUVECs and HeLa cells produced stronger shifts than those derived from the other cell types.
The t-PA –7351C>T enhancer polymorphism affects protein binding affinity. (A) EMSAs were performed following incubation of 32P-labeled wild-type C or mutant T GC-box oligonucleotides with 5 μg nuclear proteins from 4 different human cell lines that are known to express t-PA. (Lanes 1-2) HUVECs; (lanes 3-4) C11STH; (lanes 5-6) HeLa cells; (lanes 7-8) NT2 nuclear extracts; odd lanes (1,3,5,7), wild-type GC-box probe; even lanes (2,4,6,8), mutant GC-box probe. (B-C) Competition and cross-competition EMSA experiments were performed using unlabeled competitor oligomers corresponding to either the C or T allele variant to assess difference in binding affinity of proteins associating with the 2 alleles at the –7351 site. Both wild-type and mutant 32P-labeled GC-box probes were used (B and C, respectively). Arrows to the left indicate the 2 specific complexes. HUVEC nuclear extracts (5 μg) were included in all reactions, except for that in well 1. (B-C, lane 2) Migration patterns produced in the absence of competitor; (lanes 3-6) increasing concentrations of unlabeled oligomers of identical sequence to labeled counterpart (self-competition), 0.1, 1, 10, or 100 ng (approximately 0.5-, 5-, 50-, 500-fold molar excesses); (lanes 7-10) increasing concentrations of the unlabeled variant oligomer (cross-competition of the C and T variant), 0.1, 1, 10, or 100 ng (approximately 0.5-, 5-, 50-, 500-fold molar excesses); (lane 11) 100 ng (500-fold molar excess) of an unlabeled nonspecific competitor. The results indicate a 10-fold greater protein binding affinity to the t-PA –7351C allele compared with the T allele variant.
The t-PA –7351C>T enhancer polymorphism affects protein binding affinity. (A) EMSAs were performed following incubation of 32P-labeled wild-type C or mutant T GC-box oligonucleotides with 5 μg nuclear proteins from 4 different human cell lines that are known to express t-PA. (Lanes 1-2) HUVECs; (lanes 3-4) C11STH; (lanes 5-6) HeLa cells; (lanes 7-8) NT2 nuclear extracts; odd lanes (1,3,5,7), wild-type GC-box probe; even lanes (2,4,6,8), mutant GC-box probe. (B-C) Competition and cross-competition EMSA experiments were performed using unlabeled competitor oligomers corresponding to either the C or T allele variant to assess difference in binding affinity of proteins associating with the 2 alleles at the –7351 site. Both wild-type and mutant 32P-labeled GC-box probes were used (B and C, respectively). Arrows to the left indicate the 2 specific complexes. HUVEC nuclear extracts (5 μg) were included in all reactions, except for that in well 1. (B-C, lane 2) Migration patterns produced in the absence of competitor; (lanes 3-6) increasing concentrations of unlabeled oligomers of identical sequence to labeled counterpart (self-competition), 0.1, 1, 10, or 100 ng (approximately 0.5-, 5-, 50-, 500-fold molar excesses); (lanes 7-10) increasing concentrations of the unlabeled variant oligomer (cross-competition of the C and T variant), 0.1, 1, 10, or 100 ng (approximately 0.5-, 5-, 50-, 500-fold molar excesses); (lane 11) 100 ng (500-fold molar excess) of an unlabeled nonspecific competitor. The results indicate a 10-fold greater protein binding affinity to the t-PA –7351C allele compared with the T allele variant.
To assess the binding specificity and in a more quantitative manner determine the difference in binding affinity, competition and cross-competition experiments were performed. The 2 most prominent complexes formed using probes containing either polymorphic variant were specific as binding was competed by inclusion of unlabeled competitor of identical sequence (Figure 1, lanes 3-6 in panels B and C, respectively), whereas inclusion of 100 ng (approximately 500-fold molar excess) nonspecific competitor did not compete for binding (Figure 1, lanes 11 in panels B and C, respectively). Cross-competition experiments with the wild-type C probe and titrated amounts of cold T oligonucleotide (Figure 1B, lanes 7-10) and mutant T probe versus cold C oligonucleotide (Figure 1C, lanes 7-10) both indicated an approximately 10-fold greater protein binding affinity to the C allele as compared with the T allele variant. The same results were obtained when performing the experiment using nuclear extracts from C11STH, HeLa, and NT2 cells (data not shown).
Sp1 and Sp3 bind the polymorphic t-PA –7351 site
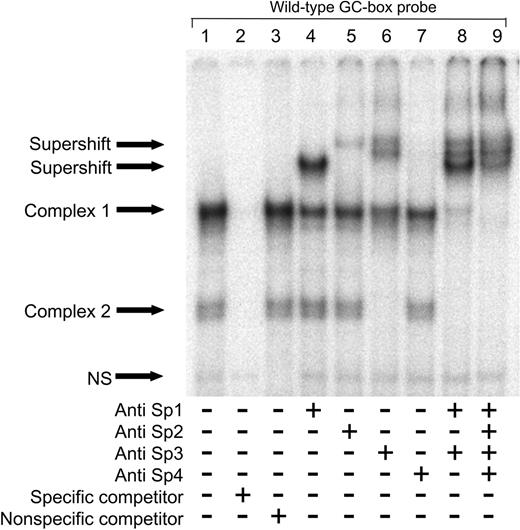
To identify the proteins binding to the polymorphic site, supershift experiments were conducted using the wild-type GC-box probe and nuclear extracts from HUVECs. As this site previously has been shown to bind recombinant Sp1,4 antibodies directed against transcription factors Sp1, Sp2, Sp3, and Sp4 were used. The same 2 complexes previously seen in Figure 1 were observed in the control EMSA (Figure 2, lane 1). The fastest migrating DNA-protein complex doublet was completely shifted by the anti-Sp3 antibody (Figure 2, lane 6). The slower migrating complex was nearly completely shifted by combining anti-Sp1 and anti-Sp3 antibodies (Figure 2, lane 8), but only partially shifted by either antibody alone, suggesting that this band represents binding of both Sp1 and Sp3 (Figure 2, lanes 4 and 6). The presence of multiple bands containing Sp3 is not surprising, since there are 3 isoforms of Sp3.23 Inclusion of anti-Sp2 antibodies produced only a minor effect on the upper complex (Figure 2, lane 5), while anti-Sp4 antibodies had no effect (Figure 2, lane 7). The experiment was repeated with nuclear extracts from C11STH, HeLa, and NT2 cells, and similar results were obtained (data not shown). Addition of anti-RARα, anti-RARβ, and RARγ antibodies had no effect (data not shown).
Sp1 and Sp3 bind to the t-PA –7351 site. Nuclear proteins (5 μg) from HUVECs were incubated with the wild-type GC-box probe with or without the addition of antibodies directed against human Sp1, Sp2, Sp3, or Sp4 as indicated. Binding specificity of nuclear proteins with the labeled oligonucleotide was confirmed by addition of excess cold GC-box specific competitor (lane 2) and nonspecific competitor (lane 3). NS indicates nonspecific.
Sp1 and Sp3 bind to the t-PA –7351 site. Nuclear proteins (5 μg) from HUVECs were incubated with the wild-type GC-box probe with or without the addition of antibodies directed against human Sp1, Sp2, Sp3, or Sp4 as indicated. Binding specificity of nuclear proteins with the labeled oligonucleotide was confirmed by addition of excess cold GC-box specific competitor (lane 2) and nonspecific competitor (lane 3). NS indicates nonspecific.
The t-PA –7351C>T polymorphism affects enhancer responsiveness to retinoic acid
To investigate whether the t-PA –7351C>T SNP has a direct effect on t-PA gene transcription, a 950-bp enhancer region of the t-PA gene (–7137 to –8087) carrying either the C or T allele was inserted upstream of the luciferase gene within the pGL3-promoter vector. The activity of these luciferase reporter gene constructs was assessed in transient transfection assays in HeLa cells. The transient transfection approach was selected for this purpose, as stable transfection, even when using pooled data to reduce the confounding effect of clone-to-clone variability, is less suitable for characterization of regulatory SNPs24,25 that typically show only 20% to 70% difference in levels of expression between alleles.26,27 HeLa cells were chosen as our EMSAs show that nuclear proteins derived from these cells produce the same binding pattern as those from HUVECs. In addition, Arts et al9 have shown that t-PA gene expression is regulated in a similar fashion in HeLa cells as compared with HUVECs.
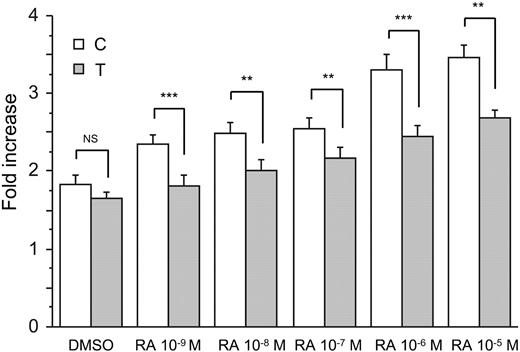
Cells transfected with constructs harboring either variant of the t-PA enhancer expressed at least 1.7-fold higher normalized luciferase activity than cells transfected with empty vector (Figure 3). To reduce the effects of endogenous retinoids and phenol red on constitutive levels of reporter gene expression, cells were maintained under serum- and phenol red–free conditions for 24 hours before luciferase activities were determined. Treatment with RA caused a dose-dependent increase in reporter gene expression with a maximal 2-fold induction (Figure 3). Cells transfected with the construct containing the wild-type C allele showed higher expression compared with cells transfected with the mutant counterpart, and this difference was statistically significant for all 5 concentrations of RA (P < .01 throughout). The maximal difference between the 2 constructs was 40%. Although we cannot exclude an activating affect of any residual endogenous agents in the culture medium, preliminary experiments performed using medium containing phenol red still revealed significant differences between the alleles, but the overall magnitude was lower.
The t-PA –7351C>T polymorphism affects enhancer responsiveness to retinoic acid. HeLa cells were transiently transfected with either the t-PA950(–7351C)-Luc or t-PA950(–7351T)-Luc construct, cotransfected with a Renilla luciferase–expressing vector, and stimulated with 5 different concentrations of RA for 24 hours. Fold increase represents the normalized increase in activity in the indicated construct relative to empty vector (pGL3-promoter vector lacking enhancer insert). As RA was dissolved in DMSO, cells were also exposed to DMSO alone as control. Results are presented as mean (n = 9) and SEM. ANOVA main effect of genotype P < .001, genotype × treatment interaction P < .01. Post hoc analysis by t test; NS indicates not significant, **P < .01, ***P < .001.
The t-PA –7351C>T polymorphism affects enhancer responsiveness to retinoic acid. HeLa cells were transiently transfected with either the t-PA950(–7351C)-Luc or t-PA950(–7351T)-Luc construct, cotransfected with a Renilla luciferase–expressing vector, and stimulated with 5 different concentrations of RA for 24 hours. Fold increase represents the normalized increase in activity in the indicated construct relative to empty vector (pGL3-promoter vector lacking enhancer insert). As RA was dissolved in DMSO, cells were also exposed to DMSO alone as control. Results are presented as mean (n = 9) and SEM. ANOVA main effect of genotype P < .001, genotype × treatment interaction P < .01. Post hoc analysis by t test; NS indicates not significant, **P < .01, ***P < .001.
A similar induction was observed in response to DEX, but in contrast to RA there was no significant difference between the 2 constructs (data not shown).
RA induces the binding of Sp1 and Sp3 to the t-PA enhancer GC-box
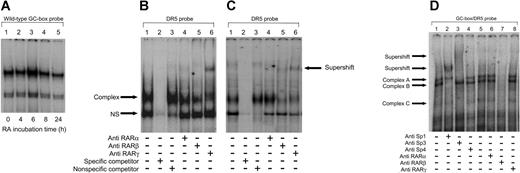
The allele-specific response to RA prompted us to study whether treatment of HUVECs with RA had any effect on the amount or relative proportions of the proteins binding to the polymorphic GC-box. For this purpose, nuclear proteins extracted from HUVECs that had been treated with 10–6 M RA for different time periods were incubated with the wild-type GC-box probe and binding profiles assessed by EMSA (Figure 4A). No differences in mobility of the proteins binding to this region were observed over time, while the nuclear proteins extracted from the HUVECs which had been treated with RA for 6 hours produced slightly stronger shifts compared to the others (Figure 4A, lane 3), suggesting that RA treatment induced the binding of Sp1 and Sp3 to this DNA region.
DNA-bound RARβ interacts with Sp1/Sp3 following RA treatment. (A) EMSA was performed following incubation of the wild-type GC-box probe with 2.5 μg nuclear proteins extracted from HUVECs that had been treated with 10–6 M RA for various time periods (0, 4, 6, 8, or 24 hours). The slight increase in complex intensity at 6 hours (A, lane 3) indicates that RA treatment enhances the binding of Sp1 and Sp3 to the t-PA –7351 site. (B-C) Nuclear proteins (15 μg) from nontreated HUVECs (B) and 6-hour RA-treated (10–6 M) HUVECs (5 μg; C) were incubated with a 32P-labeled DR5 oligonucleotide, with or without the addition of antibodies directed against human RARα, RARβ,orRARγ as indicated. Binding specificity of nuclear proteins with the labeled oligonucleotide was confirmed by addition of excess cold DR5-specific competitor (B and C, lane 2) and nonspecific competitor (B and C, lane 3). NS indicates nonspecific. The results show that RARγ binds to the DR5 site and that RARβ is present at the DR5 site during RA-treated conditions. (D) Nuclear proteins (5 μg) from 6-hour RA-treated (10–6 M) HUVECs were incubated with a 32P-labeled GC-box/DR5 oligonucleotide, with or without the addition of antibodies directed against human Sp1, Sp3, Sp4, RARα, RARβ,orRARγ as indicated. Supershifting was performed as described in the “Materials and methods,” except that the RAR antibodies were added prior to that of the labeled oligomer. Lanes 1 and 5 represent control lanes with no added antibodies. Addition of RARβ antibodies (D, lane 7) inhibits formation of the Sp1 and Sp3 containing complexes (complex A, B, and C), suggesting that RARβ is present in combination with Sp1 and Sp3 in these complexes.
DNA-bound RARβ interacts with Sp1/Sp3 following RA treatment. (A) EMSA was performed following incubation of the wild-type GC-box probe with 2.5 μg nuclear proteins extracted from HUVECs that had been treated with 10–6 M RA for various time periods (0, 4, 6, 8, or 24 hours). The slight increase in complex intensity at 6 hours (A, lane 3) indicates that RA treatment enhances the binding of Sp1 and Sp3 to the t-PA –7351 site. (B-C) Nuclear proteins (15 μg) from nontreated HUVECs (B) and 6-hour RA-treated (10–6 M) HUVECs (5 μg; C) were incubated with a 32P-labeled DR5 oligonucleotide, with or without the addition of antibodies directed against human RARα, RARβ,orRARγ as indicated. Binding specificity of nuclear proteins with the labeled oligonucleotide was confirmed by addition of excess cold DR5-specific competitor (B and C, lane 2) and nonspecific competitor (B and C, lane 3). NS indicates nonspecific. The results show that RARγ binds to the DR5 site and that RARβ is present at the DR5 site during RA-treated conditions. (D) Nuclear proteins (5 μg) from 6-hour RA-treated (10–6 M) HUVECs were incubated with a 32P-labeled GC-box/DR5 oligonucleotide, with or without the addition of antibodies directed against human Sp1, Sp3, Sp4, RARα, RARβ,orRARγ as indicated. Supershifting was performed as described in the “Materials and methods,” except that the RAR antibodies were added prior to that of the labeled oligomer. Lanes 1 and 5 represent control lanes with no added antibodies. Addition of RARβ antibodies (D, lane 7) inhibits formation of the Sp1 and Sp3 containing complexes (complex A, B, and C), suggesting that RARβ is present in combination with Sp1 and Sp3 in these complexes.
RARβ and RARγ bind to the DR5-element adjacent to the t-PA–7351 site
Since an allele-specific transcriptional effect was observed in response to RA, we also wanted to identify proteins binding to the neighboring DR5 sequence in the enhancer. To this end supershift experiments were performed using a DR5 probe (Table 1) and nuclear extracts from nontreated and 6-hour RA-treated (10–6 M) HUVECs. As shown in Figure 4B, a single specific complex was produced using extracts from nontreated cells (lanes 1-3). Addition of antibodies directed against RARα or RARβ did not produce a supershift (lanes 4 and 5, respectively). In contrast, anti-RARγ antibodies caused partial complex displacement and the generation of a supershifted complex (lane 6). When using nuclear extracts from RA-treated cells, a similar pattern was observed (Figure 4C), although RA treatment resulted in the appearance of a faint RARβ supershifted complex (Figure 4C, lane 5), together with marked complex displacement indicating that RARβ is present on this site during RA treatment. Anti-RARγ antibodies again produced a supershifted complex and significant complex displacement (Figure 4C, lane 6). Addition of anti-Sp1 or Sp3 antibodies had no effect (data not shown). These data indicate that RARγ is associated with the DR5 site in the t-PA enhancer under constitutive and RA-treated conditions. However, RARβ appears to be selectively recruited to the DR5 site by RA.
RARβ interacts with Sp1/Sp3
The DR5 sequence present in the t-PA enhancer is positioned only approximately 30 nucleotides downstream of the GC-box in which the t-PA–7351C>T SNP is located. To investigate whether the presence of both DNA elements had any affect on the properties of the proteins that bind to these sites, a 75-bp oligonucleotide spanning both the polymorphic GC-box and DR5 sequence was designed (Table 1). Since RA treatment was found to affect the binding of proteins to the DR5 probe, this experiment was performed using nuclear extracts from 6-hour RA-treated (10–6 M) HUVECs. EMSA supershift assays were performed using antibodies directed against Sp1, Sp3, Sp4, RARα, RARβ, and RARγ. Two clear complexes (A and B) and 1 faint complex (C) were observed in the control EMSA(Figure 4D, lanes 1 and 5). Of these, complex B was supershifted by the anti-Sp1 antibody (Figure 4D, lane 2). Addition of anti-Sp3 antibodies displaced the slower migrating complex (complex A) as well as the relatively weak complex C (lane 3), while anti-Sp4 antibodies had no effect (Figure 4D, lane 4). No apparent effect was seen when RARα or RARγ antibodies were added to the reaction mixture (Figure 4D, lanes 6 and 8, respectively); however, anti-RARβ inhibited formation of the Sp1 and Sp3 containing complexes (Figure 4D, lane 7). These results indicate the presence of RARβ in combination with Sp1 and Sp3 in these complexes.
RA up-regulates RARβ
To study the time profile of the RA response with respect to t-PA induction and its mediators, mRNA levels were quantified at baseline and after RA treatment of HUVECs. The results showed a strong up-regulation of RARβ that coincides and possibly even precedes the increase in t-PA mRNA (Table 2). There was a slight increase in the expression of RARα and RARγ, while Sp1 mRNA levels remained essentially unchanged (Table 2).
Effect of RA on the expression of t-PA, RARα, RARβ, RARγ, and Sp1 mRNA over time
. | Treatment-to-control ratios, mean (SEM) . | . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | 0 h . | 2 h . | 4 h . | 6 h . | 8 h . | ANOVA . | ||||
| t-PA | 1.0 | 1.3 (0.08) | 1.6 (0.22)* | 1.8 (0.23)† | 2.0 (0.14)† | < .01 | ||||
| RARα | 1.0 | 1.2 (0.07)* | 0.9 (0.11) | 0.9 (0.05) | 0.8 (0.03) | < .01 | ||||
| RARβ | 1.0 | 4.1 (1.68)* | 4.7 (1.28)† | 5.5 (1.58)† | 5.6 (1.27)† | < .01 | ||||
| RARγ | 1.0 | 1.2 (0.07) | 1.6 (0.13)‡ | 1.4 (0.03)† | 1.4 (0.06)† | < .01 | ||||
| Sp1 | 1.0 | 1.0 (0.09) | 1.0 (0.02) | 1.1 (0.03) | 1.0 (0.01) | NS | ||||
. | Treatment-to-control ratios, mean (SEM) . | . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | 0 h . | 2 h . | 4 h . | 6 h . | 8 h . | ANOVA . | ||||
| t-PA | 1.0 | 1.3 (0.08) | 1.6 (0.22)* | 1.8 (0.23)† | 2.0 (0.14)† | < .01 | ||||
| RARα | 1.0 | 1.2 (0.07)* | 0.9 (0.11) | 0.9 (0.05) | 0.8 (0.03) | < .01 | ||||
| RARβ | 1.0 | 4.1 (1.68)* | 4.7 (1.28)† | 5.5 (1.58)† | 5.6 (1.27)† | < .01 | ||||
| RARγ | 1.0 | 1.2 (0.07) | 1.6 (0.13)‡ | 1.4 (0.03)† | 1.4 (0.06)† | < .01 | ||||
| Sp1 | 1.0 | 1.0 (0.09) | 1.0 (0.02) | 1.1 (0.03) | 1.0 (0.01) | NS | ||||
n = 3. Confluent HUVEC cultures in the second passage were incubated for the indicated time periods with 10-6 M RA or DMSO vehicle (0.01%). Total cellular RNA was extracted, and mRNA was converted into cDNA. Target mRNA (cDNA) was quantified by real-time PCR and normalized relative to glyceraldehyde 3-phosphatedehydrogenase (GAPDH) mRNA. The results are expressed as fold induction compared with control cultures. Each data point represents the average of duplicate wells from 3 different HUVEC cultures, each assayed in triplicate for both target and GAPDH. Post hoc analysis was performed by contrast analysis. NS indicates not significant.
P < .05.
P < .01.
P < .001.
Discussion
We have earlier identified a common polymorphism in the t-PA enhancer, t-PA –7351C>T, which is associated with endothelial t-PA release in vivo as well as arterial thrombotic disease.4-6 The aim of the present study was, therefore, to test the hypothesis that this polymorphism is functional. Using the EMSA approach we found a specific binding of nuclear proteins to this polymorphic site. The probe corresponding to the mutant T allele displayed substantially weaker shifts than the probe corresponding to the wild-type C allele. This was observed using nuclear proteins extracted from all 4 cell types tested (HUVECs, C11STH endothelial, HeLa, and NT2 neuronal cells), and similar observations were made using extracts derived from HUVECs exposed to RA. Cross-competition experiments revealed that the binding affinity of proteins to the wild-type C allele variant was approximately 10-fold greater than that seen with the mutant T allele counterpart. Sp1 and Sp3 were identified as the GC-box binding proteins. Although our supershift experiments clearly showed that these 2 transcription factors are the major players as the complexes formed on this site were fully displaced by specific antibodies, we cannot exclude the possibility that other members of the Sp1-like/Kruppel-like protein family may bind to the polymorphic site.
The finding of a differential binding of Sp1 to the 2 alleles supports a functional role for the t-PA –7351C>T SNP. Sp1 is a positive regulator of eukaryotic gene expression and can form both homotypic and heterotypic interactions leading to multimeric complexes.23 Mutation of the 2 Sp1 sites in the t-PA promoter has been reported to completely abolish RA induction through the enhancer,11 while the RA response is independent of the intervening sequence between the enhancer and the initiation element.7 These observations suggest a DNA looping mechanism bringing the enhancer and the proximal promoter in physical contact. By self-association, Sp1 can join distant DNA segments, thereby causing the intervening DNA to loop.12-14 Sp3, however, has been shown to function as both an activator and a repressor.23 However, Merchiers et al11 reported that Sp3 could act as activator of t-PA promoter activity, both by itself and in synergy with Sp1 in transfected Drosophila SL2 cells. Therefore, Sp3 seems to function as an activator in this promoter context in vitro. It remains to be determined whether the change in protein binding affinity at this polymorphic site is solely responsible for an alteration in t-PA expression in vivo. However, our finding of a lower affinity of both Sp1 and Sp3 to the t-PA –7351T as compared with the C allele variant would provide a plausible explanation for our in vivo observation of a reduced endothelial t-PA release in carriers of the mutant T allele.
To specifically test the hypothesis that the –7351C>T SNP affects t-PA gene transcription, we performed luciferase gene reporter assays. Following treatment with RA, cells transfected with constructs harboring the T allele variant showed lower activity than cells transfected with the C allele counterpart. This was seen at all concentrations of RA and is consistent with our finding of a reduced affinity of nuclear factors binding to the T allele DNA sequence. What, then, is the significance of a differential allelic response to RA? RA (vitamin A) and its derivates (retinoids) increase t-PA gene expression in endothelial cells in vitro28,29 and enhance plasma levels of t-PA in man.30,31 Furthermore, both plasma and tissue t-PA levels are decreased in vitamin A–starved and enhanced in retinoid-fed animals.28,32,33 Interestingly, treatment with RA has also been reported to reduce clot size in a venous thrombosis model.33
Although a recent review suggests that for some genes differences as small as 20% may be physiologically important,27 it can nonetheless be questioned whether the maximal 40% difference in transcriptional activity demonstrated in our study is large enough to have a biologic effect. However, the physiologic effect of a regulatory SNP is difficult to determine by extrapolation of the magnitude of the differential responses observed in in vitro experiments to the situation in vivo.24,25 Indeed, the t-PA –7351 variant was associated with a greater (140%) difference in t-PA secretion rates when investigated in vivo.4 There are a number of possible explanations for this discrepancy. As it has been shown that RA not only increases constitutive secretion, but also t-PA storage,1,34 one explanation may be related to variations in the amount of t-PA released from the endothelial storage pool. It is thus plausible that subjects homozygous for the C allele may accumulate higher levels of stored t-PA and that this can contribute to the higher t-PA secretion rates seen in this group in vivo. Also, we cannot exclude the possible involvement of other SNPs that may be in linkage disequilibrium with the –7351 variant.
More important, however, in the present mechanistic study the response to one single agonist was assessed, whereas in vivo the functional effect of a SNP is likely to depend on a specific combination of stimuli. In this context, we recently observed a difference at the t-PA mRNA level in HUVECs homozygous for the C allele compared with cells carrying the T allele that was of similar magnitude as in the present study after stimulation with RA alone, but following stimulation with both RA and a protein kinase C (PKC) activator the difference was greater, approximating that observed in vivo (A.T.W., L. Olsson, R.L.M., C.J., unpublished observation, 2004). In vivo, the vascular endothelium is constantly exposed to PKC-activating stimuli that cannot be fully mimicked in vitro, in particular different hemodynamic forces22,35,36 acting within the vessel lumen. These are likely to act in concert with a number of other circulating endogenous agents to induce a differentiated allelic response. One would thus expect the allele-specific difference to be greater in the physiologic context compared with that seen in response to a single agonist in isolation in vitro.
In contrast to RA, we did not observe an allele-specific transcriptional effect in response to DEX. As the induction by DEX has been reported to be independent of Sp1,11 this observation further supports that Sp1 is involved in mediating the allele-specific response to RA. Results from our EMSA analyses using nuclear proteins from RA-treated HUVECs suggested that RA enhanced binding of Sp1 and Sp3 to the GC-box, even though we could not detect an increase in Sp1 gene expression. This could be due to a RA-induced change in the phosphorylation state of Sp1.37,38 Similar results have been reported with GC-box motifs derived from promoters of other genes expressed in the endothelium.37,39,40 We also identified RARβ and RARγ as DR5 binding proteins. Furthermore, when using a probe spanning both the polymorphic GC-box and DR5 sequence, anti-RARβ antibodies inhibited the formation of Sp1- and Sp3-containing complexes, which suggests that DNA-bound RARβ and Sp1/Sp3 physically interact. In contrast, no such effect was observed with the anti-RAR or anti-Sp antibodies when using the GC-box or DR5 probes alone, respectively, which indicates that the presence of both DNA elements is necessary for this interaction to take place. Although we could clearly identify RARγ when using the labeled DR5 oligomer, we found it difficult to detect RARγ by EMSA supershifting when using the 75-bp oligomer harboring the DR5 and the polymorphic region. Whether this is related to a limitation in the EMSA supershifting approach or whether RARγ in fact fails to bind to the DR5 site in the presence of the polymorphic element is presently unknown.
We also investigated the time profile of the induction of the different RAR subtypes in relation to t-PA. We observed a strong up-regulation of RARβ mRNA that coincided with that of t-PA. The induction of RARβ and t-PA mRNA by RA is consistent with earlier data by Lansink and Kooistra,41 who also showed that t-PA induction by RA occurs via a 2-step process; RARα is required for the induction of RARβ, which subsequently mediates the response.41 In line with this Bulens et al42 showed that coexpression of both RARα and RARβ, but not RARγ, increased RA-induced t-PA reporter gene expression. The fact that RARβ has been shown to be the key mediator of RA-induced t-PA gene expression strengthens the relevance of our finding of an interaction between RARβ and Sp1/Sp3.
It has earlier been demonstrated that RARs/RXRs (retinoid X receptors) can functionally interact with Sp1 bound to adjacent sites.43 RARs/RXRs and Sp1 have also been shown to functionally interact in activating promoters lacking RARE(s), and Suzuki et al39 showed in a pull-down assay that a glutathione S-transferase RAR fusion protein could bind Sp1. Despite this, several attempts to detect a DNA-protein complex containing both RAR/RXR and Sp1 have failed.38-40 However, Husmann et al,44 who studied a GC-box in the interleukin 1B promoter, detected a RAR–Sp1–GC-box complex by EMSA when using RAR fusion protein and recombinant Sp1. On the basis of the results of our study, we speculate that the polymorphic GC-box and the DR5 site may be a functionally coupled transcriptional unit that is quantitatively influenced by the SNP at position –7351.
In conclusion, we have demonstrated that the t-PA –7351C>T SNP has a functional role at the level of transcription. Since far upstream enhancers can act by Sp1-mediated DNA looping, the finding of a reduced binding affinity of Sp1/Sp3 to the T allele could explain our earlier observations of a reduced endothelial t-PA secretion and an increased risk of myocardial infarction in individuals carrying this allele.
Prepublished online as Blood First Edition Paper, October 5, 2004; DOI 10.1182/blood-2003-12-4383.
Supported by the Swedish Research Council (2002-5618), the Swedish Heart Lung Foundation, the Tore Nilsson Foundation, the Swedish Stroke Association, the Swedish Hypertension Society, the Rune and Ulla Amlövs Foundation for Neurological Research, the John and Brit Wennerström Foundation for Neurological Research, the Yngve Land Foundation for Neurological Research, and the Thorsten Westerström Foundation and by the National Health and Medical Research Council of Australia (R.L.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal