Abstract
Ischemia is a known stimulus for vascular growth. Bone marrow (BM)–derived endothelial progenitor cells (EPCs) are believed to contribute to new blood vessel growth, but the mechanism for this contribution is unknown. To elucidate how BM cells are able to form new blood vessels, a novel murine model of soft tissue ischemia was developed in lethally irradiated mice with BM reconstituted from either tie2/lacZ or ROSA/green fluorescent protein (GFP) mice (n = 24). BM-derived EPCs were recruited to ischemic tissue within 72 hours, and the extent of recruitment was directly proportional to the degree of tissue ischemia. At 7 days, there were persistently elevated levels of vascular endothelial growth factor (VEGF) (2.5-fold) and circulating VEGF receptor-2/CD11– (flk-1+/CD11–) cells (18-fold) which correlated with increased numbers of BM-derived EPCs within ischemic tissue. The cells were initially located extravascularly as proliferative clusters. By day 14, these clusters coalesced into vascular cords, which became functional vessels by day 21. In vitro examination of human EPCs from healthy volunteers (n = 10) confirmed that EPC proliferation, adhesion, and chemotaxis were all significantly stimulated in hypoxic conditions. We conclude that BM-derived cells produce new blood vessels via localized recruitment, proliferation, and differentiation of circulating cells in a sequence of events markedly different from existing paradigms of angiogenesis.
Introduction
Progenitor cells have been identified in adult bone marrow cells that possess the ability to replace resident cells throughout the human body.1 Tissues in which bone marrow–derived stem cells have been identified include liver, brain, heart, and skeletal muscle.2-5 While the contribution of progenitor cells is variable, it is becoming increasingly clear that regeneration in adults occurs by both differentiated resident cells and the recruitment of cells from the circulation.
New blood vessel growth (neovascularization) is a process currently being re-evaluated in light of recent advances in progenitor biology. With the identification of circulating endothelial progenitor cells (EPCs),6,7 neovascularization is now believed to occur via 2 possible mechanisms: the sprouting of pre-existing resident endothelial cells (angiogenesis) or the recruitment of bone marrow–derived EPCs (vasculogenesis).8 The participation of EPCs has been well documented in a number of conditions requiring neovascularization, including peripheral vascular disease,9 myocardial ischemia,10 stroke,11,12 wound healing,9,13 retinopathy,14 and tumor growth.9,15 This has led to the examination of EPC transplantation in the treatment of ischemic conditions.16-19 Since human trials have already been initiated to investigate the therapeutic20 and diagnostic21,22 utility of EPCs, it is important that their mechanism of action be more fully understood.
One of the most potent stimuli for neovascularization is hypoxia, which induces new blood vessel growth to restore adequate oxygen (O2) delivery to ischemic tissue.23 Ischemia initiates a number of angiogenic processes, including the release of cytokines,24,25 up-regulation of surface markers,26,27 and proliferation of mature endothelial cells.28,29 However, little is known regarding the effects of ischemia on EPC biology and how circulating cells are recruited and incorporated into blood vessels within ischemic tissue.9-11,13-15
In the present study, we used a novel and reproducible in vivo model of graded vascular ischemia to examine how bone marrow–derived cells produce new blood vessels and whether this represents true in situ vasculogenesis. We demonstrate that hypoxia initiates a cellular cascade with localized migration and proliferation of bone marrow–derived EPCs in vitro and in vivo, with the eventual production of new blood vessels consisting entirely of bone marrow–derived cells. This mechanism does not resemble any existing models of angiogenesis and more closely resembles the sequence of events occurring during the inflammatory cascade.
Materials and methods
In vivo models of ischemia and wound healing
A novel model of soft tissue ischemia was developed that consisted of lateral skin incisions (2.5 cm in length and 1.25 cm apart) created on the dorsal surface of mice, penetrating the skin, dermis, and underlying adipose tissue. The overlying skin was undermined, and a 0.13-mm–thick silicone sheet (Invotec International, Jacksonville, FL) was inserted to separate the skin from the underlying tissue bed. The skin was then reapproximated with 6-0 nylon sutures (Figure 1A-C). A wound-healing model was also employed, consisting of paired 6-mm–diameter biopsies on the dorsa of mice, equidistant from the midline. Following the punch biopsy, wounds were covered with an occlusive dressing to limit infection. All procedures were done in accordance with the New York University Medical Center (New York, NY) Animal Care and Use Committee.
In vivo model of graded soft tissue ischemia. (A) Schematic diagram of a novel ischemia model consisting of paired skin incisions (black lines) along the dorsal surface of mice. (B) (C) (D) Parallel incisions penetrating the skin were made, and a silicone sheet was placed beneath the undermined tissue. The tissue was then reinserted to its original position (panel C), and gross evidence of necrosis (arrow) was evident in the central portion at 1 week following surgery (panel D). (E) Oxygen levels were measured by means of an oxygen probe, and a corresponding gradient of hypoxia was noted within the incised tissue at day 4 following surgery. (F) Color laser Doppler analysis of the incised skin also confirmed the presence of an ischemic gradient, with blood flow diminishing toward the central portion; the color scale illustrates variations in blood flow from maximum perfusion (red) to minimal perfusion (dark blue).
In vivo model of graded soft tissue ischemia. (A) Schematic diagram of a novel ischemia model consisting of paired skin incisions (black lines) along the dorsal surface of mice. (B) (C) (D) Parallel incisions penetrating the skin were made, and a silicone sheet was placed beneath the undermined tissue. The tissue was then reinserted to its original position (panel C), and gross evidence of necrosis (arrow) was evident in the central portion at 1 week following surgery (panel D). (E) Oxygen levels were measured by means of an oxygen probe, and a corresponding gradient of hypoxia was noted within the incised tissue at day 4 following surgery. (F) Color laser Doppler analysis of the incised skin also confirmed the presence of an ischemic gradient, with blood flow diminishing toward the central portion; the color scale illustrates variations in blood flow from maximum perfusion (red) to minimal perfusion (dark blue).
Tissue perfusion and O2 tension levels
Blood flow (perfusion) and oxygen tensions within the tissue were measured following surgery and in the postoperative period. Perfusion was assessed with color laser Doppler analysis (Moor Instruments, Wilmington, DE),30 and oxygen levels were monitored with a tissue oxygen tension probe (Oxford Optronics, Oxford, United Kingdom).31 The oxygen probe consists of a single optical fiber and a thermocoupler (100-μm radius) that can be inserted within the tissue. The thermocoupler allows for temperature compensation, and the oxygen probe then measures fluorescence decay time and relates it to local oxygen concentration. The probe reads O2 concentrations at a rate of 10 values per second and was kept in place at each position for 60 seconds to generate an average of 600 values. All gross pictures were obtained using a Sony Cybershot DSC-F70 digital camera (5 megapixel; Sony, New York, NY).
Circulating MNCs and VEGF levels
Whole blood samples were obtained from mice at the time they were humanely killed. Circulating mononuclear cells (MNCs) were isolated by density centrifugation (Histopaque 1083; Sigma-Aldrich, St Louis, MO), and EPCs were identified with the use of previously described markers.15,32 A total of 2 × 105 MNCs were incubated for 30 minutes at 4°C with phycoerythrin (PE)–labeled fetal liver kinase–1 (flk-1). To select out macrophages/monocytes, cells were subsequently stained for macrophage marker CD11 (fluorescein isothiocyanate [FITC]–labeled). Plasma samples were analyzed for circulating levels of murine vascular endothelial growth factor (VEGF) by means of an enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN).
Bone marrow transplantation model
We used a bone marrow transplantation animal model to distinguish bone marrow–derived EPCs from resident endothelial cells.9 Bone marrow cells harvested from the tibias and femurs of transgenic FVB mice that express lacZ under the control of the endothelial-specific tie2 promoter (tie2/lacZ; Jackson Laboratory, Bar Harbor, ME) were purified by density centrifugation (Histopaque 1083; Sigma-Aldrich). Bone marrow cells were then systemically transplanted (2 × 106 cells) into wild-type mice that had been lethally irradiated with 2 doses of 6 Gy 3 hours apart. Identical bone marrow transplantation experiments were performed with donor bone marrow from mice ubiquitously expressing green fluorescent protein (GFP) (ROSA/GFP; Jackson Laboratory). All transplant-recipient mice were set aside for a minimum of 4 weeks to allow for complete reconstitution of the bone marrow. The engraftment efficiency was determined by fluorescent-activated cell sorting (FACS) for GFP expression in the bone marrow of mice receiving transplants of GFP+ bone marrow cells. Mice then underwent either the ischemia or wound healing as described in “In vivo models of ischemia and wound healing.”
Tissue harvest and immunohistochemical staining
Tissues were harvested from ischemic skin and were oriented for sectioning either parallel or perpendicular to the plane of ischemia. To reduce the role of inflammatory cells in this study, we eliminated the outer portions of the graft edge (the site of incision) from analysis. Tissues were harvested from mice at postoperative days 3, 7, 14, 21, and 28 and either snap frozen in ornithine carbamoyltransferase (OCT) or placed and fixed in 4% paraformaldehyde (PFA). LacZ+ cells were identified by incubating in X-gal solution (R&D Systems) followed by paraffin embedding. The number of lacZ+ cells per high-power field were counted in sectioned tissue. Double-blinded counts were averaged from 4 separate sections in each sample. A subset of mice were perfused via intracardiac injection with isolectin-B4 conjugated to an Alexafluor 594 fluorochrome (Molecular Probes, Eugene, OR) immediately prior to humane killing, thus enabling identification of functional, perfused blood vessels. Sections were stained with anti-CD31 antibody (1:100) (Abcam, Cambridge, United Kingdom) conjugated with an Alexafluor 488 secondary (1:100) antibody. LacZ+ cells were also identified by immunofluorescence with anti–β-galactosidase antibody (Rockland Immunochemicals, Gilbertsville, PA) at 1:100 added for 24 hours. FITC-conjugated primary antibody (Rockland Immunochemicals) at 1:100 in 5% bovine serum albumin (BSA) was added for 24 hours at 4°C. Additional tissue sections were stained for von Willebrand factor (VWF), a marker of endothelial cells,33 with rabbit polyclonal antibody (1:500) (Dako, Carpinteria, CA) at room temperature for 1 hour. Cell proliferation was identified by Ki67 staining, which identifies cells during the late G1, S, G2, and M phases of the cell cycle,34 with rabbit polyclonal antibody (1:1000) (Novocastra Laboratories, Newcastle upon Tyne, United Kingdom) at 4°C overnight. Beta-biotinylated secondary antibodies (Vector Laboratories, Burlingame, CA) were added at room temperature for 30 minutes, and signals were detected with diaminobenzidine (DAB) (Vector Laboratories). All microscope images were obtained using an Olympus BX51 light-fluorescent microscope with accessory digital camera. For light microscopy images, the microscope was set at a numeric aperture of 0.65.
Human EPC isolation
Human EPCs from 10 independent healthy donors were cultured and characterized according to techniques we and others have previously described.16,18,22,35-37 Briefly, MNCs were isolated by density centrifugation (Histopaque 1077; Sigma-Aldrich) and plated on fibronectin-coated 6-well plates in endothelial cell basal medium 2 (EBM-2) (Clonetics, San Diego, CA) supplemented with EGM-2MV single aliquots consisting of 5% fetal bovine serum (FBS), VEGF, fibroblast growth factor–2 (FGF-2), epidermal growth factor (EGF), insulin-like growth factor-1 (IGF-1), and ascorbic acid. Cells were reseeded at day 4, and all assays were performed on cells harvested with phosphate-buffered saline (PBS) plus 5 mM EDTA (ethylenediaminetetraacetic acid) on day 7. Fluorescent labeling of cultured EPCs was achieved by either incubation with dioctadecyl-tetramethylindocarbocyanine perchlorate (diI) (Molecular Probes, Eugene, OR) (2.5 μg/mL) for 5 minutes at 37°C and 15 minutes at 4°C or incubation with diI–acetylated low-density lipoprotein (diI-acLDL) (Biomedical Technologies, Stoughton, MA) for 2 hours at 37°C.
In vitro hypoxia
In vitro hypoxia experiments were performed with a customized hypoxic incubator (Reming BioInstruments, Redfield, NY) that continuously infuses a calibrated gas mixture (95% N, 5% CO2). Experiments were performed at an O2 concentration of 21% (normoxia) or 0.5% (hypoxia).
Human EPC adhesion
A monolayer of human umbilical vein endothelial cells (HUVECs) (Clonetics) (passages 4-8) was prepared in 96-well plates by adding 4 × 104 cells per well (n = 6) 24 hours prior to the assay. HUVEC monolayers were exposed to either 5% or 0.5% O2 for 6 hours, at which time DiI-labeled EPCs (5 × 104) were added to each well of HUVECs (n = 5), and the HUVECs were returned to 21% O2. A baseline fluorescence value (5 × 104 EPCs) was determined at the start of the assay using a Cytofluor 2320 (Millipore, Bedford, MA). After 3 hours at 37°C, nonadherent EPCs were removed with gentle wash with PBS, and a postadhesion fluorescence value was obtained. The percentage of EPC adherence was calculated by means of the ratio of postadhesion fluorescence to baseline fluorescence, and all values were corrected for background fluorescence (ie, autofluorescence of HUVEC monolayer alone).
Human EPC chemotaxis
EPC migration was evaluated by means of the NeuroPore transwell assay (8-μm pore size). Each membrane was coated with 5 μg/mL human fibronectin (Sigma Chemical, St Louis, MO), and 5 × 104 EPCs resuspended in EBM (plus 0.5% bovine serum albumin) were placed in the upper well overnight (n = 6). Conditioned media harvested from cultures of HUVECS or fibroblasts grown at normal (21% O2) or hypoxic (5% O2) conditions were then placed in the bottom wells, and a 6-hour EPC migration assay was performed at 37°C and 21% O2. Nonmigrating cells were removed by suction and gentle cleansing with a cotton tip applicator. The bottom surface of the membrane was fixed in formalin and stained with DAPI (4,6-diamidino-2-phenylindole) (Vector Laboratories). A total of 5 random fields of each membrane was analyzed under the fluorescent microscope (10 ×) and quantified by means of Kodak 1D 3.5 digital analysis software (Kodak, Rochester, NY).
Human EPC proliferation
EPCs (1 × 105) were plated in 12-well fibronectin-coated plates (n = 6) and serum starved for 24 hours with media containing 0.5% FBS. The media was then changed to fully supplemented media (EBM plus EGM-2MV) and cultured at varying oxygen tensions. At 18 hours, cells were pulsed with [3H] thymidine (5 μCi [185 KBq] per well) for the remaining 6 hours, and at the completion of the assay, cells were washed with PBS × 3 and 10% trichloroacetic acid × 3. Then, 2 mL 1 N NaOH was added to each well, incubated for 30 minutes, and neutralized by 2 mL 1 N HCl. Each sample was evaluated by a scintillation counter to correlate cell proliferation with the amount of radioactivity (counts per minute). HUVECs served as controls.
Statistical analysis and figure preparation
A Student t test was performed to compare data sets. Multiple pairwise comparisons were analyzed with a Tukey test. All data are presented as mean ± standard error of the mean; P less than .05 was interpreted as denoting statistical significance. For digital picture acquisition, Adobe Photoshop 7.0 was used (Adobe, San Jose, CA). Manuscript figures were created using Adobe Illustrator version 11.0 software.
Results
In vivo model of graded ischemia
To study ischemia-induced neovascularization, we used a novel murine model that results in a distinct gradient of soft tissue ischemia. Placement of 2 parallel skin incisions on the dorsal surface of mice creates a setting in which the blood supply to the incised tissue is dependent on flow from the 2 sides left intact. Positioning of a silicone sheet below the surgically elevated skin prevents capillary ingrowth from the muscle below. As a result, the most central portion of skin undergoes the most severe ischemic insult (Figure 1A-D). To confirm that this resulted directly from diminished oxygen levels, O2 tensions were assessed at constant points along the incision by means of an oxygen probe (Figure 1E). Color laser Doppler analysis of this model confirmed a reproducible ischemic gradient that ultimately led to the absence of flow and necrosis in the central portion of skin (Figure 1F), but flow analysis at 7 and 14 days revealed restoration of blood flow to the surviving portion of skin.
Evidence of total neovascularization in ischemic tissue
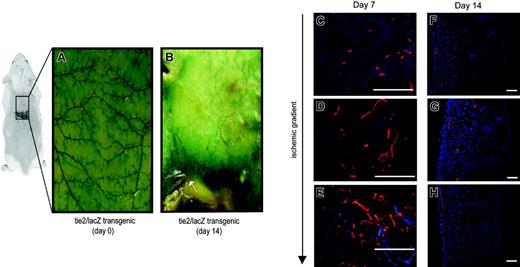
Potential explanations for the restoration of blood flow within ischemic tissue include the enlargement of existing vessels (arteriogenesis) or the formation of new vessels (neovascularization). Using tie2/lacZ transgenic mice, in which β-galactosidase (β-gal) is expressed by all endothelial cells, we could evaluate the extent of neovascularization in response to ischemia.38 Figure 2A shows the vascular anatomy of the undersurface of skin in transgenic mice (whole mounts). Prior to surgical elevation, the majority of visible vessels were oriented in a transverse fashion along dorsal surface of skin. Following induction of ischemia, there was a significant neovascular response, highlighted by the dramatic increase in β-gal intensity in these mice after 14 days (Figure 2B). Similar degrees of neovascularization were noted at the microvascular level, evidenced by β-gal antibody staining in histologic sections (Figure 2C-E). Neovascularization was most prevalent in the regions of ischemia and was less prominent in the surrounding incisions. In vivo lectin staining was also performed on animals, and histologic analysis demonstrated increased vessels within regions of ischemia (Figure 2F-H). Notably, the creation of an ischemic gradient led to a change in the orientation of visible vessels: from perpendicular to parallel to the direction of the ischemia. This directional change in large-caliber vessels in a direction toward the region of maximal ischemia was evident by 14 days.
Ischemia-induced neovascularization in tie2/lacZ mice. The ischemia model was studied in tie2/lacZ mice, in which β-galactosidase (blue staining) is expressed by all endothelial cells. (A) (B) Gross sections of the undersurface of mouse skin stained with X-gal are shown prior to and 14 days following ischemic surgery, respectively. Note the central zone of necrosis that could easily be demarcated from ischemic, but viable, tissue (arrow). (C) (D) (E) (F) (G) Histologic sections were stained for β-galactosidase (red) and demonstrated increasing microvessel density within ischemic regions at day 7 (blue indicates DAPI nuclei staining) (panels C-E). A subset of animals were injected with fluorescent lectin also (red) prior to humane killing to highlight functionally perfused vessels (panels F-H). As illustrated at day 14, neovessels formed in a direction along the gradient of ischemia toward the central portion of necrosis. Scale bars = 50 μm.
Ischemia-induced neovascularization in tie2/lacZ mice. The ischemia model was studied in tie2/lacZ mice, in which β-galactosidase (blue staining) is expressed by all endothelial cells. (A) (B) Gross sections of the undersurface of mouse skin stained with X-gal are shown prior to and 14 days following ischemic surgery, respectively. Note the central zone of necrosis that could easily be demarcated from ischemic, but viable, tissue (arrow). (C) (D) (E) (F) (G) Histologic sections were stained for β-galactosidase (red) and demonstrated increasing microvessel density within ischemic regions at day 7 (blue indicates DAPI nuclei staining) (panels C-E). A subset of animals were injected with fluorescent lectin also (red) prior to humane killing to highlight functionally perfused vessels (panels F-H). As illustrated at day 14, neovessels formed in a direction along the gradient of ischemia toward the central portion of necrosis. Scale bars = 50 μm.
Evidence of in situ vasculogenesis in ischemic tissue
In tie2/lacZ mice, β-galactosidase is under the control of the tie2 promoter, which is expressed by all endothelial cells (ie, resident and bone marrow derived), thus providing a useful tool for the study of vascular development. To limit our analysis to the contribution from bone marrow (ie, vasculogenesis), we established a bone marrow transplantation model in which bone marrow–derived EPCs could be identified by either lacZ or GFP expression (Figure 3A). After confirming the complete reconstitution of the bone marrow in a subset of mice (Figure 3B), we applied our ischemia model to transplant-recipient mice. Gross examination of tissue from tie2/lacZ+ bone marrow mice revealed intense β-gal staining that was most predominant in the ischemic zones (Figure 3C). The β-gal intensity was greatest near the region of necrosis and appeared to correlate with the degree of ischemia, with no gross staining evident in nonischemic tissue. The β-gal expression in the BM transplant–recipient mice was comparable to that seen in the initial experiments with transgenic mice (Figure 3C, right panel; versus Figure 2B), suggesting that recruitment of bone marrow cells is an important mechanism of neovascularization in ischemic tissues. The β-gal staining from bone marrow–derived EPCs was not seen in the nonischemic incisions of transplant-recipient mice or in tissue from negative controls (mice receiving transplants of wild-type bone marrow) (Figure 3C, right). Excisional wounds in tie2/lacZ+ bone marrow mice lacked gross evidence of lacZ expression at days 3, 7, and 14 (Figure 3D), suggesting that ischemia, not wounding/inflammation, is the critical determinant regulating EPC recruitment.
Contribution of bone marrow–derived EPCs to ischemia-induced neovascularization. (A) Bone marrow (BM) transplantation mice were established to assess the contribution of vasculogenesis to ischemia-induced neovascularization. In this model, BM-derived cells or EPCs can be identified by GFP or lacZ staining, respectively. A representative lacZ histologic staining in the BM transplant–recipient animals is shown (right panel). (B) FACS analysis of the bone marrow in mice receiving transplants of cells from GFP-expressing transgenic mice revealed successful reconstitution of the bone marrow at 4 weeks. The histogram on the right shows an overlay of wild-type (shaded gray), transgenic GFP (black line), and bone marrow transplant–recipient mice (green line). (C) Application of the ischemia model to mice with tie2/lacZ bone marrow showed intense β-gal expression in severely ischemic regions (left panel), while no such expression was noted in control mice receiving transplants of wild-type bone marrow (right panel). (D) Mice that had received tie2/lacZ transplants and that had undergone excisional wounding, rather than ischemic surgery, demonstrated no gross evidence of lacZ staining; tissue at 14 days after wounding is shown.
Contribution of bone marrow–derived EPCs to ischemia-induced neovascularization. (A) Bone marrow (BM) transplantation mice were established to assess the contribution of vasculogenesis to ischemia-induced neovascularization. In this model, BM-derived cells or EPCs can be identified by GFP or lacZ staining, respectively. A representative lacZ histologic staining in the BM transplant–recipient animals is shown (right panel). (B) FACS analysis of the bone marrow in mice receiving transplants of cells from GFP-expressing transgenic mice revealed successful reconstitution of the bone marrow at 4 weeks. The histogram on the right shows an overlay of wild-type (shaded gray), transgenic GFP (black line), and bone marrow transplant–recipient mice (green line). (C) Application of the ischemia model to mice with tie2/lacZ bone marrow showed intense β-gal expression in severely ischemic regions (left panel), while no such expression was noted in control mice receiving transplants of wild-type bone marrow (right panel). (D) Mice that had received tie2/lacZ transplants and that had undergone excisional wounding, rather than ischemic surgery, demonstrated no gross evidence of lacZ staining; tissue at 14 days after wounding is shown.
EPCs are preferentially recruited to ischemic tissue
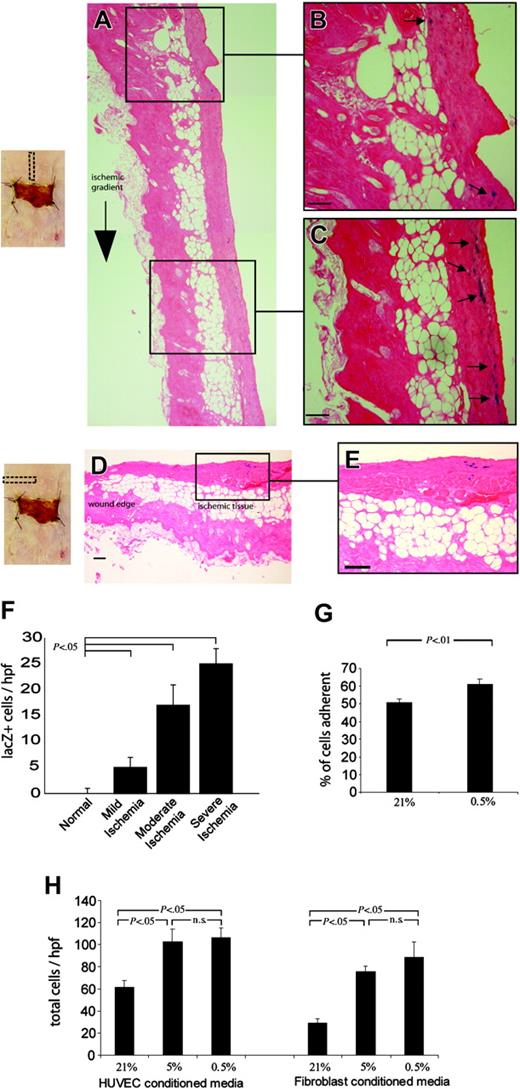
Histologic analysis of sections along the axis of ischemia confirmed that there was preferential recruitment of EPCs toward severely ischemic zones (Figure 4A-C) but not to other wounded areas (Figure 4D-E). The number of lacZ+ EPCs within the tissue correlated inversely with the degree of ischemia: 5 ± 2 cells per high-power field (hpf) in mild ischemia, 17 ± 4 cells per hpf in moderate ischemia, and 25 ± 4 in severe ischemia (P < .05) (Figure 4F). One possible mechanism for recruitment of circulating EPCs to the ischemic tissue is via hypoxia-mediated alteration of the vascular endothelium. Prior studies have demonstrated that hypoxia plays an important role in regulating the adhesion of circulating cells, such as leukocytes, to the luminal endothelium,39-41 but whether hypoxia plays an analogous role in the arrest of circulating EPCs is not known. To investigate this, we studied the ability of human EPCs (hEPCs) to adhere to HUVEC monolayers exposed to 21% and 0.5% O2. The percentage of EPCs that adhered to the monolayer was significantly increased when endothelial monolayers had been exposed to severe hypoxia (61.4% ± 2.8% of cells) versus normal conditions (50.9% ± 1.9% of cells; P < .01) (Figure 4G). To determine whether cells within ischemic tissue generate signals that lead to EPC egress, we performed EPC chemotaxis assays using conditioned media from HUVECs and fibroblasts cultured in hypoxic conditions. The migration of EPCs was significantly greater toward HUVEC media conditioned at 0.5% relative to media from cultures at 21% O2 (106 ± 8 cells per hpf versus 62 ± 6 cells per hpf; P < .05). Likewise, hEPC migration toward conditioned media from hypoxic fibroblasts was increased 2.5-fold (89 ± 14 cells per hpf versus 29 ± 4 cells per hpf; P < .05) (Figure 4H).
Selective homing of EPCs to ischemic tissue. Bone marrow–derived EPCs, identified by lacZ expression, were noted to be preferentially recruited to zones of severe ischemia. (A) (B) (C) (D) (E) Histologic sections taken along the axis of ischemia at day 7 are shown (panel A), with close-up views of the nonischemic (panel B) and ischemic (panel C) portions of the skin; lacZ+ EPCs stain blue and are highlighted with arrows. Transverse sections taken from the central portion of ischemic tissue at day 7 (panel D) reveal a considerable number of lacZ+ EPCs within the ischemic (panel E), but not incised, skin; (scale bars = 50 μm). (F) The number of lacZ+ EPCs recruited to regions of ischemia at day 7 was found to correlate inversely with the degree of ischemia; tissue sections were divided into zones of mild, moderate, and severe ischemia. (G) Human EPCs were then studied under hypoxic conditions in vitro and were noted to be significantly more adherent to endothelial monolayers exposed to 0.5% O2. (H) EPC chemotaxis was also studied by harvesting media from hypoxic cultures of endothelial cells and fibroblasts, and EPC migration toward hypoxic media (0.5% O2) from both cell types was significantly increased.
Selective homing of EPCs to ischemic tissue. Bone marrow–derived EPCs, identified by lacZ expression, were noted to be preferentially recruited to zones of severe ischemia. (A) (B) (C) (D) (E) Histologic sections taken along the axis of ischemia at day 7 are shown (panel A), with close-up views of the nonischemic (panel B) and ischemic (panel C) portions of the skin; lacZ+ EPCs stain blue and are highlighted with arrows. Transverse sections taken from the central portion of ischemic tissue at day 7 (panel D) reveal a considerable number of lacZ+ EPCs within the ischemic (panel E), but not incised, skin; (scale bars = 50 μm). (F) The number of lacZ+ EPCs recruited to regions of ischemia at day 7 was found to correlate inversely with the degree of ischemia; tissue sections were divided into zones of mild, moderate, and severe ischemia. (G) Human EPCs were then studied under hypoxic conditions in vitro and were noted to be significantly more adherent to endothelial monolayers exposed to 0.5% O2. (H) EPC chemotaxis was also studied by harvesting media from hypoxic cultures of endothelial cells and fibroblasts, and EPC migration toward hypoxic media (0.5% O2) from both cell types was significantly increased.
Hypoxia stimulates EPC cluster formation and proliferation
Magnified views of whole mounted tissue sections from tie2/lacZ+ bone marrow mice confirmed the existence of a number of lacZ+ EPCs in the ischemic tissue. Figure 5A shows a magnified view of the central ischemic zone, which demonstrates EPC cluster formation reminiscent of embryologic blood island formation.42 Notably, these clusters were in the interstitial space and not associated with existing vessels as defined by CD31 staining (data not shown). EPCs could also be seen in the ischemic regions nearest to the lines of surgical incisions, but there was no cluster formation in these areas (Figure 5B). Similarly, there was no evidence of cluster formation in histologic sections from animals that had undergone punch biopsies (data not shown). The first histologic evidence of EPC cluster formation appeared at 7 days following ischemia (Figure 5C); arrows point to costaining cells. During this time, both EPC clusters and individual foci of cells were noted in the tissue. The majority of lacZ+ cells costained with Ki67, thus suggesting that they were actively proliferating in the ischemic zones (Figure 5D). To determine whether hypoxia alone could account for the proliferation of EPCs, we exposed hEPCs to varying O2 concentrations. A thymidine-incorporation assay demonstrated enhanced proliferation of hEPCs at reduced O2 levels compared with normal oxygen tensions (2126 ± 355 cpm at 0.5% [P < .01] versus 429 ± 49 cpm at 21% O2) (Figure 5E). Identical experiments using HUVECs demonstrated significantly decreased HUVEC proliferation at 0.5% O2 relative to 21% O2 (63 ± 8 × 102 versus 86 ± 6 × 102 cpm, respectively; P < .05) (Figure 5F).
EPC cluster formation, enhanced proliferation, and elevated levels of VEGF protein in hypoxic conditions. (A) A magnified view of the central ischemic zone with EPC cluster formation reminiscent of embryologic blood island formation. (B) In contrast, a decrease of such cluster formation was seen along the nonischemic wounds. (C) (D) EPC cluster formation typically appeared at 1 week following ischemia and costained with proliferative marker Ki67 (panel D); positive staining (DAB) appears brown, and arrows point to costaining cells. (E) Exposure of human EPCs to varying O2 concentrations in vitro demonstrated enhanced proliferation at reduced O2 levels; representative pictures of DAPI-stained EPCs are shown on the right. (F) In comparison to EPCs, HUVECs showed significantly decreased proliferation at 0.5% O2. (G) The increase in circulating EPC levels also correlated with an increase in VEGF protein levels. (H) Circulating mouse MNCs were isolated at the time of killing and analyzed with FACS for flk1+/CD11b–, markers previously demonstrated to represent circulating EPCs. A significant increase in cell number was noted at 7 days following ischemic surgery.
EPC cluster formation, enhanced proliferation, and elevated levels of VEGF protein in hypoxic conditions. (A) A magnified view of the central ischemic zone with EPC cluster formation reminiscent of embryologic blood island formation. (B) In contrast, a decrease of such cluster formation was seen along the nonischemic wounds. (C) (D) EPC cluster formation typically appeared at 1 week following ischemia and costained with proliferative marker Ki67 (panel D); positive staining (DAB) appears brown, and arrows point to costaining cells. (E) Exposure of human EPCs to varying O2 concentrations in vitro demonstrated enhanced proliferation at reduced O2 levels; representative pictures of DAPI-stained EPCs are shown on the right. (F) In comparison to EPCs, HUVECs showed significantly decreased proliferation at 0.5% O2. (G) The increase in circulating EPC levels also correlated with an increase in VEGF protein levels. (H) Circulating mouse MNCs were isolated at the time of killing and analyzed with FACS for flk1+/CD11b–, markers previously demonstrated to represent circulating EPCs. A significant increase in cell number was noted at 7 days following ischemic surgery.
Ischemia leads to increased levels of VEGF and circulating flk1+/CD11b– cells
Previous studies have shown that VEGF administration leads to EPC mobilization.32,37,43,44 To determine whether VEGF played a physiologic role in the observed recruitment of bone marrow–derived EPCs, we assayed circulating VEGF levels after ischemic injury and found a significant elevation in circulating VEGF levels at day 7 relative to baseline values (0.0560 ± 0.010 ng/mL at day 7 versus 0.0208 ± 0.005 at day 0; P < .01) (Figure 5G). FACS analysis of circulating MNCs revealed that the increased VEGF levels in response to ischemia were associated with mobilization of EPCs, defined as flk1+/CD11b– cells (Figure 5H).
Overall time course of vessel formation
We observed that bone marrow–derived EPCs were recruited to ischemic tissue as early as day 3, with an increasing number of EPCs at 1 week following surgery (Figure 6A). As noted, the lacZ+ cells found in the tissue at 7 days were either individual foci or cellular clusters, but did not initially appear as vascular structures and were not associated with existing vessels (Figure 6B,D). Costaining of tie2/lacZ+ cells with VWF confirmed the endothelial phenotype of lacZ-expressing cells in the tissue; day 7 is shown (Figure 6E). By day 14, lacZ+ EPCs formed cordlike structures (Figure 6C). In contrast to previously published reports demonstrating only mosaic vessels composed of pre-existing endothelial cells with intermittent incorporation of EPCs, our experiments resulted in vessels derived entirely from EPCs (Figure 6F). Notably, bone marrow–derived vessels were predominantly aligned in the direction of the ischemic gradient (relative to the more random alignment of vessels found in nonischemic tissue), further lending support to the notion that the neovessels were formed by EPCs in response to ischemic gradients. Additionally, the costaining at day 14 with lacZ and VWF confirmed not only that the BM-derived lacZ+ cells are of the endothelial lineage, but also that within the same graft there are vessels fully formed of EPCs while others are completely devoid of BM-derived cells (Figure 6G). The identification of endothelial cells and BM-derived EPCs with antibodies against CD31 and β-gal, respectively, provided additional evidence of costained vessels (Figure 6H). To further demonstrate that the lacZ cells formed functional vessels with blood flow, in vivo staining was performed by injecting FITC-lectin prior to humane killing. Colocalization of tie2/lacZ+ structures with the in vivo lectin stain confirmed that the EPC-derived vessels were indeed functional (ie, perfusing) at day 14 (Figure 6I-J).
Coalescence of lacZ+EPCs into functional blood vessels. (A) A histologic view of recruitment of lacZ+ bone marrow–derived EPCs to ischemic tissue starting as early as day 3 after surgery. (B) LacZ+ cells in the tissue at day 7 were either individual foci or cellular clusters, but did not appear as vascular structures and were not associated with vessels by CD31 staining. (C) By day 14, lacZ+ EPCs began to form a number of vascular-like structures. (D) Magnified histologic section at day 7 demonstrating the presence of both EPC clusters (thick arrow) and individual foci of EPCs (thin arrows). (E) Costaining of tie2/lacZ+ cell clusters with VWF confirmed the endothelial phenotype of lacZ-expressing cells in the tissue. (F) Day-14 histologic section demonstrating a fully formed bone marrow–derived lacZ+ blood vessel seen in cross-section. (G) Costaining of a tie2/lacZ+ vascular structure with VWF not only confirming the endothelial phenotype of lacZ-expressing blood vessels in the day-14 graft but clearly demonstrating 2 distinct blood vessels: on the left (thick arrow), a vessel composed entirely of EPCs (in blue), and on the right (thin arrow), a vessel that is not derived from EPCs. (H) Identification of EPCs and endothelial cells with antibodies against CD31 and β-gal, respectively, yielded similar findings, as shown in a high-power view of cells costaining for CD31+ (green) and β-gal (red). (I) (J) Colocalization of tie2/lacZ+ structures with the in vivo FITC-lectin stain, seen histologically and under fluorescent microscope, show perfusing vessels at day 14. Scale bar = 20 μm.
Coalescence of lacZ+EPCs into functional blood vessels. (A) A histologic view of recruitment of lacZ+ bone marrow–derived EPCs to ischemic tissue starting as early as day 3 after surgery. (B) LacZ+ cells in the tissue at day 7 were either individual foci or cellular clusters, but did not appear as vascular structures and were not associated with vessels by CD31 staining. (C) By day 14, lacZ+ EPCs began to form a number of vascular-like structures. (D) Magnified histologic section at day 7 demonstrating the presence of both EPC clusters (thick arrow) and individual foci of EPCs (thin arrows). (E) Costaining of tie2/lacZ+ cell clusters with VWF confirmed the endothelial phenotype of lacZ-expressing cells in the tissue. (F) Day-14 histologic section demonstrating a fully formed bone marrow–derived lacZ+ blood vessel seen in cross-section. (G) Costaining of a tie2/lacZ+ vascular structure with VWF not only confirming the endothelial phenotype of lacZ-expressing blood vessels in the day-14 graft but clearly demonstrating 2 distinct blood vessels: on the left (thick arrow), a vessel composed entirely of EPCs (in blue), and on the right (thin arrow), a vessel that is not derived from EPCs. (H) Identification of EPCs and endothelial cells with antibodies against CD31 and β-gal, respectively, yielded similar findings, as shown in a high-power view of cells costaining for CD31+ (green) and β-gal (red). (I) (J) Colocalization of tie2/lacZ+ structures with the in vivo FITC-lectin stain, seen histologically and under fluorescent microscope, show perfusing vessels at day 14. Scale bar = 20 μm.
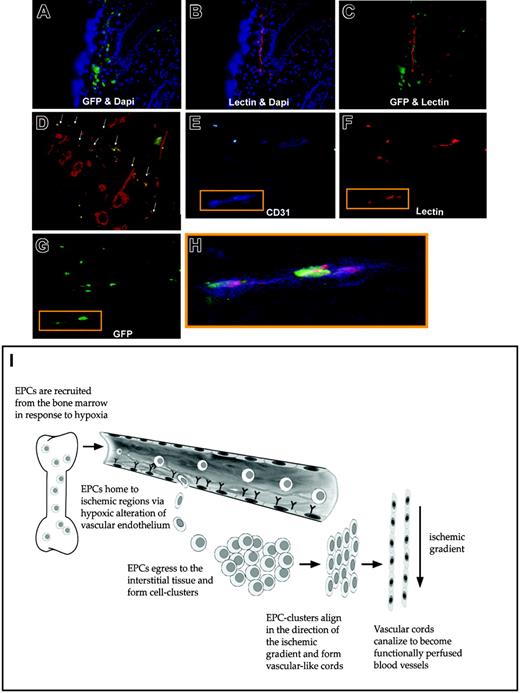
We further confirmed these findings in a model in which all bone marrow–derived cells are labeled with GFP, rather than expression being limited to tie2-expressing cells. Figure 7A-G demonstrates the contribution of GFP+ bone marrow cells to neovascularization within ischemic tissue at day 14. Again, GFP+ bone marrow–derived cells were recruited to ischemic tissue and formed vascular-like structures (Figure 7A). Lectin (red) staining again highlighted the functionality of these neovessels within the tissue (Figure 7B) and their orientation in the plane of ischemia. Analysis of the tissue sections under both green and red fluorescent windows confirmed that new vessels were lined by bone marrow–derived GFP+ cells (Figure 7C). Tissue sections taken along the plane of ischemia similarly demonstrated lectin-perfused vessels that were derived from BM-cells (ie, GFP+) (Figure 7D). Of note, BM-derived cells also exhibited staining for CD31 (blue) and lectin (red) (Figure 7D-G).
Differentiation of bone marrow–derived GFP+cells into functional endothelial cells with a schematic of ischemia-induced vasculogenesis. A second bone marrow transplantation model was employed, in which mice were reconstituted with bone marrow cells ubiquitously expressing GFP+ (ie, not restricted to tie-2+ cells). Similar findings of bone marrow progenitor involvement during ischemic neovascularization were noted in these mice. (A) GFP+ (green) bone marrow–derived cells were recruited to ischemic tissue and formed vascular-like structures by day 14; blue staining (DAPI) represents all cell nuclei in the tissue. (B) Lectin (red) staining highlighted functional neovessels within the tissue oriented in the plane of ischemia. (C) Analysis of the tissue sections under multiple fluorescent windows identified vessels lined by bone marrow–derived EPCs. (D) Tissue sections were also taken perpendicular to the plane of ischemia, which similarly identified regions of costaining of lectin-perfused blood vessels (red) and BM-derived EPCs (green); arrows point to costained vessels, which appear orange. (E) (F) (G) GFP+ cells that lined blood vessels exhibited triple staining for CD31 (blue), lectin (red), and GFP (green). (H) An overlay of the triple staining is shown. (I) Schematic representation of the proposed mechanism by which EPCs contribute to neovascularization within ischemic tissue. Tissue ischemia causes the release of growth factors/cytokines (including VEGF), leading to a systemic response and the mobilization of BM-derived EPCs. Hypoxic conditions alter the vascular endothelium, causing circulating EPCs' arrest and egress into the tissue. Once in the interstitium, EPCs form cellular clusters and proliferate to increase the pool of cells available for neovascularization. Gradients of ischemia drive the formation of vascular cords in the direction of relatively hypoxic regions. Vascular cords tubulize and unite with existing vasculature, leading to increased tissue perfusion.
Differentiation of bone marrow–derived GFP+cells into functional endothelial cells with a schematic of ischemia-induced vasculogenesis. A second bone marrow transplantation model was employed, in which mice were reconstituted with bone marrow cells ubiquitously expressing GFP+ (ie, not restricted to tie-2+ cells). Similar findings of bone marrow progenitor involvement during ischemic neovascularization were noted in these mice. (A) GFP+ (green) bone marrow–derived cells were recruited to ischemic tissue and formed vascular-like structures by day 14; blue staining (DAPI) represents all cell nuclei in the tissue. (B) Lectin (red) staining highlighted functional neovessels within the tissue oriented in the plane of ischemia. (C) Analysis of the tissue sections under multiple fluorescent windows identified vessels lined by bone marrow–derived EPCs. (D) Tissue sections were also taken perpendicular to the plane of ischemia, which similarly identified regions of costaining of lectin-perfused blood vessels (red) and BM-derived EPCs (green); arrows point to costained vessels, which appear orange. (E) (F) (G) GFP+ cells that lined blood vessels exhibited triple staining for CD31 (blue), lectin (red), and GFP (green). (H) An overlay of the triple staining is shown. (I) Schematic representation of the proposed mechanism by which EPCs contribute to neovascularization within ischemic tissue. Tissue ischemia causes the release of growth factors/cytokines (including VEGF), leading to a systemic response and the mobilization of BM-derived EPCs. Hypoxic conditions alter the vascular endothelium, causing circulating EPCs' arrest and egress into the tissue. Once in the interstitium, EPCs form cellular clusters and proliferate to increase the pool of cells available for neovascularization. Gradients of ischemia drive the formation of vascular cords in the direction of relatively hypoxic regions. Vascular cords tubulize and unite with existing vasculature, leading to increased tissue perfusion.
Discussion
Adult neovascularization has traditionally been thought to be limited to angiogenesis, which can be defined as the sprouting of vessels from pre-existing endothelial cells. Prior research has shown that this process is mediated by the release of angiogenic growth factors such as VEGF, platelet-derived growth factor (PDGF), nitric oxide, and fibroblast growth factor (FGF).45,46 These factors initiate local changes, including vasodilation and increased vascular permeability, the activation of resident endothelial cells, and degradation of the basement membrane. These cytokines/growth factors also stimulate endothelial cell migration and proliferation and the formation of capillary sprouts that ultimately lead to restored perfusion.47
However, recent advances in vascular biology have led scientists to revisit this conventional picture of angiogenesis.8 Studies using experimental models that rely on ligation of a major artery (femoral artery, coronary artery, middle cerebral artery) have suggested that endothelial progenitor cells (EPCs) may also participate in neovascularization during ischemic conditions.11,18,48 While these and other studies have demonstrated the scattered presence of EPCs in tissues undergoing neovascularization, they have failed to elucidate a putative mechanism by which circulating cells could contribute to new blood vessel growth in ischemic tissue. Some researchers believe that EPCs serve as building blocks for neovascularization, while others suggest that their primary role may be to deliver angiogenic growth factors to ischemic tissue.10,49 Further controversy remains as to whether any direct EPC contribution occurs through the established paradigm of angiogenesis or through a different process.22 The term vasculogenesis, defined as the potential differentiation of circulating endothelial precursors in situ, has been used to describe EPC contributions to blood vessel growth.8 However, while vasculogenesis and angiogenesis have been demonstrated to be independent processes during embryologic vascular development,42,50 there has been no direct evidence for true in situ vasculogenesis in adult physiology.
In the present study, we use a novel model of ischemia that is technically simple, allows for easy visualization of neovascularization, and creates a distinct, 2-dimensional gradient of ischemia. In contrast to previous reports of intermittent incorporation of bone marrow cells within ischemic tissue, we demonstrate functional vessels derived entirely from bone marrow–derived EPCs. Thus, we provide the first evidence that adult vasculogenesis is a distinct process and propose a potential mechanism by which bone marrow–derived cells are able to produce new blood vessels in response to ischemia (Figure 7I).
We demonstrate that postnatal vasculogenesis is initiated primarily as a response to local tissue ischemia. Ischemia releases growth factors/cytokines, such as VEGF, which subsequently mobilize EPCs from the bone marrow. Local tissue hypoxia alters the vascular endothelium in ischemic tissue to arrest EPCs in regions where neovascularization is needed. Adherent EPCs egress into tissues and are themselves exposed to local tissue hypoxia for the first time. Over the next few days, these hypoxic conditions stimulate EPC proliferation and the organization of cell clusters. After 14 days of injury, the increasing pool of EPCs form cordlike vascular structures. Soon after, vascular cords newly formed from bone marrow–derived cells canalize and connect to existing vasculature (through a process that remains unclear). By 2 weeks following injury, there is evidence of functional microvessels increasing perfusion in the injured tissue.
As detailed in Figure 7I, this sequence of events more closely resembles the inflammatory cascade than current models of angiogenesis. The initial hypoxia-mediated arrest of EPCs in ischemic microvessels may be similarly mediated by cell-surface vascular adhesion molecules or selectins known to be involved in the inflammatory response. In vitro, we observed that EPC adhesion was significantly increased in hypoxic endothelium (0.5% O2). It has been demonstrated that discrete cell-surface markers (ie, αvβ3 and E-selectin) are up-regulated in endothelial cells exposed to severe hypoxia.51-53 These or similarly functioning molecules are potential candidates for the selective homing of EPCs to ischemic tissue.
For adherent cells to gain access to the interstitium, they must migrate from the circulation into the tissue. Our studies demonstrate that tissue ischemia creates signals that promote the migration of arrested EPCs into ischemic tissue. The in vitro migration assay demonstrates that media harvested from fibroblasts and HUVECs stimulate EPC chemotaxis. This suggests that local hypoxia releases a soluble factor, or multiple soluble factors, that act as a chemoattractant for circulating EPCs. One likely candidate is VEGF, which has previously been shown to be locally elevated in response to hypoxia.54 Moreover, numerous experiments have demonstrated a direct role of VEGF in the mobilization of EPCs. Asahara et al32 have shown that systemic VEGF administration leads to EPC mobilization in an animal model of hind-limb ischemia. Human clinical experiments have shown similar effects of VEGF on mobilizing EPCs in human patients.37 The finding of elevated VEGF levels in our experimental animal model suggests that VEGF is likely to be playing a role in the ischemia-induced neovascularization observed in the present study.
Following the arrest and migration of EPCs to the ischemic interstitium, proliferation occurs, increasing the number of endothelial-lineage cells within the tissue. In vivo, BM-derived EPCs in ischemic regions formed cellular clusters and stained positive for proliferative marker Ki67 by day 7, and in vitro data demonstrated a significant increase in EPC proliferation at 0.5% O2. Conversely, HUVEC proliferation is significantly decreased at this level of profound hypoxia. The ability of EPCs, but not mature endothelial cells, to proliferate in severely hypoxic conditions suggests a pivotal role for EPCs as the major building block for neovessels within ischemic tissue. In support of the critical role of EPCs in ischemia-induced neovascularization is the observation that these cells appear critical for tumor growth,15 a condition largely characterized by profound hypoxia.55
Finally, we demonstrate that the gradient of hypoxia/ischemia ultimately directs EPCs to coalesce into independent vascular structures to restore tissue perfusion in the ischemic region. Although bone marrow–derived EPCs were initially found to have migrated into the interstitium of ischemic tissue and were not associated with capillaries or venules, cells began to coalesce into vascular cords by 2 weeks following injury. This process did not occur in a random fashion, but rather neovessels formed in an organized manner along the ischemic gradient toward the most severely ischemic regions. It is known that hypoxia directs blood vessel formation during embryologic vascular development,56 but this has not been previously demonstrated to occur during adult vascular growth.
Another finding suggestive of a recapitulation of the embryologic mechanism of vasculogenesis is the prevalence of EPC clusters in the regions of severe ischemia. It has previously been shown that during embryologic vasculogenesis, endothelial precursor cells (flk-1+ angioblasts) organize into clusters and extend projections that ultimately form a vascular network.42,50 Asahara et al6 described a potential in vitro correlate of embryologic blood islands in their initial paper reporting the discovery of human EPCs. In that study, cultures of mononuclear blood cells led to the formation of CD34+ cell clusters, consisting of a center of rounded cells surrounded by spindle-shaped cells at the periphery. The present study is the first, to our knowledge, to demonstrate EPC clusters in vivo, which were limited to the most severely ischemic regions of the tissue.
Recent studies using bone marrow–derived stem cells suggest that various phenotypes arise as the result of spontaneous cell fusion rather than the differentiation of progenitor cells.57,58 This raises the question of whether cell fusion could explain the results of this study. Given the rare occurrence of fusion in those studies (2 to 11 fusion events per 106 BM cells) and the high frequency of EPC involvement here, it seems unlikely that cell fusion could account for the volume of bone marrow–derived vessels observed. In support of this conclusion is recent work by Bailey et al59 demonstrating by ploidy analysis that the incorporation of bone marrow–derived cells into blood vessels was not the result of cell fusion during normal endothelial cell turnover. In addition, since hematopoietic stem cells are known to give rise to endothelial and hematopoietic lineage cells,60-63 it seems plausible that circulating hemangioblasts could differentiate into mature endothelial cells in peripheral tissues.
Our elucidation of a mechanism through which circulating EPCs contribute to ischemia-induced blood vessel growth has important diagnostic and therapeutic implications. Studies by our group and others have suggested that EPCs could be used as a cellular marker for vascular dysfunction.21,22,36 It has been proposed that these cells could be used to assess a patient's risk of poor outcome following vascular occlusion. However, before we can rationally perform meaningful functional assays, it is necessary to understand how these cells function in vivo. The present study demonstrates for the first time that essential EPC functions include cell proliferation, migration, and cluster formation, because these are important cellular actions during the proposed mechanism for in situ vasculogenesis.
Our findings also provide valuable information regarding current attempts to augment new blood vessel growth using therapeutic vasculogenesis. Over the past few decades, there has been a great deal of interest in developing techniques to promote neovascularization in ischemic disease. Most studies have examined the delivery of proangiogenic agents, many in ongoing clinical trials.64 A new modality for augmenting blood vessel growth is through EPC transplantation, which has been examined in both animals and humans,8,20 despite a limited understanding of the mechanism by which EPCs form new vessels. Our study demonstrates that hypoxia and ischemic gradients are necessary for EPCs to form independent vascular structures and suggests that timing is critical to the success of these cell-based therapies. Aicher et al65 recently demonstrated that only a small percentage (2%) of systemically delivered EPCs localize to ischemic myocardium. We believe that future experiments focusing on the molecular mediators of progenitor cell trafficking to ischemic vascular endothelium will yield productive data to increase in the homing ability of EPCs. In addition, the direct effects of hypoxia on both migration and proliferation suggest the utility of hypoxia-priming of EPCs ex vivo prior to transplantation.66
The present study used a unique ischemia model to demonstrate that bone marrow–derived EPCs play an important role in ischemia-induced neovascularization. Hypoxia is revealed as the major stimulus of EPC function, initiating a cascade of cellular events bringing progenitor cells to ischemic tissue, and resulting in the formation of neovessels constructed entirely of bone marrow–derived cells. Together, these data suggest that in situ vasculogenesis occurs in the postnatal period, and does so through a process different from existing paradigms of angiogenesis.
Prepublished online as Blood First Edition Paper, September 23, 2004; DOI 10.1182/blood-2004-03-1051.
This study was supported in part by a grant from the Juvenile Diabetes Research Foundation (O.M.T.) and by National Institutes of Health grants EB-02265 and AG-25016 (G.C.G.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal