Abstract
Motexafin gadolinium (MGd), an expanded porphyrin, is a tumor-selective redox-mediator that reacts with many intracellular reducing metabolites. Because redox mechanisms mediate apoptosis in multiple myeloma, we hypothesized that disruption of redox balance by MGd would result in cellular cytotoxicity in myeloma. We examined the effects of MGd on cellular cytotoxicity, apoptosis, reactive oxygen species (ROS) production, and intracellular drug uptake in dexamethasone-sensitive (C2E3), dexamethasone-resistant (1-310 and 1-414) chemotherapy-sensitive (8226-RPMI) and highly chemotherapy-resistant (DOX-10V) myeloma cells. We found complete inhibition of proliferation and cytotoxicity in each sensitive and resistant cell line with 24-hour exposure to clinically relevant concentrations of 50 μM MGd and 50 to 100 μM ascorbate, which was required for the effect. The mechanism of cytotoxicity was related to induction of apoptosis as demonstrated by alteration in mitochondrial membrane potential and elevated annexin V expression. This was accompanied by depletion of intracellular glutathione and increased ROS production. Moreover, catalase substantially abrogated MGd-induced cell death. Using fluorescence microscopy and flow cytometry, we found intracellular uptake of MGd and intracellular ROS production. MGd also induced apoptosis in fresh malignant cells from patients with multiple myeloma. These studies provide a rationale for clinical investigation of this novel redox-mediating agent in patients with multiple myeloma and related disorders.
Introduction
Intricate pathways that are important for the survival of multiple myeloma cells have been identified.1-5 The mitochondria are involved in many of these pathways and function as a critical mediator of cell survival and apoptosis.2,3,6 Mitochondria are the site of cellular respiration, and through that process, energy is produced through electron transfer reactions. Reactive oxygen species (ROSs) are often generated as a by-product of these reactions.3,7,8 ROSs are neutralized by intracellular antioxidant metabolites, such as glutathione (GSH), and can be degraded by enzymes such as catalase and peroxidases. We hypothesized that disruption of redox balance in the tumor cell is an attractive target for the treatment of multiple myeloma.9
Motexafin gadolinium (MGd), an expanded porphyrin, is a tumor-selective redox mediator that reacts with many intracellular reducing metabolites.10-12 MGd displays catalytic activity by accepting electrons from compounds that possess a sufficiently negative reduction potential. In the presence of oxygen, such electron transfer results in superoxide formation and regenerates MGd in a process known as futile redox cycling. Superoxide anion, in turn, disproportionates under biologic conditions to form oxygen and hydrogen peroxide.13-15
Our prior observations on redox biology in multiple myeloma motivated us to study the biologic activity of MGd in sensitive and resistant multiple myeloma cell lines.3,16 We found that MGd induces a pro-oxidant state leading to significant cytotoxicity and apoptosis in dexamethasone- and chemotherapy-sensitive and -resistant multiple myeloma cell lines, that MGd enters the cell and generates ROSs, and that the cytotoxicity can be reversed with catalase. These studies introduce a new paradigm for the treatment of multiple myeloma and other hematologic malignancies.
Materials and methods
Cell lines
We studied dexamethasone-sensitive, dexamethasone-resistant, highly dexamethasone-resistant, chemotherapy-sensitive, and highly chemotherapy-resistant myeloma cell lines (Table 1). The dexamethasone-resistant cell lines were derived from the peripheral blood of a patient with multiple myeloma who developed resistance to glucocorticoid therapy.17 Two subclones, 1-310 and 1-414, were isolated from C2E3 cells (sensitive to micromolar concentrations of dexamethasone). Both of these subclones have no measurable expression of glucocorticoid receptor and are resistant and highly resistant to dexamethasone, respectively.18 The chemotherapy-sensitive and -resistant cell lines, kindly provided by Dr William Dalton (H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL), included the following: 8226-RPMI (parental line) and 8226-MDR10V (or DOX-10V), derived from 8226-Dox40 cells through selection with verapamil. This is the most drug-resistant myeloma cell line.19,20 All cell lines were grown in RPMI 1640 with 10% heat-inactivated fetal calf serum (FCS). Cells were counted using a Model Z2 instrument (Beckman-Coulter, Miami, FL) as indicated.
Multiple myeloma cell lines
Cell line description . | Cell line . |
|---|---|
| Dexamethasone-sensitive | C2E3 |
| Dexamethasone-resistant | 1-310 |
| Highly dexamethasone-resistant | 1-414 |
| Chemotherapy-sensitive | 8226-RPMI |
| Highly chemotherapy-resistant | DOX-10V |
Cell line description . | Cell line . |
|---|---|
| Dexamethasone-sensitive | C2E3 |
| Dexamethasone-resistant | 1-310 |
| Highly dexamethasone-resistant | 1-414 |
| Chemotherapy-sensitive | 8226-RPMI |
| Highly chemotherapy-resistant | DOX-10V |
Normal peripheral blood lymphocytes
Buffy coat was prepared at the Stanford Medical School Blood Center from units of blood from healthy volunteers after they provided informed consent. After remaining overnight at room temperature, the buffy was diluted 1:3 with phosphate-buffered saline (PBS; no Ca/Mg) and aliquoted into 50-mL Falcon tubes. Lymphoprep (Axis-Shield, Oslo, Norway) was underlain beneath the buffy suspension and tubes were centrifuged according to the manufacturer's instructions. The layer containing peripheral blood mononuclear cells was removed and the cells washed twice in PBS. Cell numbers and viability were determined. Cells were plated out overnight in 10% heat-inactivated FCS in RPMI 1640 to allow removal of the monocyte fraction by attachment to the plate surface. Culture media containing lymphocytes was removed and the cells pelleted and counted. Cells were inoculated in 10-cm plates at 4 × 105/mL, and MGd with and without ascorbate was incubated for the required time.
Reagents
MGd was obtained from Pharmacyclics, Inc (Sunnyvale, CA) as a stock solution dissolved in 5% aqueous mannitol (which was the control vehicle in all experiments) at a concentration of 2.3 mg/mL (2 mM). Stock MGd was added directly to the experimental flasks to produce the indicated concentrations (10-100 μM). Ascorbate solutions were prepared fresh as a 10-mM stock solution in RPMI 1640 with 10% heat-inactivated FCS using ascorbic acid (Sigma Chemical, St Louis, MO). Where indicated, stock ascorbate was added to experimental flasks to produce the indicated concentrations (50-100 μM) immediately prior to MGd. Ascorbate was also used as a control in all experiments. Catalase (Roche Molecular Biochemicals, Indianapolis, IN) at 260 U/mL was added in certain experiments as indicated (see “Effects of MGd on apoptosis in fresh multiple myeloma cells”). All experiments were carried out in RPMI 1640, which is deficient in ascorbate.
Inhibition of cellular proliferation
Cells were inoculated into 10 mL medium in 10-cm plates at 5 × 104 cells/mL. At each time point, cells in suspension and attached to the flask were harvested, pelleted, and washed twice with PBS. Cells were resuspended in 1 mL PBS and an aliquot taken for Coulter counting. Cell viability was determined by flow cytometry using propidium iodide (PI) flow cytometric analysis. An aliquot of 3 × 105 cells was transferred to a 4-mL tube and isolated by centrifugation. Cell pellets were resuspended in PBS supplemented with 2 μg/mL PI (Sigma) and incubated for 5 minutes at ambient temperature prior to analysis. PI flow cytometry was performed on a fluorescence-activating cell sorting (FACS) Calibur instrument (BD Biosciences, San Jose, CA) using excitation at 488 nm and a 585/42-nm emission filter. Data were analyzed using the Cell Quest Pro software package (BD Biosciences).
Apoptosis assays
Annexin V. The induction of apoptosis was examined using flow cytometry to measure the levels of detectable phosphatidylserine on the outer membrane of apoptotic cells after exposure to MGd and ascorbate. Briefly, relevant cell lines were counted and plated at 5 × 104/mL. After incubation for 2 to 72 hours, cells were harvested and washed twice with chilled Hanks buffered saline. An aliquot of cells (1 × 106) was added to 500 μL diluted binding buffer from the annexin V phycoerythrin (PE) kit (BD Biosciences). Cells were pelleted, resuspended in 100 μL diluted binding buffer, and treated with the annexin V–PE reagent according to the manufacturer's protocol. Flow cytometry was performed on a FACS Calibur instrument and data were analyzed using the Cell Quest Pro software package.
Mitochondrial membrane potential. Loss of the mitochondrial membrane potential (ΔΨm) of cells was measured by the use of Mito Tracker Red (Molecular Probes, Eugene, OR), CMX rosamine (CMXros), a derivative of x-rosamine, which is a mitochondrial selective dye that is sequestered by actively respiring mitochondria.21 Cells undergoing early apoptosis lose this potential. Briefly, cells at 5 × 105 in 1 mL culture medium were treated with 10 nM CMXros and incubated at 37°C for 15 minutes. Cells were isolated by centrifugation, resuspended in 0.5 mL PBS, and assayed immediately on the flow cytometer. Flow cytometry was performed on a Coulter EPICs XL instrument and data were analyzed using the System 11 software package (Coulter, Miami, FL). Data are expressed as percentage of CMXros+ cells.
Effects of MGd on apoptosis in fresh multiple myeloma cells
Bone marrow aspirate samples were obtained from patients with newly diagnosed multiple myeloma after their informed consent was obtained. Following isolation of mononuclear cells on a Ficoll-Hypaque gradient, bone marrow cells were cultured at 4 × 105/mL in a 48-well plate with or without 100 μM ascorbic acid and 100 μM MGd. Mannitol/ascorbate and mannitol/MGd controls were used. Following 48 hours of incubation at 37°C, cells were spun down and suspended in 100 μL PBS plus 2% bovine serum albumin (BSA). Percy5.5 anti–human CD45 antibody and PE-conjugated anti–human CD38 antibody were added to the cell suspension according to the company protocol (BD Biosciences) and incubated at 4°C for 30 minutes in the dark. Cells were spun down and washed once with PBS plus 2% BSA and once with 1 × binding buffer (annexin V kit from R&D Systems, Minneapolis, MN). The cells were stained with 100 μL annexin V–fluorescein isothiocyanate (FITC) protein, incubated on ice for 10 minutes, followed by 400 μL 1 × binding buffer, and then analyzed on the flow cytometer. Flow cytometry was performed on a Coulter EPICs XL instrument. Controls used included unstained cells, cells with Percy5.5 CD45 antibody alone, PE-conjugated CD38 antibody alone, annexin V–FITC alone, and cells with all antibodies added to the tubes. Plasma cells are defined as cells that express high levels of CD38 and no or low levels of CD45 (CD38+/CD45-).
Measurement of intracellular GSH
Intracellular GSH content was measured by flow cytometric analysis using monobromobimane (MBB) and PI.22 Briefly, treated cells were suspended at 5 × 105/mL in RPMI 1640 medium alone and MBB (40 mM) was added to tubes that were then incubated for 15 minutes at 37°C. Cells were centrifuged, washed with PBS, and then suspended in 500 μL PBS. Dead cells, identified through PI staining, were excluded from the analysis. The mean fluorescence intensity of MBB represents the cellular GSH level of the live cells, and data are expressed as a percentage of cells staining for GSH. The mean fluorescence intensity for the live cells was recorded using the Beckman Coulter Epics Elite ESP flow cytometer using an excitation wavelength at 350 nm and a 450-nm emission filter. Positive controls for each GSH experiment were obtained by the addition of N-ethylmaleimide (NEM; 100 μM) to parallel samples immediately before MBB staining. NEM resulted in complete depletion of GSH in all experiments (data not shown).
Measurement of ROSs
ROSs were measured in live cells as intracellular peroxides by monitoring the oxidation of 2′7′ dichlorofluorescein-diacetate (DCFA; Molecular Probes) to 2′7′ dichlorofluorescein (DCF). Cells (1 × 106/mL) were incubated in 4 μM DCFA for 15 minutes at 37°C. The membrane-permeable dye undergoes deacetylation by intracellular esterases and oxidation by ROSs. The fluorescent intensity in live cells (PI nonpermeable) was analyzed with the flow cytometer. Tertiary butyl hydroperoxide was used as a positive control and resulted in highly elevated ROSs in all experiments (data not shown). Flow cytometry was performed on a Coulter EPICs XL instrument and data were analyzed using the System 11 software package.
Cellular uptake of MGd
The uptake of MGd was measured by flow cytometry using a 650-nm long pass filter to detect its fluorescence emission as previously reported.15 Cells were washed twice in PBS and a “live-cell” gate was drawn based on forward/side light scatter properties to allow analysis of MGd uptake into the viable cell population.
Fluorescence microscopy
C2E3 cells were incubated in RPMI 1640 medium supplemented with 10% FCS containing 50 μM MGd and 50 μM ascorbate for 24 hours. After centrifugation, cells were washed twice in PBS, counted, and resuspended at a concentration of 1 × 106 cells/mL. An aliquot of 0.5 × 106 cells was placed in a test tube and incubated with 0.25 μg/mL DCFA for 5 minutes at 37°C in the dark. Cells were washed with PBS, resuspended in 200 μL PBS, and an aliquot placed in one well of a 4-well chamber slide. The slide was then cover-slipped and cells were imaged immediately using an SD200 SpectraCube system (Applied Spectral Imaging, Carlsbad, CA) consisting of an internal Sagnac inteferometric scanner coupled to a Model 512-EFT CCD camera (Princeton Instruments Inc, Trenton, NJ). The unit is connected to the microscope with a C-mount. The fluorescence microscope is a Zeiss Axioplan-2 (Thornwood, NY) connected to an HBO100W/2 Attoarc mercury lamp (Zeiss) for excitation. Excitation and emission light is filtered using filter cube sets (Chroma Technology Corp, Rockingham, VT) specific for each fluorescence agent: for Hoechst/DAPI/AMCA, excitation 360/40, emission 460/50, and dichroic mirror 400 DCLP; for GFP, FITC/BP, excitation 480/30, emission 535/40, and dichroic mirror 515 DCLP; and for texaphrin (MGd), excitation 470/40, emission RG 665 LP, and dichroic mirror 505 DCLP. A Ph2 PLAN-Neofluar ×40 objective lens with a 0.75 numerical aperture (Zeiss) and a ×63 Plan-Apochromat oil immersion objective with a 1.40 numerical aperture (Zeiss) were used. DCF (a measure of ROS production) was detected using an excitation wavelength of 480/50 nm and a filter cube containing 505 nm and 535/40 nm dichroic and emission filters. MGd fluorescence (a measure of MGd uptake by cells) was detected using an excitation wavelength of 470/40 nm and a filter cube containing 505 nm and 665 nm dichroic and long-pass emission filters.
Results
Inhibition of cell growth and cytotoxicity in chemotherapy-sensitive and resistant myeloma cell lines
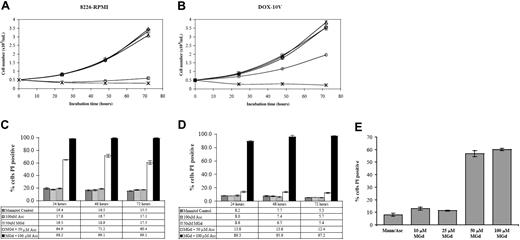
We found dose- and time-dependent inhibition of cell growth after incubation of 8226-RPMI and DOX-10V cells with MGd and ascorbate (Figure 1). Experiments were carried out with and without the addition of ascorbate. In the 8226-RPMI line, near-complete inhibition of proliferation was observed within 24 hours using 50 μM MGd and 50 μM ascorbate. In the highly chemotherapy-resistant DOX-10V line, 100 μM ascorbate (within physiologic range) was required to inhibit growth completely. Using membrane permeability to PI as an indicator of cell death, approximately 65% cytotoxicity was seen in 8226-RPMI cells at 24 hours with 50 μM MGd and 50 μM ascorbate and more than 98% cell death using an increased ascorbate concentration (Figure 1C). By contrast, 50 μM MGd and 100 μM ascorbate were necessary for appreciable cytotoxic effect in DOX-10V cells (Figure 1D). Figure 1E shows MGd dose-dependent cytotoxicity in 8226-RPMI cells that was also confirmed in resistant DOX-10V cells (data not shown). In addition, experiments were carried out in normal peripheral blood lymphocytes (PBLs). We found that with 50 μM MGd and either 50 or 100 μM ascorbate, there was loss of cellular viability as measured by PI at 20 hours that was less than with any of the myeloma lines (Table 2). When incubations were carried out to 120 hours, there was progressive loss of cellular viability in PBLs (data not shown). Interestingly, MGd-induced cytotoxicity was completely prevented when catalase was added in PBL experiments (ascorbate/mannitol, MGd/mannitol, and catalase controls resulted in approximately 10%-14% PI+ cells).
MGd cytotoxicity in chemotherapy-sensitive and highly resistant myeloma cell lines. Cell number as determined by Coulter counting following 24-hour, 48-hour, and 72-hour exposure to 50 μM MGd (▵) in the chemotherapy-sensitive cell line, 8226-RPMI (A), and the highly chemotherapy-resistant line, DOX-10V (B). Varying concentrations of ascorbate (Asc) were added to cell cultures at time 0 (50 μM, ○; 100μM, ×). Mannitol control is indicated by ⋄; ascorbate control, by □. PI was measured by flow cytometric analysis under the same conditions in 8226-RPMI (C) and DOX-10V (D). A dose-response analysis was completed at 48 hours using increasing MGd doses with a constant ascorbate concentration (50 μM) in 8226-RPMI cells (E). Control consisted of mannitol/ascorbate and MGd alone (data not shown). Results showing the mean and the SD were obtained from triplicate measurements at each time point.
MGd cytotoxicity in chemotherapy-sensitive and highly resistant myeloma cell lines. Cell number as determined by Coulter counting following 24-hour, 48-hour, and 72-hour exposure to 50 μM MGd (▵) in the chemotherapy-sensitive cell line, 8226-RPMI (A), and the highly chemotherapy-resistant line, DOX-10V (B). Varying concentrations of ascorbate (Asc) were added to cell cultures at time 0 (50 μM, ○; 100μM, ×). Mannitol control is indicated by ⋄; ascorbate control, by □. PI was measured by flow cytometric analysis under the same conditions in 8226-RPMI (C) and DOX-10V (D). A dose-response analysis was completed at 48 hours using increasing MGd doses with a constant ascorbate concentration (50 μM) in 8226-RPMI cells (E). Control consisted of mannitol/ascorbate and MGd alone (data not shown). Results showing the mean and the SD were obtained from triplicate measurements at each time point.
MGd effect on normal PBLs
Cells . | % cells staining PI+ following incubation with MGd 50 μM + AA 50 μM, mean ± SD* . | % cells staining PI+ following incubation with MGd 50 μM + AA 100 μM, mean ± SD* . |
|---|---|---|
| PBLs†‡ | 36.5 ± 15.0 | 65.3 ± 12.5 |
| PBLs + CAT† | ND | 10.6 ± 1.1 |
| 8226-RPMI§ | 64.9 ± 0.4 | 98.2 ± 0.2 |
| DOX-10V§ | 13.8 ± 1.1 | 89.3 ± 1.1 |
| C2E3§ | 97.0 ± 0.1 | ND |
| 1-130§ | 98.7 ± 0.2 | 99.3 ± 0.4 |
Cells . | % cells staining PI+ following incubation with MGd 50 μM + AA 50 μM, mean ± SD* . | % cells staining PI+ following incubation with MGd 50 μM + AA 100 μM, mean ± SD* . |
|---|---|---|
| PBLs†‡ | 36.5 ± 15.0 | 65.3 ± 12.5 |
| PBLs + CAT† | ND | 10.6 ± 1.1 |
| 8226-RPMI§ | 64.9 ± 0.4 | 98.2 ± 0.2 |
| DOX-10V§ | 13.8 ± 1.1 | 89.3 ± 1.1 |
| C2E3§ | 97.0 ± 0.1 | ND |
| 1-130§ | 98.7 ± 0.2 | 99.3 ± 0.4 |
AA indicates ascorbic acid; CAT, catalase; ND, not done.
Percent cells staining PI+ following 20 or 24 hours of incubation
Results shown (means and SD) were averaged from 3 individual healthy donors with each experiment done in triplicate
Ascorbate/mannitol, MGd/mannitol, and catalase/mannitol controls resulted in 10% to 14% PI+ cells following 24-hour incubation with PBLs
Results shown (means and SD) were averaged from 3 or more independent experiments done in triplicate for each time point
Apoptosis
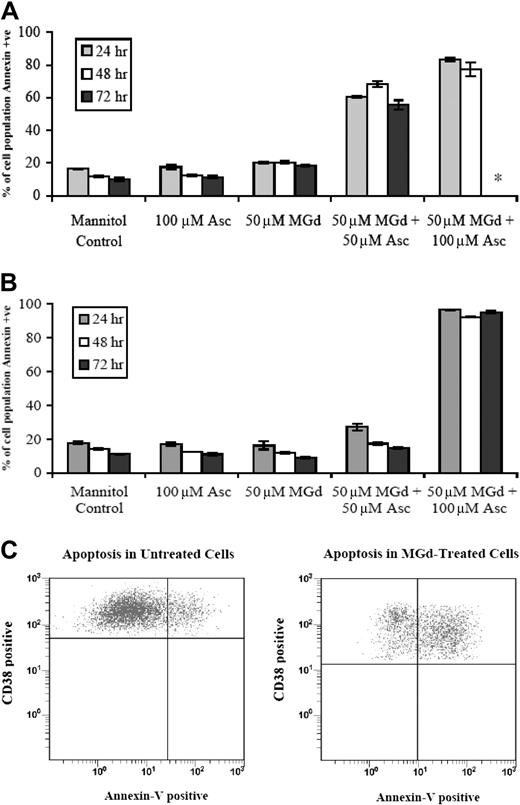
We found that the growth inhibitory effects of MGd were in part due to apoptosis in chemotherapy-sensitive and chemotherapy-resistant cell lines as demonstrated by increased expression of annexin V. More than 80% of 8226-RPMI (Figure 2A) and DOX-10V cells (Figure 2B) were apoptotic by the annexin V assay when exposed to 50 μM MGd and 100 μM ascorbate. In the 8226-RPMI cells, all cells were dead by 72 hours, so analysis by annexin V was not done, whereas this was not the case with DOX-10V cells. An increase in annexin V expression was seen as early as 2 hours (data not shown).
Apoptosis following MGd exposure. Flow cytometry to measure annexin V expression was performed following 24-hour, 48-hour, and 72-hour exposure to MGd in the (A) chemotherapy-sensitive cell line, 8226-RPMI, and the (B) highly chemotherapy-resistant line, DOX-10V; +ve indicates positive. The asterisk indicates that analysis not performed because all cells were dead at this time point. Results showing means and SD are the average of triplicate measurements at each time point. In panel C, annexin V studies were done on fresh patient myeloma cells with plasma cells defined as cells that express high levels of CD38 and no or low levels of CD45 (see “Materials and methods”). Cells were treated for 48 hours with 50μM MGd and 100 μM ascorbate (right) and ascorbate 100 μM control (left). Events in the upper right quadrant represent CD38+ cells that are annexin V+ as measured by flow cytometric analysis. Data presented are representative of independent experiments from 3 untreated multiple myeloma patients.
Apoptosis following MGd exposure. Flow cytometry to measure annexin V expression was performed following 24-hour, 48-hour, and 72-hour exposure to MGd in the (A) chemotherapy-sensitive cell line, 8226-RPMI, and the (B) highly chemotherapy-resistant line, DOX-10V; +ve indicates positive. The asterisk indicates that analysis not performed because all cells were dead at this time point. Results showing means and SD are the average of triplicate measurements at each time point. In panel C, annexin V studies were done on fresh patient myeloma cells with plasma cells defined as cells that express high levels of CD38 and no or low levels of CD45 (see “Materials and methods”). Cells were treated for 48 hours with 50μM MGd and 100 μM ascorbate (right) and ascorbate 100 μM control (left). Events in the upper right quadrant represent CD38+ cells that are annexin V+ as measured by flow cytometric analysis. Data presented are representative of independent experiments from 3 untreated multiple myeloma patients.
Fresh mononuclear cells were obtained from bone marrow aspirates of untreated patients with multiple myeloma and analyzed by flow cytometry (see “Materials and methods”). Incubation with 50 μM MGd and 100 μM ascorbate induced apoptosis of CD38+ plasma cells as measured by annexin V. Figure 2C is a representative dot plot of these data showing an increase in CD38+/annexin V+ cells from 19% of events in mannitol/ascorbate control to 56% of events with MGd/ascorbate (mannitol/MGd control 18%, mannitol/catalase control 16%, CD45 antibody alone 16%, CD38 antibody alone 15%; data not shown).
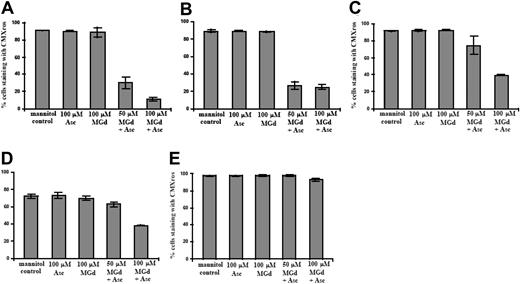
Early apoptotic events as measured by disrupted ΔΨm were seen in the dexamethasone-sensitive cell line, C2E3, and in the dexamethasone-resistant 1-310 cell line at 50 μM MGd and 100 μM ascorbate (Figure 3A-B). The percentage of cells with an intact ΔΨm was also decreased after 5 hours of MGd treatment in highly dexamethasone-resistant 1-414 and chemotherapy-sensitive 8226-RPMI cells with 100 μM MGd and 100 μM ascorbate (Figure 3C-D), whereas DOX-10V cells did not show altered ΔΨm (Figure 3E) at this time point. It is possible that alternate mechanisms are extant in this highly chemotherapy-resistant cell line, but further experiments to examine this have not been done.
Loss of mitochondrial membrane potential. Flow cytometry to detect change/loss in ΔΨm was performed on live cells following 5-hour exposure to MGd and ascorbate in the (A) dexamethasone-sensitive cell line, C2E3, the (B) dexamethasone-resistant cell line, 1-310, the (C) highly dexamethasone-resistant cell line, 1-414, the (D) chemotherapy-sensitive cell line, 8226-RPMI, and the (E) highly chemotherapy-resistant line, DOX-10V. Dead cells were gated out with PI. Results show percentage of cells staining with CMXros, with lower percentages indicating loss of ΔΨm. Results shown (means and SD) were averaged from 3 or more independent experiments done in triplicate for each time point.
Loss of mitochondrial membrane potential. Flow cytometry to detect change/loss in ΔΨm was performed on live cells following 5-hour exposure to MGd and ascorbate in the (A) dexamethasone-sensitive cell line, C2E3, the (B) dexamethasone-resistant cell line, 1-310, the (C) highly dexamethasone-resistant cell line, 1-414, the (D) chemotherapy-sensitive cell line, 8226-RPMI, and the (E) highly chemotherapy-resistant line, DOX-10V. Dead cells were gated out with PI. Results show percentage of cells staining with CMXros, with lower percentages indicating loss of ΔΨm. Results shown (means and SD) were averaged from 3 or more independent experiments done in triplicate for each time point.
MGd produces intracellular ROSs
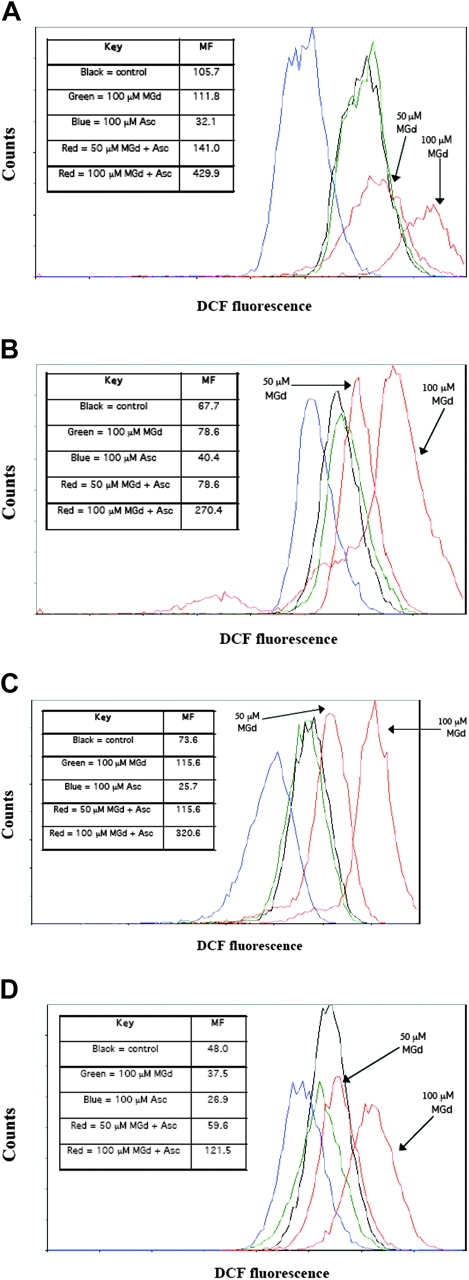
We measured the intracellular production of ROSs in live cells (as gated by PI). We found increased ROS production at 5 hours with MGd exposure at a concentration of 50 μM and 100 μM ascorbate as compared with mannitol control in both dexamethasone-sensitive/resistant and chemotherapy-sensitive/resistant cell lines (Figure 4). Increasing MGd concentrations was associated with higher ROS production. Approximately a 4-fold increase in ROS production was found in dexamethasone-sensitive cells C2E3 (Figure 4A), dexamethasone-resistant cells 1-310 (Figure 4B), chemotherapy-sensitive cells 8226-RPMI (Figure 4C), and a 2.5-fold increase in highly chemotherapy-resistant cells DOX-10V (Figure 4D) with 100 μM MGd and 100 μM ascorbate (as compared with mannitol control). Overall ROS production was less in the dexamethasone- and chemotherapy-resistant cell lines (Figure 4B,D) when compared with similar MGd/ascorbate concentrations in sensitive cell lines (Figure 4A,C). Interestingly, ascorbate inhibited ROS production in all cell lines when added without MGd, consistent with its role as a primary antioxidant to scavenge free radical species that are generated as a by-product of cellular metabolism (Figure 4A-D).
Production of ROSs. Flow cytometric analysis was used to measure the formation of ROSs. Overlay histograms of ROS formation, as detected by DCF fluorescence, are shown in live cells treated for 5 hours with MGd and ascorbate (red lines) with 2 concentrations of MGd (50 μM and 100 μM, both with 100 μM ascorbate), mannitol control (black line), MGd control (green line), or ascorbate control (blue line) in (A) dexamethasone-sensitive C2E3 cells, (B) dexamethasone-resistant 1-310 cells, (C) chemotherapy-sensitive 8226-RPMI cells, and (D) highly chemotherapy-resistant DOX-10V cells. Dead cells were gated out with PI. A shift of the curve to the right represents increased ROSs (log scale). Absolute ROS mean fluorescence (MF) levels for each cell line are shown in associated legends. Tertiary butyl hydroperoxide was used as a positive control for each experiment (see “Materials and methods”). Data presented are representative of 3 independent experiments.
Production of ROSs. Flow cytometric analysis was used to measure the formation of ROSs. Overlay histograms of ROS formation, as detected by DCF fluorescence, are shown in live cells treated for 5 hours with MGd and ascorbate (red lines) with 2 concentrations of MGd (50 μM and 100 μM, both with 100 μM ascorbate), mannitol control (black line), MGd control (green line), or ascorbate control (blue line) in (A) dexamethasone-sensitive C2E3 cells, (B) dexamethasone-resistant 1-310 cells, (C) chemotherapy-sensitive 8226-RPMI cells, and (D) highly chemotherapy-resistant DOX-10V cells. Dead cells were gated out with PI. A shift of the curve to the right represents increased ROSs (log scale). Absolute ROS mean fluorescence (MF) levels for each cell line are shown in associated legends. Tertiary butyl hydroperoxide was used as a positive control for each experiment (see “Materials and methods”). Data presented are representative of 3 independent experiments.
Analysis of intracellular reduced GSH
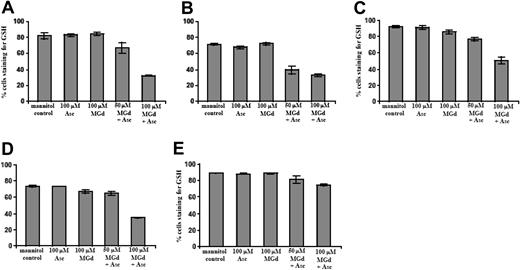
We found marked reduction of intracellular GSH in cells incubated for 5 hours with MGd and ascorbate in C2E3 and 1-310 cell lines at 50 μM MGd and 100 μM ascorbate (Figure 5A-B). For highly dexamethasone-resistant and chemotherapy-sensitive cells, 100 μM MGd and 100 μM ascorbate were needed for appreciable GSH decrease (Figure 5C-D). GSH depletion was not seen in highly chemotherapy-resistant cells at 5 hours (Figure 5E), although an approximately 45% reduction in intracellular GSH was seen following 30-hour exposure to MGd and ascorbate (data not shown). We found relatively little change in GSH in the DOX-10V cell line, which is consistent with prior observations in highly chemotherapy-resistant lines.3,16,23
Measurement of intracellular GSH content. Intracellular GSH content was measured by flow cytometric analysis using MBB and PI after a 5-hour exposure to MGd and ascorbate in the (A) dexamethasone-sensitive cell line, C2E3, the (B) dexamethasone-resistant cell line, 1-310, the (C) highly dexamethasone-resistant cell line, 1-414, the (D) chemotherapy-sensitive cell line, 8226-RPMI, and the (E) highly chemotherapy-resistant line, DOX-10V. The mean fluorescence intensity of MBB represents the cellular GSH level of the live cells, and data are expressed as a percentage of cells staining for GSH. Results show GSH content with mannitol control, 100 μM ascorbate alone, 100 μM MGd alone, concurrent 50 μM MGd and 100 μM ascorbate, or concurrent 100 μM MGd and 100 μM ascorbate exposure. Dead cells were gated out with PI. Data are expressed as percentage of cells staining for GSH, with lower percentages indicating decreased intracellular GSH. N-ethylmaleimide at 100 μM was used as a positive control for each experiment (see “Materials and methods”). Results shown (means and SD) were averaged from 3 or more independent experiments performed in triplicate for each data point.
Measurement of intracellular GSH content. Intracellular GSH content was measured by flow cytometric analysis using MBB and PI after a 5-hour exposure to MGd and ascorbate in the (A) dexamethasone-sensitive cell line, C2E3, the (B) dexamethasone-resistant cell line, 1-310, the (C) highly dexamethasone-resistant cell line, 1-414, the (D) chemotherapy-sensitive cell line, 8226-RPMI, and the (E) highly chemotherapy-resistant line, DOX-10V. The mean fluorescence intensity of MBB represents the cellular GSH level of the live cells, and data are expressed as a percentage of cells staining for GSH. Results show GSH content with mannitol control, 100 μM ascorbate alone, 100 μM MGd alone, concurrent 50 μM MGd and 100 μM ascorbate, or concurrent 100 μM MGd and 100 μM ascorbate exposure. Dead cells were gated out with PI. Data are expressed as percentage of cells staining for GSH, with lower percentages indicating decreased intracellular GSH. N-ethylmaleimide at 100 μM was used as a positive control for each experiment (see “Materials and methods”). Results shown (means and SD) were averaged from 3 or more independent experiments performed in triplicate for each data point.
Effect of catalase on MGd cell growth inhibition and cytotoxicity
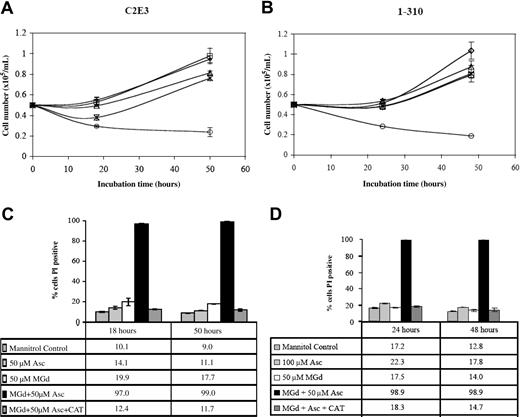
We studied the effect of catalase on MGd growth inhibition in the dexamethasone-sensitive cell line, C2E3, and the dexamethasone-resistant line, 1-310. Both cell lines were treated with and without catalase (260 U/mL final) added at time 0. Incubation in culture medium containing MGd 50 μM with 50 μM ascorbate inhibited proliferation in both dexamethasone-sensitive and -resistant lines. Catalase alone or with ascorbate resulted in no effect over background (data not shown). Catalase reversed the inhibition of cell proliferation seen with MGd and ascorbate (Figure 6A-B). The mechanism of this effect is likely due to the activity of catalase on hydrogen peroxide, which is formed along with dehydroascorbate when MGd and ascorbate are combined in the presence of oxygen.15 Hydrogen peroxide freely diffuses across cell membranes so the extracellular presence of catalase would be expected to decrease its presence both on an intracellular and extracellular level. Analysis of membrane permeability (to PI) of the catalase-supplemented cell cultures demonstrated abrogation of the cell death mediated by MGd and ascorbate in both C2E3 and 1-310 cell lines (Figure 6C-D).
Cytotoxicity is catalase-dependent. Cell number was determined by Coulter counting following 24-hour and 48-hour exposures to 50 μM MGd and 50μM ascorbate (○) in the dexamethasone-sensitive cell line, C2E3, and the dexamethasone-resistant line, 1-310. Catalase (260 U/mL) was added (*) at time 0 to C2E3 cells (A) and 1-310 cells (B). Exposure to 100 μM Asc alone is indicated by □; to 50 μ MGd alone, ▵. Mannitol control is indicated by ⋄. Cell viability by PI assessment of membrane permeability was measured following 18-hour and 50-hour exposures to 50 μM MGd in C2E3 (C), and 24-hour and 48-hour exposures to 50 μM MGd in 1-310 (D). Catalase blocks loss of cell viability induced by MGd and ascorbate as measured by PI. Results shown (means and SD) were obtained from triplicate measurements at each time point. Controls included mannitol, 50μM MGd, 100 μM ascorbate, and combined catalase/ascorbate (data not shown for the latter).
Cytotoxicity is catalase-dependent. Cell number was determined by Coulter counting following 24-hour and 48-hour exposures to 50 μM MGd and 50μM ascorbate (○) in the dexamethasone-sensitive cell line, C2E3, and the dexamethasone-resistant line, 1-310. Catalase (260 U/mL) was added (*) at time 0 to C2E3 cells (A) and 1-310 cells (B). Exposure to 100 μM Asc alone is indicated by □; to 50 μ MGd alone, ▵. Mannitol control is indicated by ⋄. Cell viability by PI assessment of membrane permeability was measured following 18-hour and 50-hour exposures to 50 μM MGd in C2E3 (C), and 24-hour and 48-hour exposures to 50 μM MGd in 1-310 (D). Catalase blocks loss of cell viability induced by MGd and ascorbate as measured by PI. Results shown (means and SD) were obtained from triplicate measurements at each time point. Controls included mannitol, 50μM MGd, 100 μM ascorbate, and combined catalase/ascorbate (data not shown for the latter).
Cellular MGd uptake and ROS production
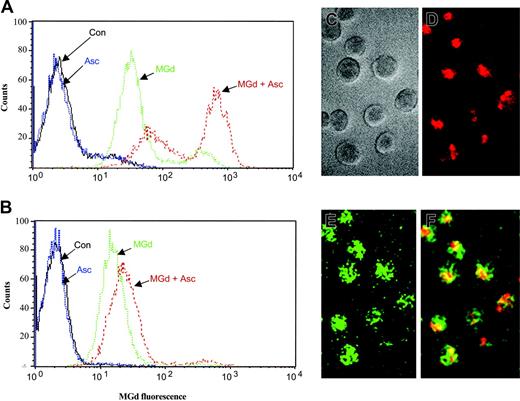
Intracellular localization of MGd in the 8226-RPMI and DOX-10V lines was measured by flow cytometry. MGd uptake into the live cell population was observed at 24, 48, and 72 hours of incubation under various conditions. MGd uptake into cells was essentially saturated after 24 hours. Representative histograms for MGd uptake into the 8226-RPMI and DOX-10V lines are shown in Figures 7A and 7B, respectively. Ascorbate enhanced cellular uptake of MGd in all lines analyzed; however, the effect observed with the DOX-10V cells was not as pronounced. The 1-310, 1-414, and C2E3 cell lines were also examined with all displaying high MGd uptake (data not shown). Using fluorescence microscopy, we confirmed intracellular uptake of MGd and increased production of ROSs (as detected by DCF) in myeloma cells treated with MGd and ascorbate (Figure 7C-F).
Intracellular MGd uptake and ROS production. Fluorescence analysis was performed on live gated cells after 24 hours of incubation with 50 μM MGd and 50μM ascorbate in the (A) chemotherapy-sensitive cell line, 8226-RPMI, and the (B) highly chemotherapy-resistant line, DOX-10V. Histograms depict fluorescence uptake in live cells, with fluorescence on the x-axis and live cell counts on the y-axis. A shift to the right indicates increased MGd fluorescence. Con indicates mannitol control. Histograms shown are representative of triplicate measurements. Intracellular uptake of MGd and intracellular ROS production in C2E3 cells was also assessed by fluorescence microscopy after 24 hours incubation with 50 μM MGd and 50 μM ascorbate: (C) myeloma cells in gray scale transmission, (D) intracellular MGd uptake with MGd delineated by red fluorescence label, (E) ROS production as delineated by green fluorescence, which results from oxidation of DCFA to DCF, (F) MGd and ROS production in the same cells as represented by the overlay of red and green fluorescence resulting in the yellow color.
Intracellular MGd uptake and ROS production. Fluorescence analysis was performed on live gated cells after 24 hours of incubation with 50 μM MGd and 50μM ascorbate in the (A) chemotherapy-sensitive cell line, 8226-RPMI, and the (B) highly chemotherapy-resistant line, DOX-10V. Histograms depict fluorescence uptake in live cells, with fluorescence on the x-axis and live cell counts on the y-axis. A shift to the right indicates increased MGd fluorescence. Con indicates mannitol control. Histograms shown are representative of triplicate measurements. Intracellular uptake of MGd and intracellular ROS production in C2E3 cells was also assessed by fluorescence microscopy after 24 hours incubation with 50 μM MGd and 50 μM ascorbate: (C) myeloma cells in gray scale transmission, (D) intracellular MGd uptake with MGd delineated by red fluorescence label, (E) ROS production as delineated by green fluorescence, which results from oxidation of DCFA to DCF, (F) MGd and ROS production in the same cells as represented by the overlay of red and green fluorescence resulting in the yellow color.
Discussion
We and others have previously demonstrated that redox mechanisms play an important role in myeloma cell survival, probably in part dependent on the manipulation of the cellular redox system through GSH depletion, production of ROSs, and induction of apoptosis.1,3,8,24 In experimental systems, MGd catalyzes the oxidation of several intracellular reducing metabolites including GSH, ascorbate, and nicotinamide adenine dinucleotide phosphate (NADPH), generating ROSs through redox cycling.12,13,15 The purpose of this study was to determine whether similar processes would be observed in multiple myeloma cell lines.
Our cell-culture experiments were done in RPMI 1640 medium, which, unlike other media would not confound analysis of ROSs and apoptosis.25 As expected, the activity of MGd was affected by the addition of ascorbate to the medium. We found that MGd, when combined with physiologic concentrations of ascorbate,26-28 caused dose-dependent cytotoxicity in myeloma cell lines. Over half the cells became permeable to PI (indicating loss of plasma membrane integrity) after a 24-hour treatment with the clinically achievable MGd concentration, 50 μM,29 and 50 μM ascorbate in C2E3 (dexamethasone-sensitive), 1-414 (highly dexamethasone-resistant), and 8226-RPMI (chemotherapy-sensitive) cultures. Comparable levels of cytotoxicity (ie, > 50%) in the chemotherapy-resistant DOX-10V line required 100 μM ascorbate in the presence of 50 μM MGd. These incubation conditions (50 μM MGd and 100 μM ascorbate) were associated with over 97% cell death at 24 hours in the other cell lines studied. Thus, increasing concentrations of ascorbate produced more cytotoxicity (Figure 1C). Ascorbate treatment is known to deplete GSH and has been shown in previous reports to be an important modulator of redox-mediated cytotoxicity in myeloma cell lines and in patients with myeloma.8,30 Ascorbate undergoes 2-electron oxidation, first to ascorbate free radical and then to dehydroascorbate, which is preferentially transported into the cell by glucose transporters.31 Ascorbate is then regenerated by redox reactions involving glutaredoxin and thioredoxin reductase, which results in GSH depletion and thus induces a pro-oxidant state in the cell. Ascorbate also reacts directly with MGd to form hydrogen peroxide.15 Studies in normal peripheral blood lymphocytes demonstrated less cell death as measured by PI compared with all the cell lines tested (Table 2). Moreover, catalase prevented MGd's effect on peripheral blood lymphocytes, supporting further the importance of ROSs for MGd cytotoxicity.
We observed that ascorbate alone appears to partially inhibit ROS production as compared to mannitol control in dexamethasone- and chemotherapy-sensitive and -resistant cells (Figure 4). Ascorbate is known to be an antioxidant as well as a prooxidant.32-34 The molecular and biologic mechanisms of these antioxidant and pro-oxidant functions are poorly understood, but probably involve intracellular redox reactions that lead to regeneration of ascorbate from dehydroascorbate. We suspect that in our system, ascorbate scavenges the small amount of ROSs generated by cell lines as a consequence of cellular metabolism in tissue culture, but in the presence of MGd, the predominant mechanism is the oxidation of ascorbate to dehydroascorbate with subsequent facilitated transport into the cell. Thus very little reduced ascorbate is available, except that which is regenerated in the cell at the expense of GSH and thioredoxin.
ROSs can react with biologic macromolecules, modify the structure and function of proteins, and cause oxidative damage to DNA.35 ROSs damage cellular DNA through destruction of pyrimidine and purine bases and by producing single-strand breaks.36 Furthermore, oxidants have been demonstrated to act on the mitochondria to trigger the initiation of apoptosis.37-39 ROSs must be neutralized by antioxidant metabolites such as GSH and enzymes that degrade hydrogen peroxide, such as catalase, to avoid potential cellular injury and induction of the apoptotic cascade.23,40-42 Tumor mitochondria are known to contain large amounts of GSH, whereas depletion of GSH has been demonstrated to sensitize tumor cells to oxidative cytolysis in several hematopoietic tumor models.23,24,41,43 Tipping the cellular redox balance through pharmacologic manipulation in favor of increasing intracellular ROSs or depleting protective reducing metabolites (such as GSH) may lead to oxidative stress and induction of apoptosis within tumor cells.3,24,30,44,45
The cytotoxic effect of MGd in the chemotherapy-sensitive and -resistant cells (and fresh patient myeloma cells) was shown in our experiments to be mediated through apoptosis with elevated expression of annexin V and loss of ΔΨm at clinically achievable concentrations of MGd and physiologic concentrations of ascorbate.26-28 Interestingly, we could not find evidence of significant ΔΨm in the DOV-10V line preceding apoptosis. It is possible that other mechanisms might be extant in this highly chemotherapy-resistant cell line, and further experiments are required to tease out this observation. Moreover, increasing concentrations of ascorbate in the presence of MGd correlated with higher levels of apoptosis in DOX-10V and 8226-RPMI cells and demonstrated dose-dependent increases of ROSs in all myeloma cell lines. At 5 hours of MGd exposure, this was associated with depletion of intracellular GSH, except in the case of DOX-10V cells. We and others have previously correlated chemotherapy resistance with higher endogenous GSH,3,23,24,43,46 so that the observed differences in levels of endogenous GSH among cell lines is consistent with the idea that protection against oxidant stress is associated with chemotherapy resistance. It is likely that other natural antioxidant species, such as thioredoxin, also modulate production of ROSs in the DOX-10V line, and possibly to some extent in the other lines as well.47 We did not formally investigate the role of thioredoxin in our experiments. That catalase reversed the growth inhibitory effect of MGd supports the conclusion that these effects are mediated in part through the extracellular production of hydrogen peroxide. The oxidation of DCFA, on the other hand, is consistent with intracellular hydrogen peroxide formation. Hydrogen peroxide and dehydroascorbate production at either site might contribute to drug activity in vivo, because hydrogen peroxide is freely diffusible across cell membranes.
It has been shown in animal tumor models and human clinical trials that MGd and other texaphyrins preferentially accumulate in cancers.29,48-50 Using fluorescence microscopy and colocalization with fluorescent probes, Woodburn50 found that MGd localizes predominantly to tumor cell lysosomes, endoplasmic reticulum, and mitochondria. We found intracellular uptake of MGd into all myeloma cell lines used in this study and demonstrated that intracellular accumulation of MGd was associated with intracellular ROS production. Interestingly, we note that the fluorescent signal due to MGd was increased in the presence of ascorbate, as seen in the data presented in Figure 7. First, there was increased occupancy of cells in the second peak in the bimodal distribution shown for the 8226-RPMI line. Despite live-cell gating based on scatter properties, we assign this peak to cells with membranes that fail to exclude drug. This peak was not observed in the DOX-10V line, consistent with viability measurements made using PI in Figure 1. There was also an increase in average fluorescent signal in the first peak (ie, in the second decade of fluorescence) in the presence of ascorbate. This was observed in both lines. We ascribe this increase to ligand-binding changes in the apical position of MGd. As described by Magda et al,15 ascorbate is not only oxidized to form dehydroascorbate by MGd under tissue culture conditions, but is further oxidized to form oxalate. The oxalate anion complexes tightly with MGd in the apical position and that complex is taken up by cells more readily. This mechanism is not well understood, though it is possible that this reaction changes the aggregation state of the drug and leads to greater cell uptake.
In summary, the novel macrocycle, MGd, induces cytotoxicity in dexamethasone-sensitive, dexamethasone-resistant, chemotherapy-sensitive, and highly chemotherapy-resistant myeloma cell lines in a dose-dependent manner. Higher ascorbate concentrations are required to affect highly chemotherapy-resistant cells. MGd-induced cytotoxicity is related to its ability to elicit changes in cellular redox state toward a more oxidative environment, with the subsequent induction of cell death by apoptosis. We also demonstrated cellular uptake of MGd with the resultant increase in intracellular production of ROSs. These data introduce a new treatment paradigm for hematologic malignancies and provide a rationale to study this novel class of agents in patients with myeloma and other hematologic cancers.
Prepublished online as Blood First Edition Paper, September 23, 2004; DOI 10.1182/blood-2004-03-0964.
Supported in part through funding from the National Cancer Institute Clinical Oncology Research Training Program (5T32 CA 79447-02; A.M.E.) and in part by the Adolph Meyer Research Fund.
R.A.M., P.L., and D.M. are employed by Pharmacyclics, Inc, and therefore have a financial interest in the product (MGd) discussed in this report.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal