Comment on Neff et al, page 997
In this issue, Neff and colleagues provide an important advance in the field of hematopoietic stem cell gene therapy: the ability to select for transduced progenitor cells over time in a large animal.
The drug selection gene used is methylguanine-DNA methyltransferase (MGMT). Originally, this gene was identified as a DNA repair gene that encodes a protein that acts solo and in a suicide manner. The MGMT protein repairs DNA adducts at the O6 position of guanine, a favored cytotoxic lesion induced by the chloroethylating nitrosoureas and methylating agents such as temozolomide. Both agents have since been shown to be stem cell toxins, making them ideal drugs for stem cell selection. To optimize the degree of resistance, the authors employed a mutant form of the MGMT gene, encoding a protein resistant to a potent inhibitor now in clinical trials, O6-benzylguanine.
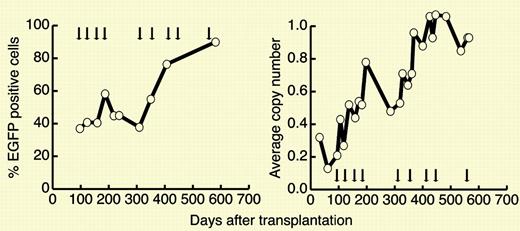
The authors transduced purified hematopoietic stem cells from 2 dogs with retrovirus containing the mutant MGMT(P140K) gene and infused the autologous cells back into the dogs after lethal irradiation. Thereafter, the dogs were treated over the course of more than a year with increasing doses of temozolomide. Over time, hematopoiesis was replaced by progeny of the transduced cells, blood counts remained stable despite increasing doses of temozolomide, and there was no evidence of marrow failure or myelodysplasia or leukemia. Analysis of insertions indicated polyclonality of the selected cells, with common insertions observed in both myeloid and lymphoid cells. A very high degree of gene transfer (over 90% with an estimated copy number approaching 1 at the end of the experiment) was observed.FIG1
In vivo selection in dog G179 after multiple cycles of O6BG and temozolomide. See the complete figure in the article beginning on page 997.
In vivo selection in dog G179 after multiple cycles of O6BG and temozolomide. See the complete figure in the article beginning on page 997.
While this has technically been done before in mice by a number of investigators, 1,2 including the more convincing validation of selection at the level of the repopulating stem cell after secondary transplantation, 3 these investigators are the first to address the issue in a large animal, where more stem and progenitor cells participate in hematopoietic reconstitution, where there is the potential for stem cell failure from “overselection,” and where longitudinal follow-up provides ample time for insertional mutagenesis and leukemic transformation. Previous studies in mice have shown a remarkable degree of selection, more than 500-fold at the level of the repopulating stem cell.3 Together, these studies suggest that after transplantation of mutant MGMT–transduced stem cells, patients will tolerate a strong degree of stem cell selection without prior myeloablation, a safer, perhaps outpatient, procedure. Furthermore, where complete stem cell replacement is not needed, drug treatment for selection could be tailored to the needed engraftment level.
Since no long-term adverse sequelae were noted by Neff and colleagues, albeit only with follow-up of slightly more than one year, these data support ongoing efforts to begin human clinical trials transducing stem cells with the MGMT(P140K) drug selection gene by retroviral and, eventually, lentiviral gene transfer for cancer and perhaps nonmalignant conditions. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal