Comment on Guo et al, page 1246
In this manuscript, Guo and colleagues describe a mechanistic role for heat shock protein 70 in Bcr-Abl–mediated resistance in leukemia cells, suggesting another promising therapeutic target for reversing drug resistance in Ph+ leukemias.
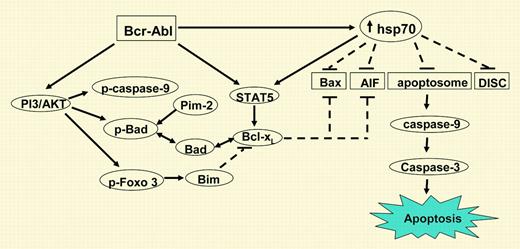
The bcr-abl oncogene generated by the t(9;22) is one of the most highly studied oncogenes. It is regarded as the hallmark for chronic myeloid leukemia (CML). The constitutive kinase activity of the bcr-abl fusion gene leads, via a myriad of signal transduction pathways, to enhanced proliferation, genomic instability, and cytoskeletal abnormalities and to decreased apoptosis. Although several molecular mechanisms resulting in the antiapoptosis properties of Bcr-Abl have been unraveled, there are still many gaps in our knowledge. Bcr-Abl expression results in resistance to apoptosis by cytostatic drugs but also inhibits the extrinsic pathway of apoptosis. In this issue of Blood, Guo and colleagues attempted to elucidate the role of heat shock protein 70 (hsp70) in Bcr-Abl–mediated antiapoptotic pathways in human leukemic cells. It was shown that Bcr-Abl expression (ectopic or endogenous) in leukemic cells results in the up-regulation of the antiapoptotic hsp70 leading to induction and activation of signal transducers and activators of transcription 5 (STAT5) and interference with several apoptosis signaling pathways at multiple levels (see figure). Attenuation of hsp70 levels either by imatinib or hsp70 antisense sensitized K562 cells to cytarabine (Ara-C), etoposide, and also Apo-2 ligand (Apo-2L)/tumor necrosis factor (TNF)–related apoptosis-inducing ligand (Apo-2L/TRAIL)–induced apoptosis. Higher levels of phosphorylated heat shock factor-1 (pHSF-1) were found in bcr-abl–positive cell lines, suggesting a regulatory role for HSF-1 in hsp70 expression. Imatinib down-regulated pHSF-1 and hsp70 expression in bcr-abl–positive leukemic cells. Remarkably, the death-inducing signaling complex (DISC), as well as apoptosis, was inhibited by hsp70. From these elegantly performed studies, the overall important conclusion can be drawn that hsp70 plays a central role in both the extrinsic and intrinsic apoptotic signaling of bcr-abl–positive leukemic cells, STAT5 dependent and independent. Most of the results were obtained in cell lines; just overexpression of hsp70 and hsp90 was shown in primary cells derived from blast crisis patients. It would be of interest to see whether these observations also apply for the CD34+ leukemic cells in chronic phase of CML or if the up-regulation of hsp70 is just restricted to later phases of the disease.FIG1
Infection with dominant negative (DN) STAT 5 sensitizes HL-60/hsp70 cells to Ara-C and etoposide-induced cell death. See the complete figure in the article beginning on page 1246.
Infection with dominant negative (DN) STAT 5 sensitizes HL-60/hsp70 cells to Ara-C and etoposide-induced cell death. See the complete figure in the article beginning on page 1246.
The successful treatment of CML with the “Bcr-Abl–specific” tyrosine kinase inhibitor imatinib leading to complete cytogenetic remissions in a high percentage of patients if applied in chronic phase has transformed this disease into a paradigm for targeted therapy.
However, primary or secondary resistance is not an infrequent event and although complete remissions can also be achieved in advanced stage CML, mostly, these are unsustained. Therefore, the search for other drugs, effectively targeting perturbed signal transduction pathways involved in the pathogenesis of CML, is warranted. Switching off not only Bcr-Abl but also other key proteins could potentially overcome primary as well as secondary resistance. Various new small molecules are currently being tested either in vitro or in vivo. Hsp70 could be a suitable target for these and, it is hoped, become the Achilles heel of the leukemic cell. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal