Abstract
Signaling through the Notch pathway plays an essential role in inducing T-lineage commitment and promoting the maturation of immature thymocytes. Using an in vitro culture system, we show that 2 different classes of Notch ligands, Jagged1 or Delta1, transmit distinct signals to T-cell progenitors. OP9 stromal cells expressing either Jagged1 or Delta1 inhibit the differentiation of DN1 thymocytes into the B-cell lineage, but only the Delta1-expressing stromal cells promote the proliferation and maturation of T-cell progenitors through the early double-negative (DN) stages of thymocyte development. Whereas the majority of bone marrow–derived stem cells do not respond to Jagged1 signals, T-cell progenitors respond to Jagged1 signals during a brief window of their development between the DN1 and DN3 stages of thymic development. During these stages, Jagged1 signals can influence the differentiation of immature thymocytes along the natural killer (NK) and γδ T-cell lineages.
Introduction
The Notch signaling pathway regulates cellular differentiation in a variety of tissue types throughout the life of multicellular organisms.1 Notch signals have been shown to affect a broad range of cellular functions, including proliferation,2,3 apoptosis,4,5 and developmental lineage choices.6-8 Within the hematopoietic system, Notch signals play a critical role in promoting T-cell development.9-11
T cells develop from hematopoietic stem cells (HSCs) that, in the adult, originate in the bone marrow and mature within the thymus. As they differentiate, HSCs undergo a series of lineage choices, beginning with the separation of myeloid and lymphoid lineages.12-14 It is not clear at what developmental stage these cells first select the T-cell lineage; however, T-cell maturation occurs almost exclusively in response to signals provided by the thymus.15,16 Within the thymus, T-cell precursors progress through a series of maturational stages that can be broadly categorized by expression of the CD4 and CD8 coreceptor molecules. The most immature thymocytes express neither CD4 nor CD8 and are termed double negative (DN). As thymocytes mature, they transiently express both CD4 and CD8 molecules and are termed double positive (DP), prior to differentiating into either the CD4 or CD8 lineage.17-20
DN thymocytes can be further separated into 4 distinct developmental stages (DN1-DN4) defined by the expression of CD44 and CD25 surface molecules.21 DN1 thymocytes (CD44+/CD25–) comprise a mixed population of precursors that retains the capacity to differentiate into T, B, natural killer (NK), and dendritic cells.22-24 DN2 thymocytes (CD44+/CD25+) lose the potential to differentiate into B cells but retain NK and dendritic cell potential,25,26 whereas DN3 thymocytes (CD44–/CD25+) are committed to the T-cell lineage.14 Thymocytes begin to rearrange the genes encoding T-cell receptor β (TCRβ), TCRγ, and TCRδ chains during the DN2/DN3 stage. During this stage, the majority of thymocytes that have successfully rearranged both TCRγ and TCRδ chain genes differentiate into the γδ T-cell lineage, whereas those that have successfully rearranged TCRβ express the pre-TCR consisting of TCRβ complexed with pre-Tα. The pre-TCR mediates β-selection, following which thymocytes undergo a massive proliferative expansion as they progress to the DN4 (CD44–/CD25–) or pre–DP stage.27-30
Notch signals have been shown to be important during several stages of T-cell development, including T-lineage commitment and during the early DN stages of T-cell maturation.10,11 Overexpression of a constitutively active form of Notch1 in HSCs results in a complete inhibition of B-cell development and the appearance of DP T cells in the bone marrow.31,32 Correspondingly, targeted deletion of Notch1 in stem cells results in the inhibition of T-cell development and an absolute increase in the number of immature B cells within the thymus.33,34 In addition to its role in promoting T-lineage commitment, Notch signals have been shown to influence the development of αβ versus γδ T cells35,36 and continue to be important throughout the DN stages of thymocyte development. Targeted deletion of Notch1 during the DN2 to DN3 stage results in a partial arrest of thymocyte development at the DN3 stage,37 and DN3 thymocytes that have received a functional pre-TCR signal continue to require Notch signals to proliferate in vitro.38
In mammals there are 4 Notch receptors and 5 Notch ligands which signal through a common mechanism. Ligand binding induces a presenilin-dependent proteolytic cleavage within the intracellular domain of Notch, releasing an active form of the receptor (Notch-IC) which enters the nucleus and induces the transcription of Notch-responsive genes by binding to C-promoter binding factor 1/recombination binding protein Jκ (CBF1/RBPJκ), a transcriptional repressor/activator that is essential for most Notch signal transduction.39 Notch ligands fall into 2 evolutionarily conserved classes: Delta (Delta1, Delta3, and Delta4) and Jagged (Jagged1 and Jagged2). The Delta and Jagged families encode related transmembrane proteins that share a conserved DSL (Delta/Serrate/Lag-2) domain that is required for binding to Notch, but they differ significantly in the overall size of their extracellular domains. Jagged molecules possess additional epidermal growth factor (EGF)–like repeats (16 rather than 8) and a unique cysteinerich domain of unknown function.40
Delta and Jagged ligands are thought to activate Notch signals similarly through the activation of CBF1/RBPJκ.41-44 Nevertheless, there is evidence that signals transmitted through Delta or Jagged can differentially affect the Notch-expressing cell. Delta or Jagged expressed on dendritic cells may influence the ability of naive CD4 T cells to adopt either the T helper 1 (Th1) or Th2 lineage.45 A study by Jaleco et al,46 examining the effects of Delta1 and Jagged1 on human CD34+ stem cells, suggested that only Delta promotes T-lineage commitment. However, because mRNAs for Delta1, Delta4, Jagged1, and Jagged2 can be detected in the thymus,47-49 it is likely that T-cell precursors encounter both classes of Notch ligands as they mature. It is therefore key to understand whether Notch signals through Delta and Jagged have distinct roles in regulating T-cell development. To address this question, we have adapted a recently described in vitro culture system47 to examine the effect of Delta1 versus Jagged1 on T-cell progenitors at specific developmental stages.
Materials and methods
Immunohistochemistry
Thymic tissue was frozen in OCT compound (Sakura Finetek, Torrance, CA), cut into 6- to 8-μm sections and collected on Frost Plus microscope slides (VWR Scientific, West Chester, PA). After air drying for at least 2 hours, the tissue was immersed for 20 minutes in cold acetone (–20°C), rinsed in phosphate-buffered saline (PBS), and then processed for immunofluorescence microscopy. Sections were stained with a cocktail of primary antibodies diluted in neat culture supernatant from either the ER-TR550 (a generous gift of W. Van Ewijk; Academic Hospital, Maastricht, The Netherlands) or CDR-151 (American Type Culture Collection [ATCC], Rockville, MD) hybridomas. Expression of Notch ligands was detected by using the following polyclonal antisera from Santa Cruz Biotechnology (Santa Cruz, CA): Jagged1 (sc-6011), Delta1 (sc-8155), and Jagged2 (sc-8158). Antisera directed against Delta1 and Jagged2 revealed staining on cortical epithelial cells, but the signal was not inhibited by preincubation with the immunizing peptides, and these data are not shown. Fluorochrome-conjugated anti-immunoglobulin (Ig) secondary antibodies, chicken anti–goat IgG Alexa 488, and rabbit anti–rat IgG Alexa 546 conjugates were purchased form Molecular Probes (Eugene, OR). Secondary antibodies were diluted in PBS containing 10 mg/mL bovine serum albumin (BSA; Sigma Chemical, St Louis, MO) and 10% normal mouse serum. Images were acquired using a Nikon Microphot-SA microscope equipped with a 20 ×/0.5 objective lens and a Nikon FX-35DX camera (Nikon, Tokyo, Japan) with SPOT 3.0.2 software (Diagnostic Instruments, Sterling Heights, MI). Image overlays were performed with Adobe Photoshop 7.0 software (Adobe, San Jose, CA).
Generation of OP9 stromal cells expressing Jagged1
The full-length cDNA for murine Jagged1 (accession BC058675) was obtained from ATCC (Image clone 6834418) and cloned into the EcoRI site of MigR1 (a kind gift from Dr W. Pear, University of Pennsylvania, Philadelphia), and the sequence of the coding region of Jagged1 was confirmed by dideoxy sequencing. The resulting expression vector encodes a bi-cistronic transcript for Jagged1 and green fluorescent protein (GFP) separated by an internal ribosomal entry sequence (IRES). Retroviral supernatants were generated in the Phoenix ecotropic packaging cell line as described previously52 and used to generate OP9 stromal cells expressing empty vector (MigR1) or Jagged1. GFP+ cells were purified by cell sorting, and expression of Notch ligands in the parental or OP9-Jagged1 stromal cells was confirmed by reverse transcription–polymerase chain reaction (RT-PCR). RNA was isolated using STAT-60 (Tel-Test, Friendswood, TX) according to the manufacturer's instructions, and cDNA was generated using Moloney murine leukemia virus (M-MuLV) reverse transcriptase (Fermentas, Hanover, MD). cDNAs were normalized by TaqMan PCR (PE Applied Biosystems, Foster City, CA) for hypoxanthine phosphoribosyltransferase (HPRT), as described previously.53 PCR was carried out on normalized cDNAs using Taq polymerase (Perkin Elmer, Boston, MA) and the following primers: Jagged1, (5′TGGTAGACAGAGAGGAGAAGG and 5′TCAATTTCCCAGCCAACC); Jagged2, (5′TGGAAACAGTTGTTATGGGTG and 5′GGTGAACTTGTGTGAGATGAACT); Delta1, (5′ACCTCGGGATGACGCCTTTG and 5′AGACCACCACAGCAGCACAG); Delta4, 5′GCACCAACTCCTTCGTCGTC and 5′TCACAAAACAGACCTCCCCA); and glyceraldehyde-3-phosphate dehydrogenase (G3PDH), (5′TGAAGGTCGGTGTGAACGGATTTGGC and 5′CATGTAGGCCATGAGGTCCACCAC). The identity of PCR products was confirmed by Southern blot hybridization, and relative expression level was estimated by comparing the level of input cDNA (5-fold serial dilutions) resulting in equivalent hybridization signals. Expression of Jagged1 protein was detected by flow cytometric analysis (CellQuest Pro software;, BD Biosciences, San Diego, CA) using a goat polyclonal antibody directed against the extracellular domain of rat Jagged1 (R&D Systems, Minneapolis, MN), or by immunoprecipitation using the above antibody, followed by Western blot using a goat polyclonal antibody specific for the intracellular domain (sc-6011) of rat Jagged1 (Santa Cruz Biotechnology).
C2C12 differentiation cultures
C2C12 myoblasts (ATCC) were maintained in Dulbecco modified Eagle medium (DMEM) containing 10% fetal calf serum (Gibco/Invitrogen, Carlsbad, CA) and 5% cosmic calf serum (Hyclone, Logan, UT). C2C12 cells were plated in 24-well culture dishes at 5 × 103 cells/well on monolayers of OP9 stromal cells that had been plated at 2.5 × 104 cells/well on the previous day. C2C12 differentiation was induced by replacing the culture medium with differentiation media, DMEM 10% horse serum (Gibco). The γ-secretase inhibitor X (Calbiochem, San Diego, CA) or 0.1% dimethyl sulfoxide (DMSO) carrier was added to selected cultures, and the growth medium was replaced every 2 to 4 days. To monitor differentiation, RNA was isolated from a single well to generate cDNA, and cDNAs were normalized by TaqMan for HPRT. The degree of C2C12 differentiation was monitored by RT-PCR for myosin light chain 2 (MLC2) as described previously.54
Isolation of stem cells
Stem cells were isolated from 4- to 12-week-old C57BL/6 mice (Taconic, Germantown, NY). For bone marrow (BM)–HSCs, bone marrow was pre-depleted with biotinylated antibodies for B220 (RA3-6B2), Mac1 (M1/70), and Gr-1 (8C5), using streptavidin-conjugated magnetic beads (Dynal Biotech, Brown Deer, WI), according to the manufacturer's instructions. Cell suspensions were incubated with Fc-block (24G2) prior to staining for cell surface markers. Cells were stained for surface expression of lineage (lin) markers using phycoerythrin (PE)–conjugated antibodies to the following antigens: Thy1.2 (53-2.1), B220 (RA3-6B2), Mac1 (M1/70), Gr1 (8C5), CD11c (HL3), Ly-76 (Ter-119), NK1.1 (PK136), TCRβ/H57-597, TCRδ/GL3, and lineage-positive cells were gated out. HSCs were identified as lin– cKithi (2B8) Sca1hi (E13-161.7). For thymus DN subsets, thymocytes were pre-depleted with biotinylated antibodies for CD4 (RM4-5) and CD8 (53.67), and lineage marker–positive cells were gated out as described for BM-HSCs, except Ter119, Gr1, and Thy1.2 antibodies were omitted and T cells were excluded with CD4 and CD8. DN1, DN3, and DN4 thymocytes were identified by expression of CD44 (IM7) and CD25 (7D4). All stem cells were sorted by using a fluorescence activated cell sorting (FACS) Vantage Cell sorter (BD Biosciences), and antibodies were obtained from Pharmingen (BD Biosciences).
Cell culture for in vitro T-cell development
Parental OP9 stromal cells and cells expressing Delta1 were a kind gift from J. C. Zuniga-Pflucker (University of Toronto, Ontario, Canada). In vitro T-cell development cultures were carried out as described previously.47,53 OP9 monolayers were prepared 1 day in advance by plating stromal cells at 2.5 × 104 cells/well in 24-well culture dishes, and stem cell populations were plated at 1000 and 10 000 cells per well onto OP9 monolayes in RPMI media (Gibco) containing 10% fetal calf serum (FCS), supplemented with L-glutamine, β-mercaptoethanol, penicillin/streptomycin, and gentamycin. Growth medium was supplemented with 5 ng/mL recombinant interleukin 7 (IL-7) and fms-like tyrosine kinase 3 ligand (Flt3L; PeproTech, Rocky Hill, NJ). The γ-secretase inhibitor X (Calbiochem) or 0.1% DMSO carrier was added to selected wells on day 0 and replaced every 3 to 4 days.
Flow cytometric analysis for cell differentiation markers
Expression of cell differentiation markers was analyzed by 4-color flow cytometry using a FACSCalibur (BD Pharmingen). Cells were incubated with Fc-block (24G2) and stained by using the following antibodies: B220-Cy-chrome (CyC; RA3-6B2), CD19-bio (ID3), CD44-fluorescein isothiocyanate (FITC; IM7) CD25-PE (7D4), CD4-CyC (RM4-5), CD8-allophycocyanin (APC; 53.67), NK1.1-PE (PK136), CD11c-FITC (HL3), TCRβ-FITC (H57-597), TCRδ-PE (GL3).
Results
Immunohistochemical analysis of Jagged1 expression in the thymus
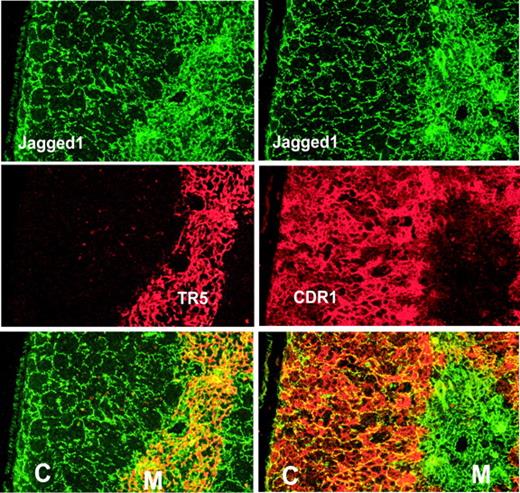
Notch ligands belonging to both the Delta and Jagged classes have been shown by PCR analysis to be expressed in the thymus.47-49 To examine the spatial organization of Notch ligands within the adult thymus, we examined their expression by immunohistochemistry. We were unable to obtain specific staining with antibodies for Delta1 or Jagged2 (described in “Materials and methods”). Results using antisera against Jagged1, for which staining was completely blocked by preincubation with the immunizing peptide (not shown), are shown in Figure 1. To differentiate between expression within the cortex or medulla, thymus sections were costained with antibodies ER-TR550 or CDR-1,51 which label medullary or cortical epithelial cells, respectively. Jagged1 is most highly expressed within the medulla, and costaining with ER-TR5 reveals almost complete overlap of the 2 markers (Figure 1, left panels). In the thymus cortex, Jagged1 is expressed on only a subset of cortical epithelial cells that form a loose network extending from the medulla to the capsule (Figure 1, right panels).
Immunohistochemical analysis of Jagged1 expression in the thymus. Frozen sections of C57BL/6 thymic lobes were stained with antibody specific for the intracellular domain of Jagged1 (green) and costained (red) with antibodies specific for medullary (TR5) or cortical epithelial cells (CDR1). Bottom panels show overlays of the 2 stains above, with the cortex (C) and medulla (M) indicated (Magnification, × 20).
Immunohistochemical analysis of Jagged1 expression in the thymus. Frozen sections of C57BL/6 thymic lobes were stained with antibody specific for the intracellular domain of Jagged1 (green) and costained (red) with antibodies specific for medullary (TR5) or cortical epithelial cells (CDR1). Bottom panels show overlays of the 2 stains above, with the cortex (C) and medulla (M) indicated (Magnification, × 20).
Generation of OP9-Jagged1 stromal cells
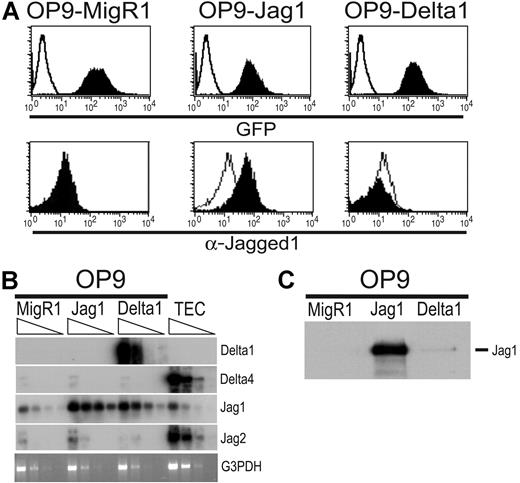
Forced expression of Delta1 on the bone marrow–derived stromal cell line OP9, which otherwise promotes only myeloid and B-cell development, renders these cells able to induce robust proliferation and maturation of T-cell precursors, up to at least the DP stage of development.47 To compare Delta versus Jagged signals, we overexpressed the full-length cDNA for murine Jagged1 in the parental OP9 using the same expression vector (MigR1) that was used to express Delta1 in these cells. This vector allows coexpression of either Delta1 or Jagged1 as a bi-cistronic message with green fluorescence protein (GFP). The similar level of GFP fluorescence in OP9-MigR1, OP9-Jagged1, and OP9-Delta147 is shown in Figure 2A. We confirmed that Jagged1 is expressed on the surface of OP9-Jagged1 cells by flow cytometric analysis using an antibody directed against the extracellular domain of Jagged1 (Figure 2A). The antibody gave similar levels of background staining on OP9-MigR1 and OP9-Delta1 cells but a clear shift in staining on OP9-Jagged1 cells.
Characterization of OP9 stromal cells expressing murine Jagged1. (A) Flow cytometric analysis of OP9-MigR1, OP9-Jagged1 (Jag1), and OP9-Delta1 for GFP expression (top row) or for cell surface expression of Jagged1 (bottom panels). Empty histograms show negative controls. For GFP, control is fluorescence in nontransduced OP9 cells (top panels). For anti-Jagged1 staining, control is OP9-MigR1 cells stained with anti-Jagged1 (bottom row). (B) Expression of Notch ligand mRNAs in OP9 stromal cells and normal thymic epithelial cells (TECs) by RT-PCR followed by Southern blot. cDNAs were normalized by TaqMan PCR for HPRT, and 5-fold serial dilutions of normalized cDNAs were subjected to PCR for different Notch ligands or G3PDH as a loading control. (C) OP9 cell lysates were immunoprecipitated with an antibody directed against the extracellular domain of Jagged1, and Western blots were probed with antisera directed against the intracellular domain of Jagged1.
Characterization of OP9 stromal cells expressing murine Jagged1. (A) Flow cytometric analysis of OP9-MigR1, OP9-Jagged1 (Jag1), and OP9-Delta1 for GFP expression (top row) or for cell surface expression of Jagged1 (bottom panels). Empty histograms show negative controls. For GFP, control is fluorescence in nontransduced OP9 cells (top panels). For anti-Jagged1 staining, control is OP9-MigR1 cells stained with anti-Jagged1 (bottom row). (B) Expression of Notch ligand mRNAs in OP9 stromal cells and normal thymic epithelial cells (TECs) by RT-PCR followed by Southern blot. cDNAs were normalized by TaqMan PCR for HPRT, and 5-fold serial dilutions of normalized cDNAs were subjected to PCR for different Notch ligands or G3PDH as a loading control. (C) OP9 cell lysates were immunoprecipitated with an antibody directed against the extracellular domain of Jagged1, and Western blots were probed with antisera directed against the intracellular domain of Jagged1.
Expression of the mRNAs for various Notch ligands was examined in the OP9 cell lines and in the total thymus epithelial cell (TEC) fraction by RT-PCR analysis (Figure 2B). TECs express detectable levels of Jagged1, Jagged2, and Delta4 mRNAs. The parental OP9 stromal cells express low levels of Jagged1 and undetectable levels of Jagged2, Delta1, or Delta4 mRNAs. As expected, Jagged1 and Delta1 mRNAs are highly expressed in OP9-Jagged1 and OP9-Delta1, respectively. Surprisingly, expression of the endogenous Jagged1 mRNA is induced by approximately 25-fold in OP9-Delta1, suggesting that OP9-Delta1 could express both Delta1 and Jagged1 proteins. However, by immunoprecipitation/Western blot analysis, we were unable to detect endogenous Jagged1 protein in the parental OP9 cells and only trace levels in OP9-Delta1 (Figure 2C).
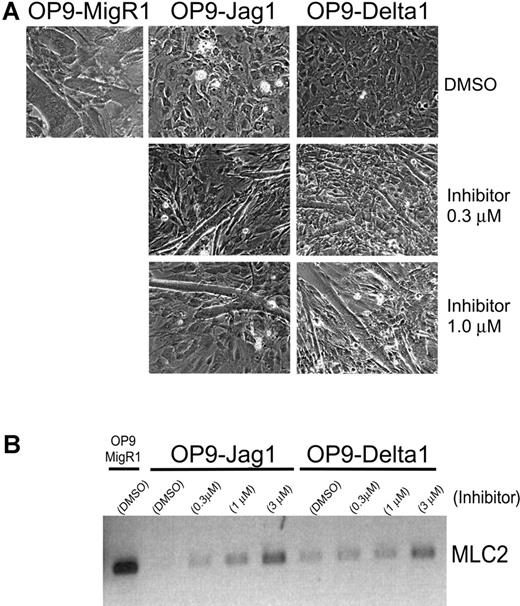
To ensure that Jagged1 is functional in OP9-Jagged1 cells and to directly compare the ability of OP9-Jagged1 and OP9-Delta1 to transmit Notch signals, we used a well-characterized system in which Notch signals prevent the differentiation of C2C12 myoblast cells into mature myotubes.54 In this system, C2C12 differentiation can be monitored either by the appearance of large multinucleated myotubes or by the induction of myotube-specific genes such as MLC2. We compared the overall level of Notch signals delivered by OP9-Jagged1 and OP9-Delta1 by examining C2C12 differentiation in the presence of increasing doses of the γ-secretase inhibitor L-685458 (inhibitor X).55 The ability of OP9-Jagged1 versus OP9-Delta1 to transmit Notch signals to C2C12 and block differentiation was assessed by examining myotube formation (Figure 3A), or more quantitatively, by monitoring the expression of MLC2 by RT-PCR analysis (Figure 3B). Both OP9-Jagged1 and OP9-Delta1 blocked C2C12 differentiation, and addition of increasing doses of the γ-secretase inhibitor X promoted C2C12 differentiation to similar degrees in OP9-Jagged1 and OP9-Delta1 cocultures. These data suggest that Jagged1 and Delta1 transmit similar levels of Notch signals in C2C12 myoblasts.
Comparison of Notch signals transmitted to C2C12 myoblasts from OP9-Jagged1 and OP9-Delta1. (A) Differentiation of C2C12 myoblast cells after 7 days coculture on OP9-MigR1, OP9-Jagged1, or OP9-Delta1 in the presence of carrier alone (0.1% DMSO) or increasing concentrations of γ-secretase inhibitor X. (B) RT-PCR analysis for expression of a myotube differentiation marker, MLC2, in C2C12 myoblasts shown in panel A. Total RNA extracted from a single well was reverse transcribed and normalized by TaqMan PCR for HPRT.
Comparison of Notch signals transmitted to C2C12 myoblasts from OP9-Jagged1 and OP9-Delta1. (A) Differentiation of C2C12 myoblast cells after 7 days coculture on OP9-MigR1, OP9-Jagged1, or OP9-Delta1 in the presence of carrier alone (0.1% DMSO) or increasing concentrations of γ-secretase inhibitor X. (B) RT-PCR analysis for expression of a myotube differentiation marker, MLC2, in C2C12 myoblasts shown in panel A. Total RNA extracted from a single well was reverse transcribed and normalized by TaqMan PCR for HPRT.
Delta and Jagged inhibit B-cell development in an early thymic precursor but only Delta promotes T-cell maturation
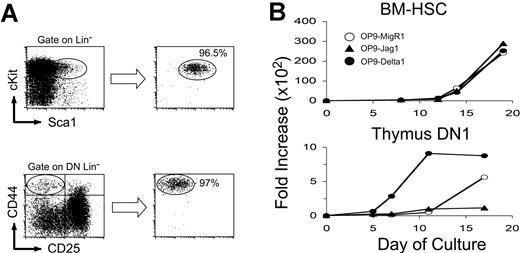
To determine whether Notch ligands differ in their ability to promote specific stages of T-cell development, we examined 2 different precursor populations: bone marrow–derived stem cells (BM-HSCs) and the earliest thymic progenitors, DN1 thymocytes (thymus DN1). BM-HSCs were isolated by gating out lineage marker–positive cells and sorting for c-Kithi/Sca1hi stem cells. DN1 thymocytes were sorted as lineage-negative CD44+/CD25– (Figure 4A). Sorted precursor populations were cultured on OP9-MigR1, OP9-Jagged1, and OP9-Delta1, and their ability to respond to Notch ligands was monitored by examining proliferation and inhibition of B-lineage commitment and T-cell maturation through the DN to DP stages.
Proliferation of BM-HSCs and DN1 thymocytes (thymus DN1) in OP9 stromal cell cocultures. (A) Isolation of stem cells from bone marrow and thymus by cell sorting. Bone marrow cells were pre-depleted for B220+, CD11b+, and Gr1+ cells, and thymocytes were pre-depleted for CD4+ and CD8+ cells by DYNAL magnetic bead separation. Stem cells were enriched to more than 96% purity by sorting for Lin– cKit+/Sca1+ (BM-HSCs) or Lin– CD44+/CD25– (thymus DN1). (B) Proliferation of BM-HSCs or thymus DN1 after coculture on OP9-MigR1, OP9-Jagged1, or OP9-Delta1. Total cell recovery is plotted as fold increase over the initial number of stem cells seeded on day 0.
Proliferation of BM-HSCs and DN1 thymocytes (thymus DN1) in OP9 stromal cell cocultures. (A) Isolation of stem cells from bone marrow and thymus by cell sorting. Bone marrow cells were pre-depleted for B220+, CD11b+, and Gr1+ cells, and thymocytes were pre-depleted for CD4+ and CD8+ cells by DYNAL magnetic bead separation. Stem cells were enriched to more than 96% purity by sorting for Lin– cKit+/Sca1+ (BM-HSCs) or Lin– CD44+/CD25– (thymus DN1). (B) Proliferation of BM-HSCs or thymus DN1 after coculture on OP9-MigR1, OP9-Jagged1, or OP9-Delta1. Total cell recovery is plotted as fold increase over the initial number of stem cells seeded on day 0.
BM-HSCs proliferated well irrespective of whether they received Notch signals (Figure 4B), although the cell type accumulating in these cultures differed dramatically (Figure 5). In contrast, DN1 thymocytes proliferated preferentially in response to Delta1 and expanded poorly on OP9-Jagged1. When cultured on OP9-MigR1, DN1 cells proliferated with delayed kinetics. It appeared from microscopic evaluation that only individual clones of DN1 thymocytes proliferated in OP9-MigR1 cultures. Limiting dilution analysis confirmed that fewer than 1 in 300 DN1 progenitors had the capacity to proliferate on OP9-MigR1 (data not shown and Porritt et al22 and Schmitt et al56 ).
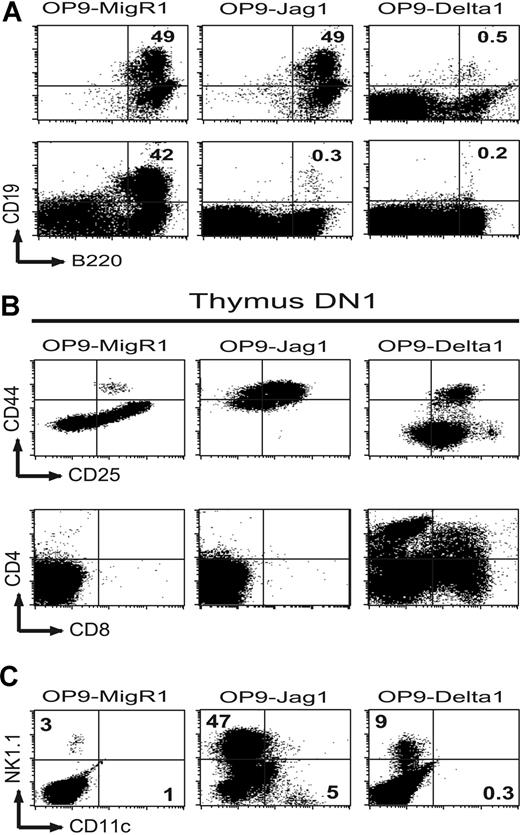
Both Delta1 and Jagged1 inhibit B-cell development in DN1 thymocytes, but only Delta1 promotes their maturation into CD4+CD8+ T cells. (A) Flow cytometric analysis for B-cell markers after 21 days of coculture of BM-HSCs (top row), or 11 days of coculture of thymus DN1 (bottom row) on OP9-MigR1, OP9-Jagged1, or OP9-Delta1. (B) Maturation of DN1 thymocytes into T cells after 26 days of coculture on OP9-MigR1, OP9-Jagged1, or OP9-Delta1. Early DN stages are distinguished by expression of CD44 and CD25, and maturation to the DP stage is monitored by expression of CD4 and CD8. (C) Differentiation of DN1 thymocytes into non–T-cell lineages after 26 days of coculture on OP9-MigR1, OP9-Jag1, or OP9-Delta1 by expression of CD11c and NK1.1. Numbers in quadrants refer to the percentage of gated populations in that quadrant.
Both Delta1 and Jagged1 inhibit B-cell development in DN1 thymocytes, but only Delta1 promotes their maturation into CD4+CD8+ T cells. (A) Flow cytometric analysis for B-cell markers after 21 days of coculture of BM-HSCs (top row), or 11 days of coculture of thymus DN1 (bottom row) on OP9-MigR1, OP9-Jagged1, or OP9-Delta1. (B) Maturation of DN1 thymocytes into T cells after 26 days of coculture on OP9-MigR1, OP9-Jagged1, or OP9-Delta1. Early DN stages are distinguished by expression of CD44 and CD25, and maturation to the DP stage is monitored by expression of CD4 and CD8. (C) Differentiation of DN1 thymocytes into non–T-cell lineages after 26 days of coculture on OP9-MigR1, OP9-Jag1, or OP9-Delta1 by expression of CD11c and NK1.1. Numbers in quadrants refer to the percentage of gated populations in that quadrant.
To examine whether coculture with OP9-Jagged1 and OP9-Delta1 inhibited B-cell development in the 2 cell populations described above, we monitored expression of B220 and CD19 (Figure 5A). As expected from previous data examining human stem cells,46 BM-HSCs developed efficiently into CD19+ B cells when cultured on OP9-MigR1 or OP9-Jagged1 but failed to differentiate into B cells on OP9-Delta1. Instead, BM-HSCs cultured on OP9-Delta1 differentiated into Thy1+ T cells (not shown). Similar to BM-HSCs, the few DN1 thymocytes that grew when cocultured with OP9-MigR1 differentiated efficiently into CD19+ B-lineage cells. However, in contrast to BM-HSCs, OP9-Jagged1 inhibited the differentiation of DN1 thymocytes into CD19+ B-lineage cells (Figure 5A).
Since Jagged1 signals to thymus DN1 appeared to inhibit B-cell development but failed to induce extensive proliferation of these cells, we examined whether Jagged1 signals were able to promote T-cell maturation through the DN to DP stages by monitoring expression of CD44, CD25, CD4, and CD8. As described previously,22 DN1 thymocytes cultured on OP9-Delta1 differentiated efficiently through the DN to DP stages, and by day 26, expressed CD4 and CD8 (Figure 5B). These cells expressed intermediate levels of TCRβ characteristic of DP thymocytes (not shown). In contrast, DN1 thymocytes cultured on OP9-Jagged1 did not mature beyond the CD44+CD25+ stage. Because DN1 progenitors can differentiate into both the NK and dendritic cell lineages, we also examined expression of NK1.1 and CD11c by these cells. In 3 independent experiments, we found increased percentages (between 20% and 65%) of NK1.1+ cells in thymus DN1 cells that had been cultured with OP9-Jagged1 (Figure 5C). Because the cells in these cultures did not proliferate well, the absolute number of NK1.1+ cells was not significantly increased in thymus DN1 that had been cultured on OP9-Jagged1 compared with OP9-MigR1 or OP9-Delta1, although we did observe a slight increase of approximately 2- to 3-fold in some experiments. Although NK1.1+ cells were gated out in our initial cell sort, it is unclear whether Jagged1 signals were able to promote the differentiation of uncommitted stem cells into the NK cell lineage, or whether Jagged1 allowed the selective survival of a rare NK1.1+ population by preventing both B- and T-cell development.
The above data raise an important question: why are signals from OP9-Jagged1 able to block B-cell development in DN1 thymocytes but unable to promote T-cell maturation? One possibility is that Notch signals initiated through Jagged and Delta are qualitatively different. For example, Jagged and Delta could activate unique sets of Notch target genes. Another possibility is that Notch signals through Jagged and Delta are quantitatively different. For example, it could be that only very weak Notch signals are required to inhibit B-cell development, while stronger Notch signals are required to promote T-cell development.
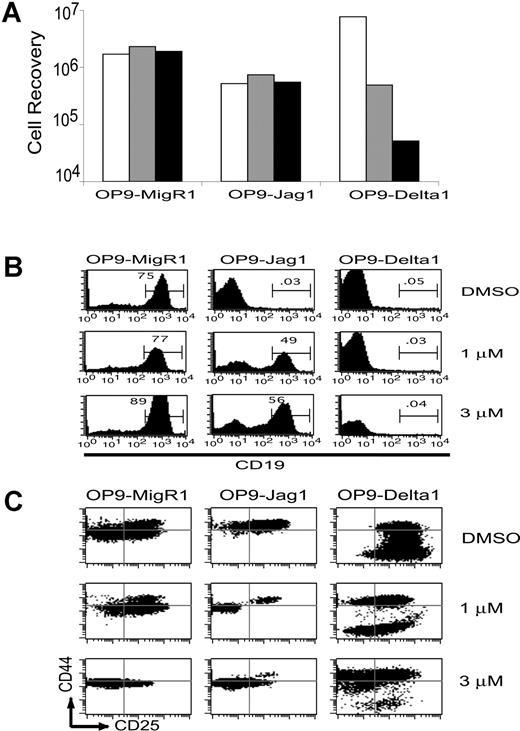
To compare the intensity of Notch signals delivered from OP9-Jagged1 versus OP9-Delta1 to DN1 thymocytes, we titrated the dose of Notch signals using the γ-secretase inhibitor X. Sorted DN1 thymocytes were cultured on OP9-MigR1, OP9-Jagged1, or OP9-Delta1 in the presence of increasing doses of inhibitor X. After 12 days, the relative intensity of Notch signals was compared by monitoring proliferation (Figure 6A), inhibition of B-cell development (Figure 6B), and maturation through the DN stages (Figure 6C). Inhibitor X, at 1 to 3 μM, did not significantly alter the recovery of DN1 thymocytes cultured on OP9-MigR1 or OP9-Jagged1 stromal cells. In contrast, inhibitor X decreased the recovery of DN1 thymocytes cultured on OP9-Delta1 in a dose-dependent manner (Figure 6A). DN1 thymocytes cultured on OP9-MigR1 differentiated efficiently into CD19+ B-lineage cells, and, as expected, this differentiation was not affected by inhibitor X (Figure 6B). As shown previously in Figure 5A, OP9-Jagged1 blocked B-cell differentiation, and even the lowest dose of inhibitor X restored B-cell development. In contrast, we were unable to completely block signals derived from OP9-Delta1. Even at the higher dose of 3 μM inhibitor X, which blocked proliferation of DN1 thymocytes by more than 100-fold (Figure 6A), we did not detect any CD19+ B cells. Higher doses of inhibitor X (9 μM) were toxic to the cells. DN1 thymocytes cultured on OP9-Delta1 in the presence of 3 μM inhibitor X appeared to arrest at the CD44+CD25+ stage (Figure 6C).
Signals from Jagged1 but not Delta1 are completely blocked by γ-secretase inhibitor X (inhibitor X). DN1 thymocytes were plated at 2000 cells/well on OP9-MigR1, OP9-Jagged1, or OP9-Delta1 in the presence of carrier (0.1% DMSO) or increasing concentrations of inhibitor X. After 12 days coculture, the cells were counted and examined for expression of differentiation markers. (A) Recovery of DN1 thymocytes per well in the presence of carrier alone (□), 1.0 μM (▦), or 3.0 μM (▪) inhibitor X. (B) Inhibition of B-cell development monitored by expression of CD19. (C) Maturation through the DN stages monitored by expression of CD44 and CD25.
Signals from Jagged1 but not Delta1 are completely blocked by γ-secretase inhibitor X (inhibitor X). DN1 thymocytes were plated at 2000 cells/well on OP9-MigR1, OP9-Jagged1, or OP9-Delta1 in the presence of carrier (0.1% DMSO) or increasing concentrations of inhibitor X. After 12 days coculture, the cells were counted and examined for expression of differentiation markers. (A) Recovery of DN1 thymocytes per well in the presence of carrier alone (□), 1.0 μM (▦), or 3.0 μM (▪) inhibitor X. (B) Inhibition of B-cell development monitored by expression of CD19. (C) Maturation through the DN stages monitored by expression of CD44 and CD25.
DN3 thymocytes respond weakly to Jagged1 signals
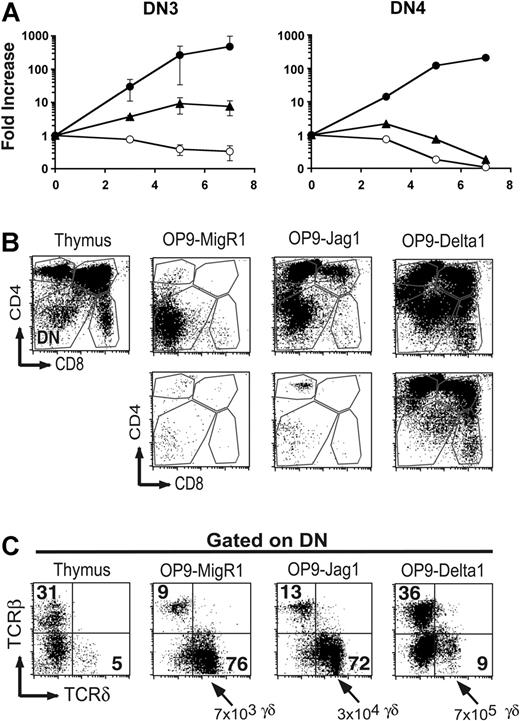
Although Jagged1 signals did not promote the differentiation of DN1 thymocytes past the DN2 stage, Jagged signals may play a role during the later DN3 or DN4 stages of T-cell development. To address this, we sorted lineage-negative, DN3 (CD44–CD25+) or DN4 (CD44–CD25–), thymocytes and examined their ability to proliferate in response to OP9-MigR1, OP9-Jagged1, or OP9-Delta1. Consistent with previous findings,38,53 DN3 and DN4 thymocytes failed to proliferate on OP9-MigR1, while they proliferated vigorously on OP9-Delta1, demonstrating that they remained dependent on Notch signals in this culture system. DN3 thymocytes proliferated weakly in response to signals from OP9-Jagged1, resulting in at least 10-fold greater cell recoveries compared with OP9-MigR1, but 50-fold lower cell recoveries compared with OP9-Delta1 (Figure 7A). DN4 thymocytes failed to proliferate in response to either OP9-Jagged1 or OP9-MigR1 (Figure 7A).
Effect of Notch ligands on the proliferation and maturation of DN3 and DN4 thymocytes. Thymocytes were depleted for CD4+ and CD8+ cells by magnetic bead separation, and DN3 and DN4 subsets were isolated by sorting for Lin– CD44–CD25+ (DN3) or CD44–CD25– (DN4) cells to more than 99% purity. Cells were cultured for 7 days on OP9-MigR1, OP9-Jagged1, or OP9-Delta1. (A) Cell recovery over time in culture. ○ indicates OP9-MigR1; ▴, OP9-Jag1; and •, OP9-Delta1. (B) Maturation of DN3 (top row) or DN4 (bottom row) thymocytes monitored by expression of CD4 and CD8 surface molecules. Expression of CD4 and CD8 molecules on normal thymocytes is shown as a staining control. (C) Expression of TCRβ and TCRδ in the recovered DN3 population shown in panel B. The total number of γδ T cells recovered per well was averaged from 3 independent experiments.
Effect of Notch ligands on the proliferation and maturation of DN3 and DN4 thymocytes. Thymocytes were depleted for CD4+ and CD8+ cells by magnetic bead separation, and DN3 and DN4 subsets were isolated by sorting for Lin– CD44–CD25+ (DN3) or CD44–CD25– (DN4) cells to more than 99% purity. Cells were cultured for 7 days on OP9-MigR1, OP9-Jagged1, or OP9-Delta1. (A) Cell recovery over time in culture. ○ indicates OP9-MigR1; ▴, OP9-Jag1; and •, OP9-Delta1. (B) Maturation of DN3 (top row) or DN4 (bottom row) thymocytes monitored by expression of CD4 and CD8 surface molecules. Expression of CD4 and CD8 molecules on normal thymocytes is shown as a staining control. (C) Expression of TCRβ and TCRδ in the recovered DN3 population shown in panel B. The total number of γδ T cells recovered per well was averaged from 3 independent experiments.
To determine whether Jagged1 signals influence the maturation of DN3 thymocytes, we examined expression of CD4, CD8, TCRβ, and TCRδ after 7 days of coculture. DN3 thymocytes matured efficiently to the DP stage in response to OP9-Delta1 and appeared to differentiate preferentially into CD4 single-positive T cells on OP9-Jagged1 (Figure 7B). These CD4 single-positive cells expressed intermediate levels of TCRβ characteristic of immature DP thymocytes (data not shown). Examination of the cells that remained DN after 7 days of culture revealed that DN3 cocultured on OP9-MigR1 or on OP9-Jagged1 differentiated preferentially into γδ T cells (Figure 7C). This resulted in a 4- to 5-fold absolute increase in the total yield of γδ T cells recovered from OP9-Jagged1 cultures compared with OP9-MigR1 but did not improve the yield of γδ T cells when compared with OP9-Delta1. These data demonstrate that DN3 and DN4 thymocytes continue to require Notch signals to proliferate in vitro, and that Jagged1 signals are less potent than Delta1 signals in promoting this proliferation. DN3 thymocytes that are stimulated with no or weak Notch signals, provided by either OP9-MigR1 or OP9-Jagged1, differentiate preferentially into γδ T cells.
Discussion
The existence of 2 classes of Notch ligands is highly conserved through evolution.40,57 Whether this is simply to allow their differential tissue expression, or whether the ligands transmit distinct signals is unknown. Our data suggest that Jagged and Delta transmit distinct signals to T-cell progenitors and influence the ability of thymocytes to develop along the NK, or the αβ versus γδ T-cell lineages.
Several previous reports have proposed that Jagged signals are unable to promote T-cell development from hematopoietic stem cells.46,58 Our data reveal that Jagged signals prevent the development of B-lineage cells from DN1 thymocytes. However, it remains unclear how, or during which maturational stage, B-cell development is inhibited. Analysis of mice in which Notch signaling is activated in the bone marrow, through expression of a constitutively active Notch-IC31 suggest that Notch signals inhibit B-lineage commitment prior to the B220+ stage. We have also observed that expression of Notch-IC in bone marrow–derived stem cells results in complete inhibition of B220+ cells in vitro (data not shown). In contrast, activation of Notch signals through Delta1 or Jagged1 permits the development of a significant B220+ population (Figure 5). It is not clear whether these cells represent B-cell progenitors; however, it is known that the B220+CD19– fraction in normal bone marrow is not committed to the B-cell lineage and includes a common lymphoid precursor that retains T-cell, B-cell, and NK cell potential.25
DN1 thymocytes stimulated with Jagged1 fail to differentiate into either B or T cells and develop preferentially into NK cells. Our immunohistochemical analysis suggests that Jagged1 is expressed in the appropriate anatomic location to influence this decision in vivo. Uncommitted lymphoid precursors are thought to enter the thymus through blood vessels near the corticomedullary junction,59,60 and we observed high levels of Jagged1 expression on the endothelial cells lining thymic blood vessels (Figure 1 and data not shown).
In the case of committed T-cell precursors, we observed that Jagged signals can influence the differentiation of DN3 thymocytes into the αβ versus γδ T-cell lineage. The notion that Notch signals can influence γδ T-cell development is supported by evidence obtained in 3 independent knockout studies. First, T-cell progenitors derived from Notch1+/– mice differentiate preferentially into the γδ T-cell lineage.35 Second, inhibition of Notch signals during the DN2/DN3 stage through targeted deletion of CBF1/RBPJκ results in increased development of γδ T cells.36 And third, Jagged2 signals appear to be necessary for generating normal numbers of γδ T cells, as Jagged2–/– mice produce reduced numbers of γδ T cells in the fetal thymus.61 Together, these data suggest that reduced Notch signals, or signals through Jagged, favor γδ T-cell development.
Although the above data clearly suggest that signals from Delta versus Jagged influence the differentiation of thymic T-cell precursors, it remains unclear how these signals promote cell lineage choices. Our observation that stromal cells expressing Jagged alone favor different differentiative fates from DN1 versus DN3 progenitors provides an important clue. Our data suggest that Jagged signals do not actively induce specific cell lineage choices but favor the selection of a default fate. DN1 thymocytes have the potential to differentiate into T cells, B cells, and NK cells. In the absence of Notch signals, a subset of progenitors found within the DN1 population differentiate into rapidly proliferating B-lineage cells, whereas potent Delta signals promote T-cell maturation. In this case, Jagged signals favor NK cell development by preventing the development of B-lineage precursors but failing to promote T-cell maturation. Recent data examining the differentiative potential of DN2 thymocytes have shown that DN2 thymocytes differentiate into NK cells in the absence of Notch-Delta signals.56
In the case of DN3 thymocytes, our data also suggest that Jagged signals favor γδ T-cell development by promoting a default cell fate. DN3 thymocytes, which are committed to the T-cell lineage, require Notch signals from Delta to proliferate in vitro, even after they have received a productive pre-TCR signal.38 DN3 thymocytes differentiate preferentially into γδ T cells when cultured on either OP9-MigR1 or on OP9-Jagged1 (Figure 7C). However, DN1 thymocytes do not differentiate preferentially into the γδ T-cell lineage in response to Jagged signals (data not shown). Together, these data suggest that Jagged signals favor γδ T-cell development by providing an essential survival signal to DN3 thymocytes (Figure 7A), whereas only Delta signals promote the massive proliferation of TCRβ-selected DN3 and DN4 thymocytes.
Future experiments will be necessary to address the question of how Notch signals emanating from Jagged and Delta differ at the molecular level. We envision 2 likely models. They differ qualitatively, through the activation of distinct downstream events, or quantitatively. Our culture system is not ideally suited to addressing these questions because OP9 stromal cells express a low level of Jagged1 mRNA that is further induced in OP9-Delta1. Therefore, it is unclear whether our results obtained using OP9-Delta1 reflect the coordinated actions of both Jagged1 and Delta1 signals. Furthermore, we cannot rule out the possibility that expression of Notch ligands in OP9 stromal cells alters the expression of other genes that could influence the ability of the cells to support T-cell development.
Jagged1-mediated inhibition of B-cell development was completely blocked by the presenilin inhibitor, while Delta1-mediated inhibition of B-cell development was not (Figure 6), raising the possibility that Delta1 could activate a presenilin-independent pathway. We were unable to isolate sufficient quantities of DN1 thymocytes to test directly whether Jagged1 and Delta1 differentially activate Notch responsive genes. However, this model appears unlikely because it would imply that Delta and Jagged inhibit B-cell development through different pathways. Another possibility is that signals through Delta and Jagged regulate the intensity of Notch signals, for example, through the action of Fringe molecules which are thought to selectively inhibit signals through Jagged while permitting Notch-Delta signals.62 Our observations comparing the ability of OP9-Jagged1 and OP9-Delta1 to inhibit B-lineage commitment in DN1 thymocytes support this hypothesis, as Jagged1 signals are more easily inhibited by the presenilin inhibitor X (Figure 6B). Examination of the effect of presenilin inhibitors on DN1 thymocyte proliferation is complicated by the fact that, while developing T cells require Notch signals to proliferate, B-cell proliferation is independent of Notch signals. Since inhibition of Notch signals promotes B-lineage commitment, the cultures depicted in Figure 6A contain vastly different cell populations that may or may not require Notch signals to proliferate. However, examination of DN3 thymocytes reveals that OP9-Jagged1 induces significantly weaker proliferation compared with OP9-Delta1 (Figure 7A).
The existing in vivo studies examining the role of Jagged signals on developing thymocytes are complicated by potential redundancy with other Notch ligands, and the fact that both Jagged1- and Jagged2-deficient mice have severe developmental abnormalities (reviewed in Kojika and Griffin57 ). Our observation that Jagged1 and Delta1 differ in their ability to inhibit B-cell development and promote the maturation of T-cell precursors should facilitate future efforts to dissect how Notch signals are able to regulate these functions.
Prepublished online as Blood First Edition Paper, October 14, 2004; DOI 10.1182/blood-2004-08-3257.
Supported by National Institutes of Health (grant AI29802), the Howard Hughes Medical Institute, and a predoctoral training grant (GM07270).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Juan Carlos Zuniga-Pflucker for the OP9 stromal cell line expressing Delta1. We are also grateful to Alena Gallegos, Pamela Fink, and Gabriela Hernandez-Hoyos for helpful discussions and critical reading of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal