Abstract
Fibromodulin (FMOD) was shown to be highly overexpressed in chronic lymphocytic leukemia (CLL) cells compared with normal B lymphocytes by gene expression profiling. Therefore FMOD might serve as potential tumor-associated antigen (TAA) in CLL, enabling expansion of FMOD-specific T cells. In CLL samples derived from 16 different patients, high expression of FMOD by real-time reverse transcriptase–polymerase chain reaction (RT-PCR) was detectable in contrast to normal B lymphocytes. We used unpulsed native CLL cells and CD40 ligand (CD40L)–stimulated CLL cells as antigen-presenting cells (APCs) to expand autologous T cells from 13 patients. The number of T cells during 4 weeks of in vitro culture increased 2- to 3.5-fold and the number of T cells recognizing FMOD peptides bound to HLA-A2 dimers increased 10-fold. The expanded T cells also were able to secrete interferon-γ (IFN-γ) upon recognition of the antigen demonstrated by IFN-γ ELISPOT assays. T cells not only recognized HLA-A2–binding FMOD peptides presented by transporter-associated with antigen-processing (TAP)–deficient T2 cells, but also FMOD overexpressing autologous CLL cells in an HLA-A2–restricted manner. In summary, FMOD was shown for the first time to be naturally processed and presented as TAA in primary CLL cells, enabling the expansion of autologous tumor-specific T cells.
Introduction
It is well established that T lymphocytes are able to specifically recognize tumor cells, which is the principle of antigen-specific immunotherapy.1,2 It is suggested that most if not all tumors express antigens that cytotoxic T lymphocytes (CTLs) can potentially attack. According to Vonderheide et al, the requirements for a tumor-associated antigen (TAA) are to be overexpressed or de novo expressed in tumor cells compared to normal cells, to include peptide sequences that bind to major histocompatibility (MHC) molecules, to be processed by tumor cells such that antigen-derived peptides are available for binding to MHC molecules, to be recognized by the T-cell repertoire in an MHC-restricted fashion, and to permit the expansion of naive CTL precursors bearing specific T-cell receptors.3
Fibromodulin (FMOD) is one of the members of the leucinerich repeat protein family, first described as a 59-kDa collagen binding protein.4 FMOD exhibits a wide tissue distribution, with the highest abundance observed in articular cartilage, tendon, and ligament. FMOD is involved in the assembly of the extracellular matrix by virtue of its ability to interact with type I and type II collagen fibrils.5 Furthermore, interactions of FMOD with the transforming growth factor TGF-β were described, and FMOD may be a biologically relevant modulator of TGF-β activity.6,7
FMOD was identified as the gene with the highest fold difference in expression in CLL cells compared with normal B lymphocytes in 3 independent gene expression profiling analyses.8-10 The gene expression of CLL cells was compared with the expression of memory B cells, germinal center B cells, and naive B cells and with peripheral blood B lymphocytes of age-matched healthy donors.8,9 The overexpression also was demonstrated by RT-PCR.9 Recently, in a large series comprising 60 patients with CLL and 7 patients with mantle cell lymphoma, overexpression of FMOD was observed for all leukemic samples on the mRNA level, while expression of FMOD was not detected in 13 chronic myeloid leukemia (CML) patients, 11 acute lymphoblastic leukemia (ALL) patients, and in peripheral blood mononuclear cells (PBMCs) of 70 healthy donors tested. Overexpression of FMOD was also confirmed on the protein level by flow cytometric and Western blot analyses.11
Based on these findings suggesting that FMOD might serve as a unique tumor-associated antigen expressed in B-CLL, we wanted to investigate whether T cells from CLL patients are able to specifically recognize and respond to naturally processed and presented HLA-A0201 binding peptides of FMOD.
Patients, materials, and methods
Patients and B-CLL samples
After informed consent, peripheral blood was obtained from patients satisfying diagnostic criteria for B-CLL.12 Mononuclear cells were isolated on a Ficoll/Hypaque (Seromed, Berlin, Germany) density gradient by centrifugation, depleted of monocytes by adherence to plastic tissue-culture flasks, and cryopreserved in liquid nitrogen in the presence of 10% dimethyl sulfoxide (DMSO; Sigma-Aldrich, Steinheim, Germany). More than 90% of isolated cells coexpressed CD5 and CD19, as assessed by flow cytometry. Patients were either untreated or had not received cytoreductive treatment for a period of at least 3 months before investigation. The study included 16 HLA-A0201–positive patients (4 women and 12 men; 44 to 82 years of age, Binet A, 1; Binet B, 4; Binet C, 11).
Real-time polymerase chain reaction (PCR) analysis
Total RNA was extracted from cells using the Trifast reagent (PEQLAB, Erlangen, Germany) according to the manufacturer's protocol. cDNA synthesis and real-time PCR were performed with some modifications as previously described.13 For detection of FMOD-specific cDNA the following primer pairs were used: 5′-CAACACCTTCAATTCCAGCA-3′ and 5′-ACCTGCAGCTGGGAGAAGT-3′, resulting in a product size of 186 bp with a melting point of 86°C. Samples were normalized for GAPDH RNA expression with the following primer pairs: 5′-GCACCACCAACTGCTTAGCACC-3′, 5′-GTCTGAGTGTGGCAGGGACTC-3′ (product size: 637 bp) and for CD19 RNA expression with the following primer pairs: 5′-CTCCTTCTCCAACGCTGAGT-3′,5′-TGGAAGTGTCACTGGCATGT-3′.
Cell lines and peptides
Mec-1 cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD). T2 cells, a hybridoma of T- and B-lymphoblastoid cells with a deficient TAP transporter, were a generous gift from Dr Elfriede Nössner, Institute of Molecular Immunology, GSF, in Munich.
HLA-A0201–binding peptides from the sequence of FMOD were identified using 2 different publicly available data bases: http://syfpeithi.bmi-heidelberg.com/ and http://bimas.dcrt.nih.gov/molbio/hla_bind/.14 The following 4 peptides were used: F1 (7-17): LLLAGLFSL, F2 (206-215): YLQHNEIQEV, F3 (250-259): YMEHNNVYTV and F4 (226-235): YLLDLSYNHL. As controls, the MAGE-3–derived, HLA-A0201–binding peptide MAGE-3 (271-279): FLWGPRALV and as a peptide not binding to HLA-A0201, the immunoglobulin-derived peptide A98-Id (AHTKDGFNF) were used.3,15 All peptides were synthesized and purified by high-performance liquid chromatography (HPLC) by Dr G. J. Arnold at the Gene Center, Ludwig-Maximilians-University, in Munich. The peptides were dissolved in DMSO. All 4 FMOD peptides were shown to bind HLA-A0201 in a binding assay using the TAP-deficient cell line T2 (data not shown).
Immunophenotyping
Immunophenotyping was performed with the following monoclonal antibodies (mAbs) conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), or phycoerythrin cyanine 5 (PE-Cy5): CD3, CD4, CD5, CD8, CD19, CD30, CD54, CD58, CD80, HLA-DR (Immunotech/Coulter, Marseille, France), CD38, CD86, HLA-ABC (PharMingen, Hamburg, Germany). Fluorescence was measured using a FACScan (Coulter Electronics, Miami, FL).
B-CLL stimulation with CD40 ligand (CD40L)
For CD40L-induced activation, B-CLL cells were cultured with some modifications as previously described.16 Briefly, CD40L stably transfected mouse fibroblast L cells (CD40L-L cells) were γ-irradiated at 100 Gy, plated at 3.5 × 105 cells per well in 6-well plates in basal Iscove medium (Biochrome, Berlin, Germany), supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin and incubated overnight at 37°C in a 5% CO2 humidified atmosphere.17 B-CLL cells were cultured at 1 to 2 × 106 cells/mL in basal Iscove medium for 72 hours.
Generation of effector T cells
At least 2 × 105 CD3-positive T cells from CLL patients were incubated with native or CD40L-stimulated autologous CLL cells in the presence of interleukin-2 (IL-2: 40 U/mL, Cellconcepts, Umkirch, Germany) and interleukin-7 (IL-7; 10 ng/mL, Cellconcepts). Stimulation was performed on days 0, 7, 14, and 21. As indicated, native or stimulated CLL cells were incubated with either F1 plus F2 peptide (termed F1/2) or F3 plus F4 peptide (F3/4) (40 μg/mL) in the presence of β2-microglobulin (1.5 μg/mL) (Sigma-Aldrich, Steinheim, Germany) in basal Iscove medium for 4 to 6 hours prior to coincubation with T cells.
ELISPOT assay
The ELISPOT assay used to quantify peptide epitope-specific, IFN-γ–releasing effector cells was performed as described previously.18 Briefly, nitrocellulose-bottomed 96-well plates (MultiScreen MAHA; Millipore, Bedford, CA) were coated with anti–IFN-γ antibody (1-D1K; Mabtech, Sweden) overnight at 4°C. Cells were added in duplicates together with the peptides (10 μg/mL) and, as indicated, with the anti-MHC class I monoclonal antibody (mAb) (W6/32; Acris, Germany) and incubated overnight at 37°C. The biotinylated secondary antibody 7-B6-1-Biotin (Mabtech), streptavidin-alkaline phosphatase (Mabtech), and the alkaline phosphatase substrate pNPP (Bio-Rad, Hercules, CA) were used. Spots were counted in a blinded manner. As negative controls, stimulator and effector cells were incubated in the presence of a CLL-unrelated, HLA-A0201 binding peptide derived from MAGE-3; or with a peptide (A98-Id), which does not bind to HLA-A0201; or with DMSO. The background, which was the number of spots detected by coincubation of T cells and T2 cells in the presence of DMSO or the number of spots detected by coincubation of the T cells with native or CD40L-stimulated CLL cells in the presence of DMSO, respectively, was substracted from the experimental values to obtain the number of FMOD-specific spots. The ELISPOT assays were performed on days 14 and 21 of stimulation. To obtain the precursor frequency of FMOD-specific T cells in the peripheral blood of CLL patients, the ELISPOT assay was performed on day 0.
T-cell staining by HLA-A2–dimerX technology
DimerX staining was performed according to the manufacturer's instructions. DimerX HLA-A2:Ig fusion protein (1 μL/3 × 105 cells; Becton Dickinson, Heidelberg, Germany) was incubated with peptide (600 μg/mL) in phosphate-buffered saline overnight at 37°C. T cells were stained with the HLA-A0201-dimer/peptide complexes for 1 hour at 4°C. As a negative control, DimerX was treated overnight with DMSO in phosphate-buffered saline at the same concentration used for peptide incubation. The secondary rabbit anti–mouse immunoglobulin/R-phycoerythrin (RPE) F(ab′)2 antibody (DakoCytomation, Copenhagen, Denmark) was used. In addition, T cells were stained with anti–CD8-PE-Cy5 (Immunotech/Coulter, Marseille, France) and analyzed by flow cytometry. HLA-A2-dimer/peptide staining was performed on day 28. To obtain the precursor frequency of FMOD-specific T cells in the peripheral blood of CLL patients, HLA-A2-dimer/peptide staining was performed on day 0 without further enrichment of the T cells.
Statistics
Because of non-normality, statistical associations between dependent subgroups were carried out by the Wilcoxon test, and for independent subgroups the Mann-Whitney U test was applied. Correlations were calculated using Spearman correlation coefficient. A statistical significance was accepted for P < .05. The statistical calculations were performed using SPSS Version 11.5 (SPSS, Chicago, IL).
Results
mRNA overexpression of FMOD in CLL cells
Using real-time RT-PCR we detected FMOD expression in all 16 CLL samples tested and in the prolymphocytic leukemia cell line Mec-1. Normal B lymphocytes, that is, PBMCs derived from 3 different healthy donors and tonsillar B cells did not express FMOD mRNA. CLL samples were tested for FMOD expression before and after 72 hours of CD40 activation by CD40 ligand (CD40L) expressing mouse fibroblast L cells. There was no significant difference (Wilcoxon test: – 0.535; P = .593) in the number of cycles needed for detection of FMOD-specific transcripts of native CLL cells and CD40L-stimulated CLL cells (Table 1). Thus, CD40L stimulation did not alter FMOD RNA expression.
Real-time RT-PCR for detection of fibromodulin-specific mRNA
Sample . | Product at Tm = 86°C . | Cycles . |
|---|---|---|
| CLL-1 | + | 29.2 |
| CD40L—CLL-1 | + | 30.1 |
| CLL-2 | + | 24.7 |
| CLL-3 | + | 34.7 |
| CLL-4 | + | 31.0 |
| CD40L—CLL-4 | + | 29.6 |
| CLL-5 | + | 26.6 |
| CLL-6 | + | 30.3 |
| CLL-9 | + | 31.7 |
| CD40L—CLL-9 | + | 30.9 |
| D-1 | - | 44.3 |
| D-2 | - | 47.0 |
| D-3 | - | 42.0 |
| T-1 | - | 47.0 |
| Mec-1 | + | 28.3 |
Sample . | Product at Tm = 86°C . | Cycles . |
|---|---|---|
| CLL-1 | + | 29.2 |
| CD40L—CLL-1 | + | 30.1 |
| CLL-2 | + | 24.7 |
| CLL-3 | + | 34.7 |
| CLL-4 | + | 31.0 |
| CD40L—CLL-4 | + | 29.6 |
| CLL-5 | + | 26.6 |
| CLL-6 | + | 30.3 |
| CLL-9 | + | 31.7 |
| CD40L—CLL-9 | + | 30.9 |
| D-1 | - | 44.3 |
| D-2 | - | 47.0 |
| D-3 | - | 42.0 |
| T-1 | - | 47.0 |
| Mec-1 | + | 28.3 |
Given are the numbers of cycles needed for detection of fibromodulin-specific mRNA of unstimulated CLL samples, CD40L-stimulated CLL samples (CD40L-CLL), PBMCs of healthy donors (D), tonsillar B cells (T), and Mec-1 cells.
Native CLL cells are able to specifically expand autologous T cells
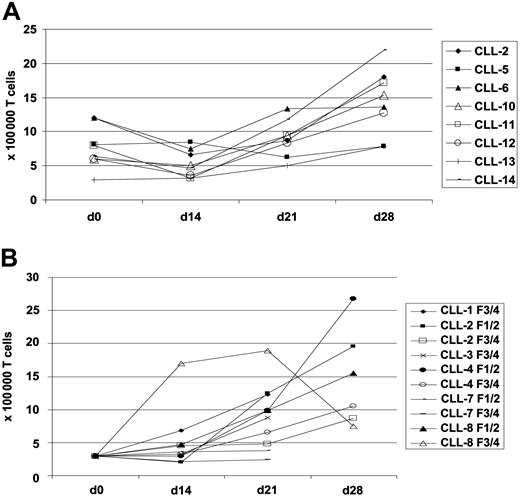
We wanted to find out whether FMOD RNA overexpression results in enhanced presentation of FMOD-derived peptides on the surface of CLL cells, which then could be recognized by autologous T cells in an HLA-A0201–restricted manner. We first used native CLL cells as APCs, which were not pulsed with FMOD peptides to expand autologous T cells from CLL patients. In this setting, we successfully expanded T cells in 7 of 14 cases tested (Figure 1A). In the first 2 weeks we observed a decrease in the number of T cells, but then the surviving T cells proliferated, resulting in a 2-fold increase in the absolute numbers after 4 weeks of in vitro culture.
Proliferation of autologous T cells derived from 8 CLL patients upon stimulation with native unpulsed CLL cells in the presence of IL-2 and IL-7. Shown are T-cell numbers (× 100 000) using native unpulsed CLL cells (A) and CD40L-stimulated, FMOD peptide–pulsed CLL cells (B) as APCs.
Proliferation of autologous T cells derived from 8 CLL patients upon stimulation with native unpulsed CLL cells in the presence of IL-2 and IL-7. Shown are T-cell numbers (× 100 000) using native unpulsed CLL cells (A) and CD40L-stimulated, FMOD peptide–pulsed CLL cells (B) as APCs.
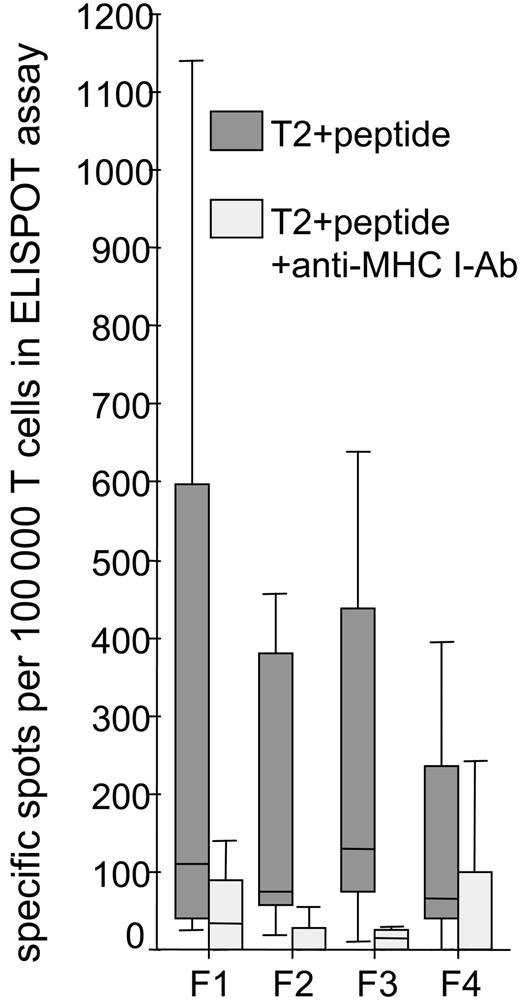
When T cells were obtained, we performed an IFN-γ–ELISPOT assay after 14 to 21 days of in vitro culture. In the ELISPOT assay, we used as stimulator cells T2 cells pulsed with the HLA-A0201–binding, FMOD-derived peptides F1, F2, F3, and F4. T2 cells pulsed with DMSO or an HLA-A0201–binding peptide derived from the CLL-unrelated antigen MAGE-3 served as negative controls. To test the antigen specificity, blocking experiments were performed with an MHC class I–specific monoclonal antibody (W6/32) (Table 2; Figure 2). Using T2 cells as APCs in the ELISPOT assay, we detected 0 to 1140 of 100 000 (median: 113 of 100 000) CD3-positive FMOD-specific T cells after 2 to 3 weeks of in vitro culture. In all the patients tested (7 of 8), HLA-A0201 restriction was shown by blocking with an MHC class I–specific monoclonal antibody (W6/32) (Wilcoxon test: – 4.542; P < .001). The rate of unspecific spots detected by adding the unrelated MAGE-3 peptide was low (median, 30; range, 0-54 spots per 100 000 T cells). This experiment proves that T cells from different CLL patients recognize all 4 tested FMOD-derived peptides in an HLA-A0201–restricted manner.
Specific recognition of fibromodulin-derived peptides presented on T2 cells by expanded T cells from CLL patients
Patient . | Peptide . | T2 + peptide . | T2 + peptide + anti—MHC-1 mAb . |
|---|---|---|---|
| CLL-2 | |||
| F1 | 110 | 35 | |
| F2 | 55 | 0 | |
| F3 | 35 | 30 | |
| F4 | 0 | 0 | |
| MAGE-3 | 30 | ND | |
| CLL-5 | |||
| F1 | 35 | 0 | |
| F2 | 20 | 0 | |
| F3 | 10 | 0 | |
| F4 | 30 | 0 | |
| MAGE-3 | 0 | ND | |
| CLL-6 | |||
| F1 | 25 | 0 | |
| F2 | 60 | 0 | |
| F3 | 115 | 0 | |
| F4 | 75 | 0 | |
| MAGE-3 | 50 | ND | |
| CLL-10 | |||
| F1 | 45 | 0 | |
| F2 | 75 | 0 | |
| F3 | 130 | 0 | |
| F4 | 65 | 0 | |
| MAGE-3 | ND | ND | |
| CLL-11 | |||
| F1 | 375 | 40 | |
| F2 | 305 | 55 | |
| F3 | 235 | 15 | |
| F4 | 50 | 0 | |
| MAGE-3 | 0 | ND | |
| CLL-12 | |||
| F1 | 1140 | 140 | |
| F2 | 1060 | 0 | |
| F3 | 640 | 20 | |
| F4 | 800 | 200 | |
| MAGE-3 | 0 | ND | |
| CLL-13 | |||
| F1 | 129 | ND | |
| F2 | 54 | ND | |
| F3 | 139 | ND | |
| F4 | 169 | ND | |
| MAGE-3 | 54 | ND | |
| CLL-14 | |||
| F1 | 820 | 243 | |
| F2 | 456 | 304 | |
| F3 | 1033 | 91 | |
| F4 | 395 | 243 | |
| MAGE-3 | 30 | ND |
Patient . | Peptide . | T2 + peptide . | T2 + peptide + anti—MHC-1 mAb . |
|---|---|---|---|
| CLL-2 | |||
| F1 | 110 | 35 | |
| F2 | 55 | 0 | |
| F3 | 35 | 30 | |
| F4 | 0 | 0 | |
| MAGE-3 | 30 | ND | |
| CLL-5 | |||
| F1 | 35 | 0 | |
| F2 | 20 | 0 | |
| F3 | 10 | 0 | |
| F4 | 30 | 0 | |
| MAGE-3 | 0 | ND | |
| CLL-6 | |||
| F1 | 25 | 0 | |
| F2 | 60 | 0 | |
| F3 | 115 | 0 | |
| F4 | 75 | 0 | |
| MAGE-3 | 50 | ND | |
| CLL-10 | |||
| F1 | 45 | 0 | |
| F2 | 75 | 0 | |
| F3 | 130 | 0 | |
| F4 | 65 | 0 | |
| MAGE-3 | ND | ND | |
| CLL-11 | |||
| F1 | 375 | 40 | |
| F2 | 305 | 55 | |
| F3 | 235 | 15 | |
| F4 | 50 | 0 | |
| MAGE-3 | 0 | ND | |
| CLL-12 | |||
| F1 | 1140 | 140 | |
| F2 | 1060 | 0 | |
| F3 | 640 | 20 | |
| F4 | 800 | 200 | |
| MAGE-3 | 0 | ND | |
| CLL-13 | |||
| F1 | 129 | ND | |
| F2 | 54 | ND | |
| F3 | 139 | ND | |
| F4 | 169 | ND | |
| MAGE-3 | 54 | ND | |
| CLL-14 | |||
| F1 | 820 | 243 | |
| F2 | 456 | 304 | |
| F3 | 1033 | 91 | |
| F4 | 395 | 243 | |
| MAGE-3 | 30 | ND |
ND indicates not done.
T cells from CLL patients were expanded using autologous, native, unpulsed CLL cells as APCs. IFN-γ—ELISPOT assays were performed on days 14 or 21 with T2 cells as APCs. The T2 cells were pulsed with HLA-A0201 binding fibromodulin-derived peptides (F1-F4) or with a CLL unrelated control peptide derived from MAGE-3. HLA-A0201 restriction of the T cells was shown by adding an anti-MHC class 1 mAb (W6/32). Coincubation of T2 cells with DMSO and the patients' T cells served as negative controls, and the number of spots detected were subtracted from the experimental values. Given are the numbers of fibromodulin-specific spots per 100 000 T cells.
HLA-A0201–restricted recognition of fibromodulin-derived peptides presented on T2 cells by expanded T cells from 8 CLL patients. The numbers of spots detected and presented in Table 2 were pooled to show the immunogenicity of the 4 fibromodulin-derived peptides and the HLA-A0201 restriction of the T cells by blockade with an anti-MHC class I mAb (W6/32).
HLA-A0201–restricted recognition of fibromodulin-derived peptides presented on T2 cells by expanded T cells from 8 CLL patients. The numbers of spots detected and presented in Table 2 were pooled to show the immunogenicity of the 4 fibromodulin-derived peptides and the HLA-A0201 restriction of the T cells by blockade with an anti-MHC class I mAb (W6/32).
Next, we wanted to investigate if the expanded T cells also recognize tumor cells overexpressing FMOD. Therefore we used autologous, native CLL cells as APCs in the ELISPOT assay. In the assay, CLL cells were pulsed with a mix of all 4 FMOD peptides. We detected 0 to 2717 spots (median, 73) per 100 000 T cells, which were FMOD specific. Again, the background value, obtained by adding DMSO to the APC, was subtracted from the experimental values. With one exception, the number of FMOD-specific spots detected was lower than observed with T2 cells. HLA-A0201–negative, but FMOD-overexpressing CLL cells from 2 different patients and HLA-A0201–positive nonmalignant cells from 5 different donors, like PBMC and tonsillar B cells, were recognized by the patients' T cells only in the range of background level (Table 3).
Specific recognition of HLA-A0201—binding fibromodulin-derived peptides present on native CLL cells by autologous T cells
. | Native autologous T cells . | Native CLL cells pulsed with F1-F4 . | HLA-A0201—negative, FMOD-overexpressing CLL cells . | HLA-A0201—positive healthy donors . | . | |
|---|---|---|---|---|---|---|
| Patient . | . | . | . | PBMCs . | Tonsillar B cells . | |
| CLL-2 | 0 | 0 | 0 | 0 | ND | |
| 0 | ||||||
| CLL-5 | 0 | ND | 0 | 0 | ND | |
| ND | ||||||
| CLL-6 | 0 | 20 | 3 | ND | ND | |
| 0 | ||||||
| CLL-10 | 0 | 73 | 25 | 0 | 0 | |
| 0 | ||||||
| CLL-11 | 0 | 15 | 0 | 0 | 0 | |
| 0 | ||||||
| CLL-12 | 0 | 2717 | 0 | 143 | 0 | |
| 0 | ||||||
| CLL-13 | 0 | 300 | 31 | 15 | 0 | |
| 0 | ||||||
| CLL-14 | 0 | 200 | 24 | 0 | 0 | |
| ND | ||||||
. | Native autologous T cells . | Native CLL cells pulsed with F1-F4 . | HLA-A0201—negative, FMOD-overexpressing CLL cells . | HLA-A0201—positive healthy donors . | . | |
|---|---|---|---|---|---|---|
| Patient . | . | . | . | PBMCs . | Tonsillar B cells . | |
| CLL-2 | 0 | 0 | 0 | 0 | ND | |
| 0 | ||||||
| CLL-5 | 0 | ND | 0 | 0 | ND | |
| ND | ||||||
| CLL-6 | 0 | 20 | 3 | ND | ND | |
| 0 | ||||||
| CLL-10 | 0 | 73 | 25 | 0 | 0 | |
| 0 | ||||||
| CLL-11 | 0 | 15 | 0 | 0 | 0 | |
| 0 | ||||||
| CLL-12 | 0 | 2717 | 0 | 143 | 0 | |
| 0 | ||||||
| CLL-13 | 0 | 300 | 31 | 15 | 0 | |
| 0 | ||||||
| CLL-14 | 0 | 200 | 24 | 0 | 0 | |
| ND | ||||||
T cells from CLL patients were expanded using autologous, native, unpulsed CLL cells as APCs. IFN-γ—ELISPOT assays were performed on days 14 or 21 with autologous, native CLL cells as APCs. HLA-A0201 binding fibromodulin-derived peptides (mix of all 4 peptides: F1-4) were added to obtain the number of fibromodulin-specific spots. Coincubation of native CLL cells with DMSO and autologous T cells served as negative controls, and the number of spots detected were subtracted from the experimental values. Given are the numbers of fibromodulin-specific spots per 100 000 T cells. HLA-A0201—negative CLL cells (n = 2) overexpressing fibromodulin and nonmalignant cells, for instance, PBMCs (n = 3), or tonsillar B cells (n = 2) from HLA-A0201—positive healthy donors were not recognized specifically. The precursor T-cell frequency was investigated by coincubation of native CLL cells and unstimulated autologous T cells together with the individual fibromodulin-derived peptides.
HLA-A2–dimer/peptide staining specific for FMOD of expanded T cells
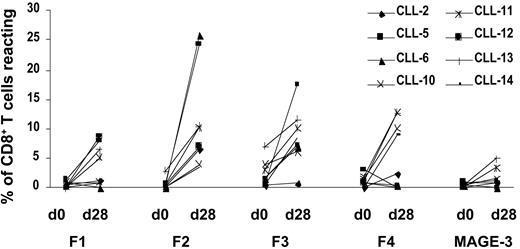
To further validate the data obtained by ELISPOT analysis, the expanded T cells were stained with HLA-A2-dimer/peptide complexes specific for FMOD and MAGE-3 on day 28, which is another method to identify specific T cells. Background values were obtained by staining cells with the HLA-A2–dimeric complex “loaded” with solvent only, and they were subtracted from the experimental values. Staining with HLA-A2–dimeric complexes carrying an unrelated peptide from MAGE-3 was low (median, 1.1%; range, 0%-5.0%). In contrast, the FMOD peptides were recognized by up to 26.1% (median, 5.1%) of all the CD8-positive T cells that were viable on day 28 (Table 4; Figure 3; Figure 4).
HLA-A2-dimer/peptide staining of CD8+ T cells from CLL patients before and after 28 days of in vitro culture
Patient . | Peptide . | Percentage of specific T cells, day 0 . | Percentage of specific T cells, day 28 . |
|---|---|---|---|
| CLL-2 | |||
| F1 | 0.46 | 1.30 | |
| F2 | 0 | 6.70 | |
| F3 | 0.50 | 0.80 | |
| F4 | 0.11 | 2.40 | |
| MAGE-3 | 0.99 | 1.00 | |
| CLL-5 | |||
| F1 | 1.31 | 2.90 | |
| F2 | 0.33 | 24.30 | |
| F3 | 0.49 | 15.70 | |
| F4 | 3.00 | 0 | |
| MAGE-3 | 0.90 | 1.10 | |
| CLL-6 | |||
| F1 | 0.99 | 0 | |
| F2 | 0 | 26.10 | |
| F3 | 1.77 | 7.10 | |
| F4 | 0.96 | 0.50 | |
| MAGE-3 | 0.50 | 0 | |
| CLL-10 | |||
| F1 | 0.88 | 1.00 | |
| F2 | 0.21 | 4.00 | |
| F3 | 3.95 | 6.00 | |
| F4 | 1.47 | 10.00 | |
| MAGE-3 | 0.40 | 1.60 | |
| CLL-11 | |||
| F1 | 0.11 | 5.10 | |
| F2 | 0.40 | 10.20 | |
| F3 | 3.03 | 10.10 | |
| F4 | 1.81 | 12.70 | |
| MAGE-3 | 0.15 | 3.40 | |
| CLL-12 | |||
| F1 | 0 | 8.80 | |
| F2 | 0.52 | 7.00 | |
| F3 | 1.54 | 7.20 | |
| F4 | 1.04 | 0.10 | |
| MAGE-3 | 0 | 0 | |
| CLL-13 | |||
| F1 | 0 | 6.40 | |
| F2 | 2.81 | 10.40 | |
| F3 | 7.08 | 11.60 | |
| F4 | 1.26 | 12.70 | |
| MAGE-3 | 0.33 | 5.00 | |
| CLL-14 | |||
| F1 | 0 | 1.00 | |
| F2 | 0 | 3.50 | |
| F3 | 0.40 | 8.40 | |
| F4 | 0.02 | 9.00 | |
| MAGE-3 | 0 | 0.90 |
Patient . | Peptide . | Percentage of specific T cells, day 0 . | Percentage of specific T cells, day 28 . |
|---|---|---|---|
| CLL-2 | |||
| F1 | 0.46 | 1.30 | |
| F2 | 0 | 6.70 | |
| F3 | 0.50 | 0.80 | |
| F4 | 0.11 | 2.40 | |
| MAGE-3 | 0.99 | 1.00 | |
| CLL-5 | |||
| F1 | 1.31 | 2.90 | |
| F2 | 0.33 | 24.30 | |
| F3 | 0.49 | 15.70 | |
| F4 | 3.00 | 0 | |
| MAGE-3 | 0.90 | 1.10 | |
| CLL-6 | |||
| F1 | 0.99 | 0 | |
| F2 | 0 | 26.10 | |
| F3 | 1.77 | 7.10 | |
| F4 | 0.96 | 0.50 | |
| MAGE-3 | 0.50 | 0 | |
| CLL-10 | |||
| F1 | 0.88 | 1.00 | |
| F2 | 0.21 | 4.00 | |
| F3 | 3.95 | 6.00 | |
| F4 | 1.47 | 10.00 | |
| MAGE-3 | 0.40 | 1.60 | |
| CLL-11 | |||
| F1 | 0.11 | 5.10 | |
| F2 | 0.40 | 10.20 | |
| F3 | 3.03 | 10.10 | |
| F4 | 1.81 | 12.70 | |
| MAGE-3 | 0.15 | 3.40 | |
| CLL-12 | |||
| F1 | 0 | 8.80 | |
| F2 | 0.52 | 7.00 | |
| F3 | 1.54 | 7.20 | |
| F4 | 1.04 | 0.10 | |
| MAGE-3 | 0 | 0 | |
| CLL-13 | |||
| F1 | 0 | 6.40 | |
| F2 | 2.81 | 10.40 | |
| F3 | 7.08 | 11.60 | |
| F4 | 1.26 | 12.70 | |
| MAGE-3 | 0.33 | 5.00 | |
| CLL-14 | |||
| F1 | 0 | 1.00 | |
| F2 | 0 | 3.50 | |
| F3 | 0.40 | 8.40 | |
| F4 | 0.02 | 9.00 | |
| MAGE-3 | 0 | 0.90 |
HLA-A2–dimer/peptide staining of T cells from a representative CLL patient (CLL-14) before and after 28 days of in vitro culture recognizing FMOD-derived peptides and MAGE-3–derived peptide. T cells were expanded using native CLL cells as APCs without the addition of exogenous peptide. The numbers given are the percentage of CD8-positive T cells reacting with the FMOD(F1-F4)–or MAGE-3–specific HLA-A2-dimer/peptide.
HLA-A2–dimer/peptide staining of T cells from a representative CLL patient (CLL-14) before and after 28 days of in vitro culture recognizing FMOD-derived peptides and MAGE-3–derived peptide. T cells were expanded using native CLL cells as APCs without the addition of exogenous peptide. The numbers given are the percentage of CD8-positive T cells reacting with the FMOD(F1-F4)–or MAGE-3–specific HLA-A2-dimer/peptide.
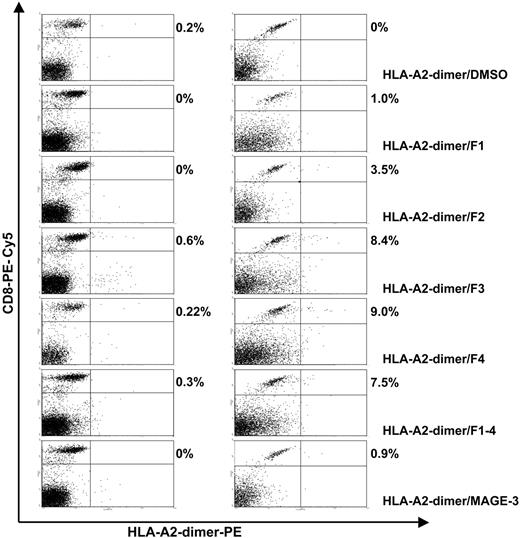
HLA-A2-dimer/peptide staining of precursor T cells and T cell being stimulated for 28 days of in vitro culture recognizing FMOD-derived peptides and the MAGE-3–derived peptide. A representative CLL patient (CLL-14) is shown. The precursor T-cell frequency of fibromodulin recognizing autologous T cells was tested without stimulation of the T cells. Then T cells were stimulated weekly for 28 days using autologous, native unpulsed CLL cells as APCs. The numbers given are the percentage of CD8-positive T cells costained with HLA-A2-dimer/peptide.
HLA-A2-dimer/peptide staining of precursor T cells and T cell being stimulated for 28 days of in vitro culture recognizing FMOD-derived peptides and the MAGE-3–derived peptide. A representative CLL patient (CLL-14) is shown. The precursor T-cell frequency of fibromodulin recognizing autologous T cells was tested without stimulation of the T cells. Then T cells were stimulated weekly for 28 days using autologous, native unpulsed CLL cells as APCs. The numbers given are the percentage of CD8-positive T cells costained with HLA-A2-dimer/peptide.
Because of the high frequency of T cells detected that recognized specifically FMOD peptides after 4 weeks of in vitro culture, we wanted to define the precursor frequency of T cells in CLL patients recognizing FMOD peptides. We performed an HLA-A2-dimer/peptide staining with PBMCs of CLL patients without stimulation. The frequency of CD8-positive T cells being able to recognize FMOD was between 0% and 7.1% (median, 0.5%) (Table 4). Taken together, the relative amount of FMOD-recognizing T cells increased 10-fold by stimulation of the T cells with autologous native CLL cells during 4 weeks of in vitro culture.
Relationship between FMOD-specific T cells detected by HLA-A2–dimer/peptide staining and ELISPOT analysis
In 2 patients (CLL-12, CLL-14) the number of HLA-A2-dimer/peptide–positive T cells recognizing all 4 FMOD peptides corresponded very well with the number of specific spots detected by the IFN-γ–ELISPOT assay with T2 cells as APCs. The ratio between spots in the ELISPOT assay and dots in the HLA-A2-dimer/peptide staining was 1:2 to 1:3. In 4 other patients (CLL-2, CLL-6, CLL-10, and CLL-11) some FMOD peptides were recognized in both assays with comparable frequency (1:3 to 1:10) and other FMOD peptides with a much lower frequency in the ELISPOT (1:11-1:83). In another 2 patients (CLL-5, CLL-13) only 1:50 to 1:300 of the T cells recognizing the HLA-A2-dimer/peptide reacted by IFN-γ secretion in the ELISPOT assay.
Despite the high frequency of FMOD-specific T cells from CLL patients that we observed in the HLA-A2-dimer/peptide staining without prior stimulation, no reactivity was seen using these T cells in the IFN-γ–ELISPOT assay. No specific spots were generated using T2 cells or native CLL cells as APCs in the assay (Table 3).
CD40L-stimulated CLL cells as antigen-presenting cells (APCs) during T-cell expansion
In 7 of 14 CLL patients we were not able to expand T cells using native CLL cells as APCs. We detected the expression of adhesion molecules (ICAM-1 [CD58], LFA-3 [CD54]), costimulatory molecules (B7-1 [CD80], B7-2 [CD86]), and activation markers (MHC-I and MHC-II) on native and CD40L-stimulated CLL cells. The only significant difference between samples that allowed expansion of T cells using native CLL cells compared with samples that showed poor APC characteristics was the amount of MHC class II–positive CLL cells before stimulation. The median percentage of MHC class II–positive high-quality unmanipulated stimulator cells was 34.0% (0.3%-88.4%) compared with 2.7% (1.3%-9.8%) of MHC class II–positive cells within the poor quality stimulator CLL cells (P = .042). All other examined parameters were comparable between the 2 groups.
The poor quality unmanipulated stimulator CLL cells were then activated upon stimulation with CD40L to increase the expression of adhesion and costimulatory molecules. Stimulation with CD40L increased significantly (Wilcoxon test: each, – 2.023; P = .043) the expression of adhesion and costimulatory molecules on CLL cells. Interestingly, also the expression of MHC class II molecules was significantly augmented by CD40L stimulation (P = .043).
We then used these activated CLL cells, without further pulsing with FMOD peptides, as APCs to expand autologous T cells. In all the cases investigated (5 of 6) we were able to expand 20 to 884 of 100 000 (median, 60) FMOD-specific T cells, as detected in the ELISPOT assay on day 14 or day 21 (Table 5). As APCs in the ELISPOT assay we used CD40L-stimulated CLL cells. To further increase the number of expanded FMOD-specific T cells, we also pulsed CD40L-stimulated CLL cells with FMOD peptides prior to coincubation with the T cells. We achieved a 3.5-fold expansion of T cells during 4 weeks of in vitro culture (Figure 1B). We then detected 4 to 1303 of 100 000 (median, 124 of 100 000) FMOD-specific T cells in the ELISPOT assay after 2 to 3 weeks of in vitro culture. The number of specific spots detected in the IFN-γ–ELISPOT assay was significantly higher using peptide-pulsed compared with unpulsed CD40L-stimulated CLL cells as APCs in the T-cell expansion (Wilcoxon test: Z = – 2.366; P < .018).
Specific recognition of HLA-A0201 binding fibromodulin-derived peptides present on CD40L-stimulated CLL cells by autologous T cells
. | . | FMOD-specific T cells (per 100 000) by ELISPOT . | . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Native autologous T cells . | T-cell expansion without pulsing the APCs . | APCs were pulsed with F1/2 or F3/4 to expand T cells . | . | . | FMOD-specific CD8+ T cells by dimer-peptide staining (in %) . | ||||
| Patient . | FMOD peptides . | . | . | Elispot: APCs pulsed with F1/2 or F3/4 . | Elispot: APCs pulsed with F1 or F3 . | Elispot: APCs pulsed with F2 or F4 . | . | ||||
| CLL-1 | F3/4 | 12 | 111 | 140 | 215 | 420 | 15.6 | ||||
| CLL-2 | F1/2 | 4 | 54 | 160 | 22 | 8 | 30.4 | ||||
| F3/4 | 4 | 20 | 42 | 26 | 56 | ND | |||||
| CLL-3 | F3/4 | 0 | ND | 108 | ND | ND | ND | ||||
| CLL-4 | F1/2 | 230 | 884 | 1303 | 1228 | 1164 | 12.2 | ||||
| F3/4 | 414 | 388 | 976 | 0 | 560 | ND | |||||
| CLL-7 | F1/2 | 0 | ND | 24 | ND | ND | ND | ||||
| F3/4 | 0 | ND | 4 | ND | ND | ND | |||||
| CLL-8 | F1/2 | 2 | 60 | 740 | 470 | 360 | ND | ||||
| F3/4 | 0 | 40 | 68 | 0 | 88 | 6.1 | |||||
. | . | FMOD-specific T cells (per 100 000) by ELISPOT . | . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Native autologous T cells . | T-cell expansion without pulsing the APCs . | APCs were pulsed with F1/2 or F3/4 to expand T cells . | . | . | FMOD-specific CD8+ T cells by dimer-peptide staining (in %) . | ||||
| Patient . | FMOD peptides . | . | . | Elispot: APCs pulsed with F1/2 or F3/4 . | Elispot: APCs pulsed with F1 or F3 . | Elispot: APCs pulsed with F2 or F4 . | . | ||||
| CLL-1 | F3/4 | 12 | 111 | 140 | 215 | 420 | 15.6 | ||||
| CLL-2 | F1/2 | 4 | 54 | 160 | 22 | 8 | 30.4 | ||||
| F3/4 | 4 | 20 | 42 | 26 | 56 | ND | |||||
| CLL-3 | F3/4 | 0 | ND | 108 | ND | ND | ND | ||||
| CLL-4 | F1/2 | 230 | 884 | 1303 | 1228 | 1164 | 12.2 | ||||
| F3/4 | 414 | 388 | 976 | 0 | 560 | ND | |||||
| CLL-7 | F1/2 | 0 | ND | 24 | ND | ND | ND | ||||
| F3/4 | 0 | ND | 4 | ND | ND | ND | |||||
| CLL-8 | F1/2 | 2 | 60 | 740 | 470 | 360 | ND | ||||
| F3/4 | 0 | 40 | 68 | 0 | 88 | 6.1 | |||||
T cells from CLL patients were expanded using autologous, CD40L-stimulated CLL cells as APCs. IFN-γ-ELISPOT assays were performed on days 14 or 21 with autologous CD40L-stimulated CLL cells as APCs. HLA-A0201 binding fibromodulin-derived peptides were added to obtain the number of fibromodulin-specific spots. Either a mix of F1/2 or F3/4 or the single peptides were added. Coincubation of CD40L-stimulated CLL cells with DMSO and autologous T cells served as negative controls, and the number of spots detected were subtracted from the experimental values. Given are the numbers of fibromodulin-specific spots per 100 000 T cells. The precursor T-cell frequency was investigated by coincubation of CD40L-stimulated CLL cells and unstimulated autologous T cells together with the fibromodulin-derived peptides (F1/2 or F3/4). The percentage of CD8+ T cells on day 28 recognizing fibromodulin-derived peptides bound to HLA-A2—dimer is given after subtraction of the negative control HLA-A2—dimer coincubated with DMSO. T cells were obtained using CD40L-stimulated fibromodulin-pulsed CLL cells as APCs.
If enough T lymphocytes could be expanded after 14 to 21 days of in vitro culture (CLL-1, CLL-2, CLL-4, CLL-8), we tested which FMOD-derived peptide was responsible for the reactivity observed in the ELISPOT assay (Table 5). There was specific recognition of each of the 4 tested peptides with interindividual differences.
In 2 of 8 patients (CLL-4, CLL-8) the antigen specificity was tested by blocking experiments with an MHC class I–specific monoclonal antibody (W6/32) (data not shown). Adding W6/32 resulted in a reduction (median, 78.5%) of the number of spots detected by ELISPOT. Tonsillar or peripheral B cells from HLA-A0201–positive healthy donors were not specifically recognized by the T cells expanded (data not shown).
The number of FMOD-reactive T cells was further validated and quantified by HLA-A2-dimer/peptide staining in 4 of the CLL samples that were expanded by peptide-pulsed CD40L-stimulated APCs (CLL-1, CLL-2, CLL-4, CLL-8). We detected FMOD-reactive CD8+ T lymphocytes at a frequency of 6.1% to 30.4% (median, 13.9%) (Table 5).
To determine the frequency of FMOD-specific CTL from CLL patients without prior stimulation, we performed an IFN-γ–ELISPOT assay with native CLL cells and CD40L-stimulated CLL cells as APCs. With native CLL cells there was no specific reactivity observed. But with CD40L-stimulated CLL cells we detected 0 to 414 of 100 000 T cells reacting specifically against FMOD-derived peptides.
Discussion
In the present study we provide evidence that autologous FMOD-specific T lymphocytes derived from CLL patients can be expanded. Furthermore the relative amount of T cells recognizing FMOD peptides bound to HLA-A2-dimers increases by stimulation using autologous unpulsed native CLL cells as APCs. The expanded T cells are able to recognize 4 different HLA-A0201–binding FMOD-derived peptides, which is shown both by ELISPOT analysis and HLA-A2-dimer/peptide staining. Importantly, there is no response against nonmalignant cells, that is, PBMC-derived from HLA-A0201–positive healthy donors or tonsillar B cells. Also, HLA-A0201–negative CLL cells, overexpressing FMOD are not recognized. Therefore, we conclude that FMOD serves as a novel tumor-associated antigen (TAA) in CLL.
In our setting we use unmodified CLL cells as APCs, which induce T-cell proliferation. Since the CLL cells used for T-cell cultivation were not treated with exogenous antigenic peptide or protein, the only available source of FMOD epitopes was FMOD protein endogenously processed and presented by the native CLL cells themselves. After in vitro stimulation, up to 26.1% of the expanded CD8-positive T cells specifically recognize FMOD-derived peptides by HLA-A2-dimer/peptide staining and up to 1.14% of all T cells expanded react specifically against an FMOD-derived peptide in the IFN-γ–ELISPOT assay. Peptide/MHC class I multimer staining is known to be exquisitely epitope specific.19 We think that cross-staining of T cells recognizing similar but different epitopes from a different origin than FMOD is highly improbable, more so because HLA/peptide dimer staining demonstrated the expansion of T cells specific for more than one FMOD epitope, but no expansion of T cells specific for CLL-unrelated epitopes like MAGE-3 in the context of HLA-A2. This provides evidence that CLL cells do not only express FMOD mRNA, but also endogenously process and present FMOD-derived peptides in an HLA-A0201–restricted fashion in sufficient amounts. Furthermore, this reveals that autologous T cells are able to recognize and to respond specifically to the antigenic stimulus provided by unpulsed native CLL cells, shown by proliferation of CD8+ T cells.
Interestingly, we are able to expand autologous CD8+ T cells from CLL patients using native CLL cells as APCs in half of the cases investigated, despite a low expression of ICAM-1 (CD58), LFA-3 (CD54), B7-1 (CD80), and B7-2 (CD86). Therefore, we suggest that there must be other than the CD28/B7 costimulatory pathway involved in CLL, like ICOS/LICOS, 4-1BB/4-1BBL, CD27/CD70, and CD26, which seem to play important roles in tumor rejection and immunity.20-26 In our cohort, the expression of MHC class II molecules on the surface of CLL cells is significantly higher in the cases in which unmanipulated CLL cells could be used as APCs for an effective expansion of T cells. It is well known that IFN-γ is able to up-regulate MHC class II expression in APCs.27 One could hypothesize that CLL cells with a higher fraction of MHC class II–positive cells are surrounded by a T helper-1 milieu, which results in an up-regulated IFN-γ concentration, thus increasing the expression of MHC class II molecules on the surface of a subset of CLL samples and allowing expansion of autologous T cells. Another hint for a relatively well-preserved antigen-presenting capacity of a subset of CLL samples is the observation that also native CLL cells, which are pulsed with FMOD peptides and are used as APCs in the ELISPOT assays, are recognized by specific T cells, although in most cases to a lesser extent compared with T2 cells.
By HLA-A2-dimer/peptide staining we detect up to 7% of all CD8-positive T cells reacting specifically with the FMOD peptides without prior stimulation, which is an unexpected high number of T cells. We can detect FMOD-specific T cells in all the CLL samples tested. But in the ELISPOT assay we did not observe any reactivity against T2 cells or native CLL cells if the patients' T cells were not prestimulated in vitro. This suggests that antigen-specific T cells are present in the peripheral blood of CLL patients, but they are not able to secrete IFN-γ upon contact with the antigen. We hypothesize that these T cells are not naive precursor T cells and might have been already primed in vivo, being in a dormant state that is overcome by stimulation under in vitro conditions.
We observed that up to 26.1% of all CD8-positive T cells after in vitro culture specifically recognized FMOD-derived peptides bound to the HLA-A2-dimer. After in vitro expansion, T cells also secreted IFN-γ in the ELISPOT in an antigen-specific manner, although numbers of T cells reacting in the ELISPOT were lower than numbers of cells stained by HLA-A2-dimer/peptide complexes. This observation is in accordance with results of other groups.18,28 This suggests that after appropriate stimulation or preactivation, T cells from CLL patients should be able to specifically react against antigens presented by CLL in vivo as well. It seems that the T-cell function of 6 of 8 of our patients is not impaired, because T cells recognized FMOD bound to HLA-A2-dimers, and corresponding numbers of T cells recognized FMOD presented by T2 cells or native CLL cells. In 2 patients we observed a greater discrepancy between the T cells recognizing FMOD peptides in the HLA-A2-dimer/peptide staining and additionally secreting IFN-γ, which could be due to major T-cell defects in those patients, which is suggested by others.29
In 50% of our CLL samples tested, native CLL cells were not able to expand autologous T cells, probably due to their poor antigen-presenting capacity. In these cases we stimulated the CLL cells prior to coincubation with the T cells with CD40L-expressing feeder cells, which increased significantly the expression of adhesion and costimulatory molecules on CLL cells as has been previously described, and therefore restore the impaired antigen-presenting capability of native CLL cells.16,30 Using CD40L-stimulated CLL cells as APCs we were able to expand specific T cells even better compared with other experiments using native CLL cells as stimulators. Again we were able to expand FMOD-specific T cells without pulsing the APCs with the FMOD peptides. Even higher numbers of FMOD-specific spots were detectable after 2 to 3 weeks of in vitro culture, when FMOD peptide–pulsed CD40L-stimulated CLL cells were used as APCs. While already achieving a specific T-cell response against autologous CLL cells using native CLL cells as APCs, an even higher response in terms of proliferation of T cells resulted using CD40L-stimulated CLL cells as APCs. In line with this observation, we hypothesize that a further increase in the autologous T-cell response might be seen if CLL cells are directly transduced by gene vectors coding for CD40L. It can be speculated that here the bystander effect of CD40L-transduced CLL cells could be advantageous because transduced CLL cells not only up-regulate adhesion and costimulatory molecules on their own surface, they also trigger neighboring CLL cells to up-regulate those molecules. This could prolong the activated status of CLL cells and thus increase their antigen-presenting capacity.31,32
In contrast to the numerous TAAs described in melanoma, only a small number of TAAs have been found in CLL so far. In CLL, first, the hypervariable sequences and somatic mutations of the lymphoma immunoglobulin variable regions, which result in individual tumor-specific antigens, were identified.33 Therefore, the clonotypic surface immunoglobulin receptor expressed by malignant B cells, also termed idiotype, represents an attractive target structure for antitumor immunity.34,35 The disadvantage of an anti-idiotypic immunization approach is the laborious development of a specific vaccine for every individual patient. In search for a more general applicable immunotherapy in CLL, Trojan et al investigated framework region–derived peptides as TAAs in CLL.36 More recently survivin, a member of the inhibitor of apoptosis protein family, was found to serve as TAA in CLL.37-39 For survivin, the specific recognition of autologous tumor cells by survivin-specific CTLs was only shown for a small fraction of CLL patients, while reaction against allogeneic tumor cells was frequently demonstrated. In contrast, autologous T-cell immunity against fibromodulin could be induced in the majority of CLL patients in our study.
Besides the provided evidence that fibromodulin can serve as TAA in CLL, more research suggests it might be involved at an important step in the pathophysiology of CLL. So far, it was shown by different groups that fibromodulin-specific mRNA is highly overexpressed in CLL cells, but the role of this overexpression has to be elucidated. Fibromodulin is able to bind to TGF-β and to modulate its activity.6,7 TGF-β, a multifunctional cytokine, which is involved in regulation of cell growth and survival, immune functions, and wound healing is an important endogenous growth inhibitor of B lymphocytes.40-42 Fibromodulin, which is overexpressed in CLL cells, might bind to TGF-β, thus inhibiting the functions of TGF-β and contributing to a sustained proliferation and expansion of the leukemic clone.
In summary, we could demonstrate that fibromodulin is a naturally processed and presented antigen in CLL that can serve as a TAA in this disease, for instance, for clinical vaccination trials or adoptive T-cell transfer. Fibromodulin seems to be an ideal TAA for a CLL-specific immune monitoring in the context of vaccination approaches including CD40L-gene–modified autologous leukemic cells.
Prepublished online as Blood First Edition Paper, October 7, 2004; DOI 10.1182/blood-2004-04-1233.
Supported by grants from the Deutsche Forschungsgemeinschaft (SFB 455), Wilhelm-Sander-Stiftung (95-056-2), Bayerische Forschungstiftung (AZ 490-02), and Friedrich Baur-Stiftung (C.M., A.M., M.H., and C.-M.W.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Susan King and Elfriede Nössner (GSF, Munich) for critical reading of the manuscript and for stimulating discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal