Abstract
To enhance the in vivo antitumor activity of adoptively transferred, CD19-specific chimeric antigen receptor (CAR)–redirected cytotoxic T lymphocytes (CTLs), we studied the effect of restimulating CAR+ CTLs through their endogenous virus-specific T-cell antigen receptor (TcR) by the cotransfer of engineered T-cell antigen–presenting cells (T-APCs). Using influenza A matrix protein 1 (MP1) as a model antigen, we show that ex vivo–expanded CD4+ and CD8+ T-APCs expressing a hygromycin phosphotransferase-MP1 fusion protein (HyMP1) process and present MP1 to autologous human leukocyte antigen (HLA)–restricted, MP1-specific CD4+ and CD8+ CTL precursors. The MP1-specific CTLs are amenable to subsequent genetic modification to express a CD19-specific CAR, designated CD19R, and acquire HLA-unrestricted reactivity toward CD19+ leukemia and lymphoma tumor targets while maintaining HLA-restricted MP1 specificity. The restimulation of MP1×CD19 dual-specific CTLs in vivo by the adoptive transfer of irradiated HyMP1+ T-APCs resulted in the enhanced antilymphoma potency of bispecific effector cells, as measured by elimination of the biophotonic signal of established firefly luciferase–expressing Burkitt lymphoma xenografts in nonobese diabetic/severe combined immunodeficiency (NOD/scid) animals compared with control groups restimulated by Hy+MP1neg T-APCs. Engineered T-APCs are a novel and versatile antigen-delivery system for generating antigen-specific T cells in vitro and enhancing the in vivo effector functioning of CAR-redirected antitumor effector cells.
Introduction
Adoptive transfer of ex vivo–expanded T cells specific for immunodominant viral epitopes into immunocompromised hosts can reconstitute protective antiviral immunity and can result in the long-term persistence of transferred cells.1-5 In contrast, the application of adoptive T-cell transfer to the successful cellular immunotherapy of malignancy has proved to be significantly more challenging, in part because of the difficulty of isolating high-affinity, tumor-specific T cells that can mediate effective antitumor in vivo effector functions and the potential for tumors to evade immunologic clearance through a variety of escape mechanisms, including the down-regulation of restricting HLA molecules.6-8 Several groups, including ours, are developing alternative strategies for targeting tumors using genetically modified T cells that are endowed with redirected antigen specificity through the expression of chimeric antigen receptors (CARs), such as a CD19-specific chimeric immunoreceptor. These chimeras typically use HLA-independent, high-affinity antigen recognition domains consisting of extracellular single-chain immunoglobulin variable fragments (scFvs) linked to cytoplasmic T-cell activation domain(s), such as CD3-ζ.9-19
Strategies to enhance the antitumor activity of adoptively transferred CAR+ cytotoxic T lymphocytes (CTLs) and to overcome the potentially deleterious impact of in vivo recycling of these cells solely through CAR-redirected engagement of tumor cells will likely be critical for achieving therapeutic efficacy. Because CAR-redirected T cells retain the specificity and function of their endogenous T-cell antigen receptor (TcR), expressing CARs on virus-specific T cells, such as commonly acquired latent viruses (Epstein-Barr virus [EBV] and cytomegalovirus [CMV]), is a potential approach to maintain persistence in vivo through re-encounter of these bispecific T cells with viral antigen presented by professional antigen-presenting cells (APCs).9,20,21 Although the timing and magnitude of latent virus reactivation makes the in vivo restimulation of bispecific T cells difficult to control, we hypothesize that the grafting of antitumor CARs to T cells specific for common nonlatent viruses and the delivery of a viral antigen vaccine boost(s) after adoptive transfer (transfer-boost strategy) is an approach amenable to iatrogenic regulation.
Here we describe the usefulness of ex vivo–expanded CD8+ and CD4+ T cells to function as APCs by their genetic modification to express a model viral antigen (influenza A MP1) for eliciting the in vitro expansion of MP1-specific CTLs and for augmenting the clearance of CD19+ Daudi lymphoma in vivo, by CD19×MP1–bispecific CTLs by post-transfer boosting. Our finding that human T cells are amenable to genetic modification for expressing and presenting viral antigens makes this transfer boost system well suited to augment the antilymphoma/leukemia effect of adoptively transferred CD19-specific CTLs in a variety of clinical settings.22
Materials and methods
Plasmid expression vectors
The pMG expression vector (InvivoGen, San Diego, CA) was modified by site-directed mutagenesis to remove a PacI restriction enzyme (RE) site at position 307 to generate pMGP̂ac (Figure 1A). The plasmid vector CD19R/HyTK-pMG was derived from pMGP̂ac and has been described previously.23 The HyMP1 fusion gene was assembled by polymerase chain reaction (PCR) splice overlap extension and consists of the 5′ 972 base pairs (bps) of hygromycin phosphotransferase (Hy) gene and the 3′ 759 bps of the influenza virus A/WSN/33 MP1 gene (GenBank accession number M19374), kindly provided by Dr Adolfo Garcia-Sastre (Mount Sinai School of Medicine, NY). This construct incorporates unique 5′ NheI and 3′ BamHI RE sites for subcloning into plasmid ELF1α promoter and Kanamycin-resistance gene (pEK) to generate the plasmid HyMP1-pEK (Figure 1A). The pEK plasmid was modified from pcDNA3.1+ by replacing the CMV promoter with human elongation factor 1α (hEF1α) promoter and replacing neomycin- and ampicillin-resistance genes with the kanamycin-resistance (KanR) gene. The bifunctional ffLucZeo fusion gene that coexpresses the North American firefly (Photinus pyralis) luciferase (ffLuc) and zeomycin-resistance genes (Zeo) were cloned from the plasmid pMOD-LucSh (InvivoGen) into pcDNA3.1+ (Invitrogen, Carlsbad, CA), to create the plasmid ffLucZeo-pcDNA. Truncated CD19 (tCD19), lacking the cytoplasmic domain, was expressed from the plasmid pCI-rCD19, kindly provided by Dr Michio Kawano (Yamaguchi University School of Medicine, Japan).24
Genetic modification of T-APCs. (A) Schematic of HyMP1 cDNA and plasmid expression vectors. The HyMP1 cDNA consisting of a 5′ hygromycin phosphotransferase segment and a 3′ influenza A matrix protein-1 (HyMP1) is shown with its flanking NheI and BamHI restriction enzyme (RE) sites. This transgene was cloned into the multiple cloning site (MCS) under control of the hEF1α hybrid promoter in the plasmid HyMP1-pEK. Plasmid pMGP̂ac contains the hygromycin phosphotransferase (Hy) cDNA, under control of the human CMV immediate early (IE) promoter. The bovine growth hormone (bGhpA), late SV40 poly A sites (SV40pA), synthetic poly A and pause site (SpAn), Escherichia coli origin of replication (ori ColE1), and unique RE sites are shown. The PacI RE site was used to linearize the plasmids before electroporation. (B) Chemiluminescence Western immunoblot of recombinant HyMP1. Whole-cell protein lysates from T-APCs genetically modified with HyMP1-pEK (lane 1) or pMGP̂ac (lane 2) plasmids, along with molecular weight controls (not shown), were resolved by PAGE under reducing conditions. Western blotting with MP1-specific antibody was used to detect the approximately 176-kDa HyMP1 fusion protein. (C) Phenotype of T-APCs by flow cytometry. Histograms of binding of fluorescence-labeled cell-surface marker–specific mAbs (bold line) relative to isotype control or unstained cells (dotted line) to CD8+ T-APCs genetically modified with HyMP1-pEK are shown. T-APCs were stained and analyzed by flow cytometry between days 10 and 14 of a 2-week in vitro OKT3 stimulation cycle. The flow cytometry histograms are almost identical for CD8+ Hy+MP1neg T-APCs genetically modified with pMGP̂ac (data not shown). The relative percentage of cells in each gate is indicated.
Genetic modification of T-APCs. (A) Schematic of HyMP1 cDNA and plasmid expression vectors. The HyMP1 cDNA consisting of a 5′ hygromycin phosphotransferase segment and a 3′ influenza A matrix protein-1 (HyMP1) is shown with its flanking NheI and BamHI restriction enzyme (RE) sites. This transgene was cloned into the multiple cloning site (MCS) under control of the hEF1α hybrid promoter in the plasmid HyMP1-pEK. Plasmid pMGP̂ac contains the hygromycin phosphotransferase (Hy) cDNA, under control of the human CMV immediate early (IE) promoter. The bovine growth hormone (bGhpA), late SV40 poly A sites (SV40pA), synthetic poly A and pause site (SpAn), Escherichia coli origin of replication (ori ColE1), and unique RE sites are shown. The PacI RE site was used to linearize the plasmids before electroporation. (B) Chemiluminescence Western immunoblot of recombinant HyMP1. Whole-cell protein lysates from T-APCs genetically modified with HyMP1-pEK (lane 1) or pMGP̂ac (lane 2) plasmids, along with molecular weight controls (not shown), were resolved by PAGE under reducing conditions. Western blotting with MP1-specific antibody was used to detect the approximately 176-kDa HyMP1 fusion protein. (C) Phenotype of T-APCs by flow cytometry. Histograms of binding of fluorescence-labeled cell-surface marker–specific mAbs (bold line) relative to isotype control or unstained cells (dotted line) to CD8+ T-APCs genetically modified with HyMP1-pEK are shown. T-APCs were stained and analyzed by flow cytometry between days 10 and 14 of a 2-week in vitro OKT3 stimulation cycle. The flow cytometry histograms are almost identical for CD8+ Hy+MP1neg T-APCs genetically modified with pMGP̂ac (data not shown). The relative percentage of cells in each gate is indicated.
Cell lines and primary human T-cell propagation
Lymphoblastoid cells (LCLs) and Daudi,25 T2,26 and K56227 cells were obtained from ATCC (Manassas, VA) and were maintained in media consisting of RPMI 1640 (Irvine Scientific, Santa Ana, CA) supplemented with 2 mM l-glutamine (Irvine Scientific), 25 mM (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) HEPES (Irvine Scientific), 100 U/mL penicillin, 0.1 mg/mL streptomycin (Irvine Scientific), and 10% heat-inactivated defined fetal calf serum (FCS) (Hyclone, Logan, UT), hereafter referred to as culture media (CM). The U251T human glioblastoma cell line was kindly provided by Dr Waldemar Debinski (Wake Forest University, NC), and were cultured in Dulbecco modified Eagle medium (Irvine Scientific) supplemented with 10% heat-inactivated FCS and antibiotics, as described. All cells were maintained at 37 °C in a humid atmosphere of 5% CO2 in air.
Human T-cell lines were derived from peripheral blood mononuclear cells (PBMCs) of healthy volunteers, who gave their consent, and were cultured using previously described methods.23 Briefly, stimulation/expansion cultures were established using 106 T cells, 30 ng/mL anti-CD3ϵ (OKT3; Ortho Biotech, Raritan, NJ), 50 × 106 γ-irradiated PBMCs (3500 cGy), and 107 γ-irradiated LCLs (8000 cGy) in 50 mL CM. Recombinant human interleukin-2 (rhIL-2) (Chiron, Emeryville, CA) was added to culture at 25 U/mL every 48 hours, beginning on day 1 of each 14-day expansion cycle. To obtain and expand CD4+ T-APC, the cultures were sorted for binding of anti-CD4 28 days after electroporation. Antigen-specific expansion of MP1-specific T cells used γ-irradiated autologous T-APCs (3500 cGy) expressing HyMP1 gene at a 5:1 responder/stimulator ratio. Every 48 hours, 5 U/mL rhIL-2 was added to cultures beginning on day 1 of each 7-day culture cycle.
Flow cytometry
The following fluorescein isothiocyanate (FITC)–, phycoerythrin (PE)–, and CyChrome-conjugated reagents were obtained from BD Biosciences (San Jose, CA) and used according to the manufacturer's instructions: anti–T-cell receptorαβ (anti-TCRαβ), anti-CD2, anti-CD3ϵ, anti-CD4, anti-CD8, anti-CD10, anti-CD11a, anti-CD18, anti-CD19, anti-CD27, anti-CD28, anti-CD50, anti-CD54, anti-CD58, anti-CD70, anti-CD80, anti-CD86, anti-HLA ABC, anti-HLA DR, and anti-NKG2D. A F(ab')2 fragment of FITC-conjugated goat anti–human Fcγ, (Jackson ImmunoResearch, West Grove, PA) was used at a 1:20 dilution to detect cell-surface expression of CD19R. Tetramer studies used the PE-conjugated MP1(GILGFVFTL)–HLA-A2*0201 tetramer from Beckman Coulter (BC Immunomics Operations, San Diego, CA).28-30 The biotin-conjugated monoclonal antibody (mAb) specific for TCR Vβ17 (Immunotech; Beckman Coulter, Fullerton, CA) was used with CyChrome-conjugated streptavidin (BD Biosciences). In some experiments, CyChrome-conjugated mAbs were replaced with 1 μg/mL propidium iodide (PI) to exclude nonviable cells from analyses. Data acquisition was performed on a FACScalibur (BD Biosciences), and the percentage of cells in a region of analysis was calculated using CellQuest version 3.3 (BD Biosciences). Fluorescence-activated cell sorting using a MoFlo MLS (Dako-Cytomation, Fort Collins, CO) was used to isolate CD4+ T-APCs and MP1-HLA A2 tetramer+ T cells.
Electrotransfer of plasmid vectors
To obtain T-APCs, OKT3-activated human PBMCs were genetically modified by electroporation using the Eppendorf Multiporator device (Eppendorf AG, Hamburg, Germany) based on the method previously described.31 Briefly, PBMCs (harvested from cultures on day 3 after OKT3 stimulation) were resuspended in hypo-osmolar buffer (Eppendorf) at 8 × 106/mL, and aliquots were apportioned into 0.2-cm cuvettes containing 10 μg linearized pMGP̂ac or HyMP1-pEK DNA plasmids in a total volume of 400 μL. Each cuvette received a single 40-microsecond pulse of 250 V, followed by 5-minute room-temperature incubation, after which cells were placed back into rhIL-2–supplemented CM at 5 × 105 cells/mL. On day 2 after electroporation and on day 5 for subsequent stimulation cycles, hygromycin B (Stratagene, Cedar Creek, TX) was added to cultures at a cytocidal concentration of 0.2 mg/mL active drug.
To obtain bispecific T cells, 8 × 106 MP1-tetramer+ in vitro–expanded T cells were electroporated per cuvette with linearized (at the PacI RE site) CD19R/HyTK-pMG plasmid DNA, as described in the preceding paragraph. The T cells were then stimulated on the same day with OKT3, 50 × 106 γ-irradiated PBMCs, and 107 γ-irradiated LCLs. A cytocidal concentration of hygromycin B was added beginning 5 days after the addition of OKT3. Beginning the day after the addition of OKT3, 25 U/mL rhIL-2 was added to all T-cell cultures every other day. After 2 weeks of culture, the T cells were restimulated for numerical expansion every 14 days, as described, in the presence of cytocidal concentrations of hygromycin B.
Daudi lymphoma cells in log-phase growth were electroporated with linearized ffLucZeo-pcDNA using the same conditions to express the ffLucZeo gene. Two days after electroporation, zeocin (Invivogen) was added to the culture at a concentration of 0.2 mg/mL.
U251T (HLA A2+) was transfected in log-phase growth using 2 μg pMGP̂ ac, pCI-rCD19-neo, or HyMP1-pEK linearized DNA in 2 μL lipofectamine (Invitrogen) and/or was expanded in cytocidal concentrations of hygromycin B (0.2 μg/mL) to obtain Hy+ and HyMP1+ U251T and/or G418 (0.25 μg/mL) to obtain tCD19+HyMP1+ U251T or tCD19+ U251T, respectively.
Western blots
T cells (2 × 107) were lysed on ice in 1 mL RIPA buffer (phosphate-buffered saline [PBS], 1% nonidet P40 (NP40), 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]) containing 1 tablet/10 mL Complete protease inhibitor cocktail (Boehringer Mannheim, Penzberg, Germany). After 60 minutes, aliquots of centrifuged supernatant were boiled in an equal volume of loading buffer under reducing conditions and subjected to SDS–polyacrylamide gel electrophoresis (SDS-PAGE) on precast 12% acrylamide gels (Bio-Rad Laboratories, Hercules, CA). After transfer to nitrocellulose, membranes were blocked for 2 hours in Blotto solution containing 0.07 g/mL nonfat dry milk. Membranes were washed in T-TBS (0.05% Tween 20 in Tris-buffered saline, pH 8.0) and were incubated for 2 hours with 5 to 10 μg goat antihuman influenza A MP1 (Immune Systems Ltd, Paignton, United Kingdom). After washing in T-TBS, the membranes were incubated for 1 hour with a 1:500 dilution of alkaline phosphatase–conjugated mouse antibody specific for goat immunoglobulin G (IgG). After rinsing in T-TBS, the membranes were developed with 30 mL AKP solution (Promega, Madison, WI) according to the manufacturer's instructions. Chemiluminescence was measured over a 2-hour period using an EpiChemi II Darkroom (UVP Inc, Upland, CA), and the images were processed using LabWorks version 4.0.0.8 (UVP Inc).
Chromium-release assay
The cytolytic activity of T cells was determined by 4-hour chromium-release assay (CRA) using triplicate V-bottom wells in a 96-well plate (Costar, Cambridge, MA) containing Na51CrO4 (MP Biomedicals, Orangeburg, NY)–labeled Daudi, T2, T-APC, primary acute lymphoblastic leukemia (ALL) blast, or K562 target cells. Effector cells were harvested 10 to 14 days after stimulation with OKT3, washed, and incubated with 5 × 103 target cells in triplicate, and the percentage of specific cytolysis was calculated from the release of 51Cr, as described earlier, using a Cobra II AutoGAMMA (Canberra Packard Ltd, Pangbourne, Berks, United Kingdom) or TopCount NXT (PerkinElmer Life and Analytical Sciences, Inc, Boston, MA).23 Data are reported as mean ± SD.
Proliferation assay
T-cell responder cells were cocultured for 72 hours at a 1:1 ratio with γ-irradiated autologous T-APCs (3500 cGy) or mitomycin C–treated (10% for 1 hour) HLA-restricted U251T stimulators in CM without rhIL-2. During the last 18 hours, cultures were pulsed with 1 μCi (0.037 MBq)/well 3H-thymidine. Cells were then harvested with a Packard Cell Harvester onto unifilter plates, and cell-associated radioactivity was measured by scintillation counting (Topcount NXT). Data are reported as mean ± SD.
Analysis of cytokine production
T-cell responder cells (106 cells) were cocultured at a 1:1 ratio in 12-well tissue culture plates with γ-irradiated Daudi (8000 cGy), T-APC (3500 cGy), or mitomycin C–treated HLA-restricted U251T stimulators in 2 mL CM. After a 48-hour incubation at 37°C, the conditioned media were assayed by cytometric bead array (CBA) using the human TH1/TH2 cytokine kit and array software according to the manufacturer's instructions (BD Biosciences) on a FACScalibur.
Xenograft tumor model
On day 0, 6- to 10-week-old female NOD/scid (NOD/LtSz-Prkdcscid/J) mice (Jackson Laboratory, Bar Harbor, ME) were injected in the peritoneum with 5 × 106 ffLuc+ Daudi cells. Beginning on day 2, tumor engraftment was evaluated by biophotonic imaging (see “Biophotonic tumor imaging”) and was defined as increasing tumor ffLuc-mediated flux over at least 2 imaging sessions. Mice with progressively growing tumors were segregated into 4 treatment groups (5 mice/group) receiving combinations of intraperitoneal rhIL-2 (25 000 U/mouse on a Monday-Wednesday-Friday schedule), 20 × 106 effector T cells, and 5 × 106 γ-irradiated (3500 cGy) Hy+MP1– or HyMP1+ T-APCs by additional separate intraperitoneal injections through 28-gauge hypodermic needles.
Biophotonic tumor imaging
Anesthetized mice were imaged using a Xenogen IVIS 100 series system beginning approximately 15 minutes after intraperitoneal injection of 150 μL (4.29 mg/mouse) of a freshly thawed aqueous solution of D-luciferin potassium salt (Xenogen, Alameda, CA). Each animal was serially imaged in an anterior-posterior orientation at the same relative time point after d-luciferin injection. Previous experiments established that the photon flux from these ventral views of the abdomen was constant within 6.32% ± 8.11% (mean ± SD) for mice bearing ffLuc+ intraperitoneal Daudi xenografts imaged over a 15-minute period (data not shown). Photons emitted from ffLuc+ Daudi xenografts were quantified using the software program Living Image (Xenogen), and the bioluminescence signal was measured as total photon flux normalized for exposure time and surface area and expressed in units of photons (p) per second per cm2 per steradian (sr). For anatomic localization, a pseudocolor image representing light intensity (blue, least intense; red, most intense) was superimposed over a digital grayscale body-surface reference image.
Statistical methods to analyze biophotonic data
To measure the differences between mouse treatment groups, we considered a primary end point evaluating tumor biophotonic signal over time. By calculating a cumulative area under the curve (AUC) for each mouse, an end point was generated that rewarded treatments that not only shrank tumors but also kept them small over the course of the study. Mean AUCs between treatment groups were compared using an exact permutation test under the Hothorn and Hornik exactRankTests package for the R language.32-35 Details for deriving the permutation P values in general are discussed in Streitberg and Röhmel.36 Given the mouse data time points and the photon flux, we plotted the connected points using time as the x-axis and the end point as the y-axis. For any sequential time points, (xi,xj), and their corresponding end points, (yi, yj), the area under this curve was calculated by using the area of a trapezoid: 0.5 × (xj – xj) × (yi + yj). The cumulative AUC for the duration of the experiment was the sum of trapezoids. We considered cumulative AUCs as an outcome for purposes of comparisons among groups. Groups with small y-values (ie, tumor flux) have small mean AUCs. When a mouse was killed for excessive tumor burden, we carried the last measured tumor size through the end of the study. We chose a threshold of 3.4 × 106 p/s per cm2/sr as the threshold for detectable tumor. This was the mean of the maximum flux of mice with no evidence of tumor after day 31 and the minimum flux of mice with tumor after day 31. We defined the time from initial treatment until the bioluminescence fell below the lower threshold as a time to remission end point. Similarly, we defined a progression-free survival end point as the time from day 0 until tumor growth increased so that the mouse was killed for excessive tumor burden. Mice that went into remission were censored at the time of last evaluation. Based on these end points, we estimated time until remission and progression-free survival.
Results
Generation of MP1+ T-APCs
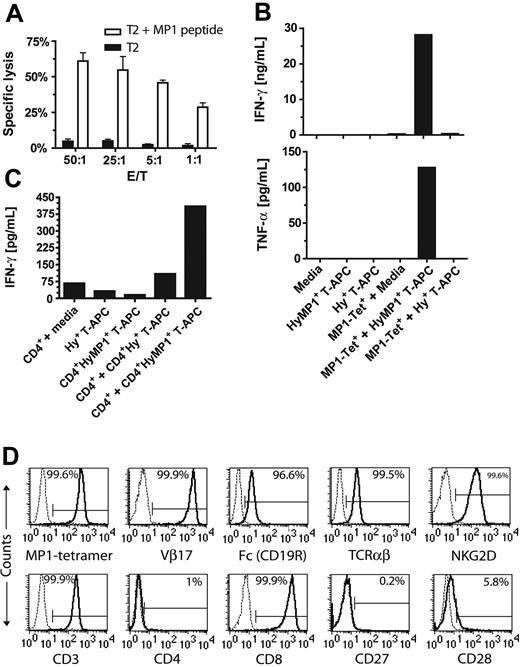
The influenza A cDNA encoding MP1 was fused to the 3′ end of the hygromycin phosphotransferase cDNA by PCR splice overlap extension yielding an approximately 1746-bp transgene, designated HyMP1 (Figure 1A). The HyMP1 cDNA was subcloned into the mammalian expression vector pEK under the transcriptional control of a modified human EF-1α promoter.37 HyMP1+ and Hy+ T-APCs were derived from OKT3-activated PBMCs by electrotransfer of the HyMP1-pEK and pMGP̂ac plasmids, respectively, followed by selection and expansion in the presence of cytocidal concentrations of hygromycin B, as previously described.23 Repeated stimulation using this method typically results in preferential in vitro expansion of CD8+ T cells, but genetically modified CD4+ T cells can be grown if isolated by flow cytometry sorting early in the ex vivo–expansion process. Western blot analysis using an MP1-specific antibody probe identified the expressed HyMP1 fusion protein with an electrophoretic mobility consistent with the expected molecular weight of approximately 176 kDa (Figure 1B, lane 1). A control T-APC line genetically modified with the plasmid pMGP̂ac to express the Hy gene failed to exhibit a band at this molecular weight (Figure 1B, lane 2).
T-APCs were subjected to flow cytometric analyses of their cell-surface phenotype toward the end of 14-day stimulation cycles (days 12-14). Expanded CD4+CD8–HyMP1+, CD8+CD4–HyMP1+, CD4+CD8–Hy+, and CD8+CD4–Hy+ T-APC lines coexpressed HLA-ABC, HLA-DR, CD2, CD11a, CD18, CD50, CD54, CD58, CD70, and, to a variable extent, CD80 and CD86 (Figure 1C; data not shown). Analysis of the CD8+ T-APCs for expression of 4-1BBL, OX40L, or MICA at the end of an ex vivo expansion cycle, when they were used as stimulators, failed to detect expression over isotype and unstained controls (data not shown).
HyMP1+ T-APCs stimulate the in vitro expansion of MP1 tetramer+ CD8+ T cells from PBMCs
The ability of autologous CD4+ and CD8+ HyMP1+–irradiated T-APCs to stimulate MP1-specific precursors in PBMCs was investigated by following the numbers of tetramer-positive responding cells emerging from the coculture. By day 7 of coculture with CD4+ and CD8+ T-APCs, the percentage of HLA A2+ MP1-tetramer+ T cells had increased to 2%, which compares favorably with the expansion of MP1-tetramer+ T cells cultured on mature dendritic cells (DCs) infected with live influenza virus (Figure 2A-B).29 The percentage of MP1-tetramer+ CD8+ T cells continued to rapidly increase to approximately 50% after 21 days of continuous coculture with CD8+HyMP1+ T-APCs (Figure 2A). The outgrowth of MP1-tetramer+ T cells could be explained by preferential expansion of these cells in response to stimulation with MP1 antigen—studies demonstrated that the T-APCs were capable of supporting MP1-specific proliferation of the MP1-tetramer+ T cells (Figure 2C). Furthermore, enumeration data demonstrated that viable MP1-tetramer+ CD8+ T cells expanded up to 630-fold in a 3-week interval (Figure 2D). HLA-A2+ PBMC responders were cocultured under identical conditions without T-APC cells or, with Hy+MP1– T-APCs, failed to expand MP1-tetramer+ CD8+ CTLs.
Generation of MP1-specific T cells by coculture with T-APCs. (A) Expansion of MP1-tetramer+ T cells by coculture with autologous CD8+HyMP1+ T-APCs. PBMCs from a homozygous HLA-A2+ donor were cocultured for 21 days with 5 U/mL rhIL-2 in the presence of (top row) CM alone or (middle row) a 5:1 (responder/stimulator) ratio of autologous γ-irradiated hygromycin B–resistant CD8+Hy+MP1– T-APCs (transfected with the plasmid pMGP̂ac), or (bottom row) autologous γ-irradiated CD8+HyMP1+ T-APCs (transfected with the plasmid HyMP1-pEK). Cultures were supplemented with irradiated T-APCs every 7 days. Responding cells were analyzed at the indicated time points by flow cytometry using FITC-conjugated anti-CD8 and PE-conjugated MP1-HLA-A2*0201 tetramer. Propidium iodine+ cells were excluded from analysis. T-APCs themselves are MP1-tetramerneg (data not shown). The percentage of tetramer+ T-cells is shown after electronic gating on CD8+ T cells. (B) Expansion of MP1-tetramer+ T cells by coculture with autologous CD4+ HyMP1+ T-APCs. PBMCs from a heterozygous HLA-A2+ donor were cocultured for 7 days with 5 U/mL rhIL-2 in the presence of (top row) CM alone, (middle row) a 5:1 (responder/stimulator) ratio of autologous γ-irradiated hygromycin B–resistant CD4+Hy+MP1– T-APCs (transfected with the plasmid pMGP̂ac), or (bottom row) autologous γ-irradiated CD4+HyMP1+ T-APCs (transfected with the plasmid HyMP1-pEK). Responding cells were analyzed at the indicated time points by flow cytometry using anti–CD8-FITC and PE-conjugated MP1-HLA-A2*201 tetramer. The percentage of tetramer+ T cells is shown after electronic gating on CD8+ T cells. (C) Proliferation of CD8+MP1-tetramer+ T cells on T-APCs. 5 × 104 HLA A2+ CD8+ MP1-tetramer+ T-cells were cocultured at a 1:1 (responder/stimulator) ratio with media or with thawed γ-irradiated autologous CD8+Hy+ and CD8+HyMP1+ T-APCs. The analysis was performed in quadruplicate, and the mean incorporated 3H-thymidine is shown along with SD. (D) Numerical expansion of CD8+MP1-tetramer+ T cells by T-APCs. 25 × 106 HLA A2+ PBMCs were cocultured for 21 days at a 5:1 (responder/stimulator) ratio in low-dose rhIL-2 with thawed γ-irradiated autologous CD8+HyMP1+ T-APCs. Cultures were supplemented with irradiated T-APCs every 7 days. Viable cells were counted based on the trypan blue dye exclusion method.
Generation of MP1-specific T cells by coculture with T-APCs. (A) Expansion of MP1-tetramer+ T cells by coculture with autologous CD8+HyMP1+ T-APCs. PBMCs from a homozygous HLA-A2+ donor were cocultured for 21 days with 5 U/mL rhIL-2 in the presence of (top row) CM alone or (middle row) a 5:1 (responder/stimulator) ratio of autologous γ-irradiated hygromycin B–resistant CD8+Hy+MP1– T-APCs (transfected with the plasmid pMGP̂ac), or (bottom row) autologous γ-irradiated CD8+HyMP1+ T-APCs (transfected with the plasmid HyMP1-pEK). Cultures were supplemented with irradiated T-APCs every 7 days. Responding cells were analyzed at the indicated time points by flow cytometry using FITC-conjugated anti-CD8 and PE-conjugated MP1-HLA-A2*0201 tetramer. Propidium iodine+ cells were excluded from analysis. T-APCs themselves are MP1-tetramerneg (data not shown). The percentage of tetramer+ T-cells is shown after electronic gating on CD8+ T cells. (B) Expansion of MP1-tetramer+ T cells by coculture with autologous CD4+ HyMP1+ T-APCs. PBMCs from a heterozygous HLA-A2+ donor were cocultured for 7 days with 5 U/mL rhIL-2 in the presence of (top row) CM alone, (middle row) a 5:1 (responder/stimulator) ratio of autologous γ-irradiated hygromycin B–resistant CD4+Hy+MP1– T-APCs (transfected with the plasmid pMGP̂ac), or (bottom row) autologous γ-irradiated CD4+HyMP1+ T-APCs (transfected with the plasmid HyMP1-pEK). Responding cells were analyzed at the indicated time points by flow cytometry using anti–CD8-FITC and PE-conjugated MP1-HLA-A2*201 tetramer. The percentage of tetramer+ T cells is shown after electronic gating on CD8+ T cells. (C) Proliferation of CD8+MP1-tetramer+ T cells on T-APCs. 5 × 104 HLA A2+ CD8+ MP1-tetramer+ T-cells were cocultured at a 1:1 (responder/stimulator) ratio with media or with thawed γ-irradiated autologous CD8+Hy+ and CD8+HyMP1+ T-APCs. The analysis was performed in quadruplicate, and the mean incorporated 3H-thymidine is shown along with SD. (D) Numerical expansion of CD8+MP1-tetramer+ T cells by T-APCs. 25 × 106 HLA A2+ PBMCs were cocultured for 21 days at a 5:1 (responder/stimulator) ratio in low-dose rhIL-2 with thawed γ-irradiated autologous CD8+HyMP1+ T-APCs. Cultures were supplemented with irradiated T-APCs every 7 days. Viable cells were counted based on the trypan blue dye exclusion method.
HyMP1+ T-APCs elicit functional CD8+ and CD4+ MP1-specific CTLs
To demonstrate that MP1-tetramer+ CD8+ CTLs elicited by in vitro stimulation with HyMP1-expressing T-APCs are functionally intact, we assessed their lytic activity against MP1 peptide (GILGFVFTL)–loaded T2 target cells (Figure 3A). MP1-tetramer+ effector CTLs lysed peptide-loaded T2 targets in an effector/target (E/T) dose-dependent manner with negligible background killing of T2 targets without peptide. In addition, these effector cells specifically lysed HyMP1+ CD8+ and CD4+ T-APCs, confirming that these APCs process and present MP1 antigen through classical HLA class 1 (data not shown). Next, we evaluated the capacity of HyMP1-elicited MP1-tetramer+CD8+ and CD4+ effector T cells to be activated for cytokine secretion. Culture supernatants of these responders, incubated for 48 hours with autologous irradiated CD8+ or CD4+ HyMP1+ T-APCs, were harvested and assayed for cytokine content by CBA. The interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α) cytokines were 11-fold and 7-fold, respectively, increased over MP1 tetramer+CD8+ responders cultured in media alone or with autologous irradiated CD8+Hy+MP1– T-APCs (Figure 3B), and IFN-γ was 6-fold and 4-fold, respectively, increased over CD4+ responders cultured in media alone or with autologous CD4+Hy+MP1– T-APCs (Figure 3C). The CD4+HyMP1+ T-APCs were also capable of specifically eliciting IFN-γ by CD8+MP1-tetramer+ T cells (data not shown). Control cultures of irradiated T-APC stimulators alone did not contain detectable levels of these cytokines. These data are consistent with the processing and presentation of MP1 by T-APCs through the HLA class 1 and class 2 pathways.
Function and phenotype of MP1-specific T cells. (A) T cells expanded on HyMP1+ T-APCs exhibited HLA-restricted MP1-specific cytolytic response. 4-hour CRA using T-APC–elicited MP1-tetramer+ effector T cells and 51Cr-labeled HLA A2+ T2 targets were performed to compare the lytic activity against T2 targets loaded in serum-free media with 10 μM of the MP1-derived peptide GILGFVFTL (□) compared with mock-loaded targets (▪). Representative results of mean ± SD specific lysis of triplicate wells having E/T ratios of 50:1 to 1:1 are shown. (B) MP1-specific T cells elicited by HyMP1+ T-APCs recognize endogenously processed and presented HLA class 1 MP-1 epitopes and are activated for cytokine secretion. CD8+MP1-tetramer+ responder T cells cocultured with autologous CD8+HyMP1+ T-APCs, followed by OKT3-based rapid expansion, were incubated at a 1:1 responder/stimulator ratio with γ-irradiated autologous CD8+Hy+ or CD8+HyMP1+ T-APCs. Controls included T-APCs without addition to culture of responders and responders incubated in CM without stimulators. After 48 hours, cell-free supernatants from these cultures were harvested and subjected to CBA analysis for quantifying IFN-γ and TNF-α content. (C) MP1-specific T cells elicited by HyMP1+ T-APCs recognize endogenously processed and presented HLA class 2 MP-1 epitopes and are activated for cytokine secretion. CD4+ responder T cells cocultured for 21 days on autologous CD4+HyMP1+ T-APCs, followed by OKT3-based rapid expansion, were incubated at a 1:1 responder/stimulator ratio with γ-irradiated autologous CD4+Hy+ or CD4+HyMP1+ T-APCs. The CD4+HyMP1+ T-APCs can also stimulate MP1-specific secretion of IFN-γ by CD8+MP1-tetramer+ responder T cells (data not shown). Controls included T-APCs without addition to culture of responders and responders incubated in CM without stimulators. After 48 hours, cell-free supernatants from these cultures were harvested and subjected to CBA analysis for quantifying IFN-γ content. (D) Phenotype of hygromycin-resistant, CD19R/HyTK-pMG–transfected MP1-specific CTLs. Histograms showing binding of mAbs specific for T-cell cell-surface markers (bold lines), relative to isotype control or unstained cells (dotted lines), to HyMP1+ T-APC–elicited CD8+ MP1-tetramer+ T cells, genetically modified with CD19R/HyTK-pMG and expanded by repeated OKT3-based 14-day stimulation cycles in the presence of cytocidal concentrations of hygromycin B. The relative percentage of cells in each gate is indicated.
Function and phenotype of MP1-specific T cells. (A) T cells expanded on HyMP1+ T-APCs exhibited HLA-restricted MP1-specific cytolytic response. 4-hour CRA using T-APC–elicited MP1-tetramer+ effector T cells and 51Cr-labeled HLA A2+ T2 targets were performed to compare the lytic activity against T2 targets loaded in serum-free media with 10 μM of the MP1-derived peptide GILGFVFTL (□) compared with mock-loaded targets (▪). Representative results of mean ± SD specific lysis of triplicate wells having E/T ratios of 50:1 to 1:1 are shown. (B) MP1-specific T cells elicited by HyMP1+ T-APCs recognize endogenously processed and presented HLA class 1 MP-1 epitopes and are activated for cytokine secretion. CD8+MP1-tetramer+ responder T cells cocultured with autologous CD8+HyMP1+ T-APCs, followed by OKT3-based rapid expansion, were incubated at a 1:1 responder/stimulator ratio with γ-irradiated autologous CD8+Hy+ or CD8+HyMP1+ T-APCs. Controls included T-APCs without addition to culture of responders and responders incubated in CM without stimulators. After 48 hours, cell-free supernatants from these cultures were harvested and subjected to CBA analysis for quantifying IFN-γ and TNF-α content. (C) MP1-specific T cells elicited by HyMP1+ T-APCs recognize endogenously processed and presented HLA class 2 MP-1 epitopes and are activated for cytokine secretion. CD4+ responder T cells cocultured for 21 days on autologous CD4+HyMP1+ T-APCs, followed by OKT3-based rapid expansion, were incubated at a 1:1 responder/stimulator ratio with γ-irradiated autologous CD4+Hy+ or CD4+HyMP1+ T-APCs. The CD4+HyMP1+ T-APCs can also stimulate MP1-specific secretion of IFN-γ by CD8+MP1-tetramer+ responder T cells (data not shown). Controls included T-APCs without addition to culture of responders and responders incubated in CM without stimulators. After 48 hours, cell-free supernatants from these cultures were harvested and subjected to CBA analysis for quantifying IFN-γ content. (D) Phenotype of hygromycin-resistant, CD19R/HyTK-pMG–transfected MP1-specific CTLs. Histograms showing binding of mAbs specific for T-cell cell-surface markers (bold lines), relative to isotype control or unstained cells (dotted lines), to HyMP1+ T-APC–elicited CD8+ MP1-tetramer+ T cells, genetically modified with CD19R/HyTK-pMG and expanded by repeated OKT3-based 14-day stimulation cycles in the presence of cytocidal concentrations of hygromycin B. The relative percentage of cells in each gate is indicated.
HyMP1+ T-APC–expanded CD8+ MP1-specific T cells are amenable to genetic modification by plasmid electrotransfer and express a CD19-specific chimeric immunoreceptor
The genetic modification of T cells to be specific for CD19 was accomplished using nonviral electrotransfer of a DNA expression plasmid designated CD19R/HyTK-pMG, which directs the expression of a CD19-specific scFvFc:ζ CAR and the bifunctional selection suicide gene consisting of hygromycin phosphotransferase and herpesvirus thymidine kinase (HyTK).23 After electroporation, hygromycin selection and expansion using OKT3, feeder cells, and rhIL-2, the MP1-tetramer+ T-cell line was analyzed by flow cytometric analyses for CAR expression. The CD19R/HyTK-pMG–transfected MP1-tetramer+ lines retained MP1-tetramer binding and were also TcR Vβ17+ (Figure 3D), consistent with the finding that TcR Vβ17 is the dominant Vβ segment used by HLA-A2–restricted CTLs recognizing MP158-66.38-40 (The presence of cell surface CD19R CAR was documented by flow cytometry using an antihuman Fc-specific antibody. Ninety-six percent of the expanded hygromycin-resistant MP1-tetramer+ CTLs coexpressed CD19R CAR.) These T-cell lines were TCRαβ+, NKG2D+, CD3+, CD4–, CD8+, CD27–, and CD28low (Figure 3D) and were CD2+, CD11a+, CD18+, CD54+, CD58+, as previously shown.23
MP1-tetramer+CD19R+CD8+ T cells are functionally bispecific
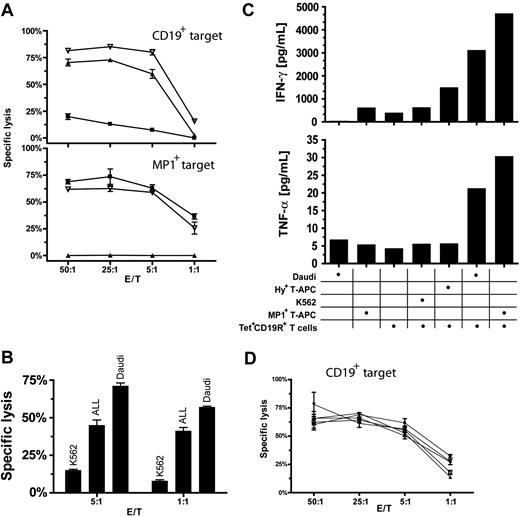
We investigated the ability of genetically modified MP1 tetramer+CD19R+ CD8+ T cells to be activated through their endogenous TcR and introduced CD19-specific CAR. Results from 4-hour CRAs revealed that the MP1-tetramer+CD19R+ CD8+ T cells could lyse both CD19+ tumor targets in an HLA-unrestricted manner and MP1+ targets in an HLA A2–restricted manner. In contrast, an MP1-tetramerneg CTL expressing CD19R could lyse only the CD19+ targets, and MP1-tetramer+ CTL could lyse only the MP1+ targets (Figure 4A). MP1-tetramer+CD19R+ CTLs recognize and lyse primary B-lineage acute ALL blasts isolated from clinical specimens (Figure 4B).
Function of MP1- and CD19-bispecific T cells. (A) MP1-tetramer+CD19R+ T cells lyse MP1+ or CD19+ target cells. 51Cr-labeled targets (top) CD19+ Daudi cells or (bottom) autologous HyMP1+ HLA A2+ T-APCs were incubated with anti-CD19 CAR-redirected (▴), HLA-A2–restricted MP1-specific (▪), or MP1×CD19–bispecific (▿) effector T cells. Representative results of mean ± SD specific lysis of triplicate wells having E/T ratios of 50:1 to 1:1 are shown. (B) MP1-tetramer+CD19R+ T cells lyse primary B-lineage ALL blasts. 51Cr-labeled thawed ALL blasts, CD19+ Daudi cells, and CD19–K562 cells were incubated with MP1- and CD19-bispecific effector T cells. After a 4-hour incubation period, supernatants were harvested, and CPM was quantified. Mean ± SD specific lysis was calculated from triplicate wells. The ALL blasts (CD19+CD10+CD45–) represented 56% of the total population and 78% of the lymphoid-gated population of these specimens. (C) MP1-tetramer+CD19R+ T cells can be activated by MP1+ or CD19+ stimulator cells for Tc1 cytokine secretion. (top) IFN-γ and (bottom) TNF-α secreted by HLA A2+ MP1- and CD19-bispecific T cells incubated at 37°C with CD19–K562 cells, autologous Hy+ T-APCs, autologous HyMP1+ T-APCs, or CD19+ Daudi cells. The relative ratio of responder T cells to γ-irradiated stimulator cells is shown. After 48 hours, cytokine concentration was determined by CBA. (D) MP1-tetramer+CD19R+ T cells can lyse CD19+ target cells after previous exposure to MP1+ or CD19+ target cells, or both. HLA A2+ MP1- and CD19-bispecific effector T cells were incubated at 37°C in media (□) or at a 1:1 ratio with γ-irradiated autologous Hy+ T-APCs (▴), MP1+ T-APCs (▾), CD19+ Daudi cells (♦), or 1:1 mixture of MP1+ T-APCs and CD19+ Daudi cells (•). After 5 days, 51Cr-labeled Daudi cells were added, and mean ± SD specific lysis was calculated after 4 hours. Lysis of CD19–K562 cells under these conditions at an E/T ratio of 25:1 was 6% to 13% (data not shown).
Function of MP1- and CD19-bispecific T cells. (A) MP1-tetramer+CD19R+ T cells lyse MP1+ or CD19+ target cells. 51Cr-labeled targets (top) CD19+ Daudi cells or (bottom) autologous HyMP1+ HLA A2+ T-APCs were incubated with anti-CD19 CAR-redirected (▴), HLA-A2–restricted MP1-specific (▪), or MP1×CD19–bispecific (▿) effector T cells. Representative results of mean ± SD specific lysis of triplicate wells having E/T ratios of 50:1 to 1:1 are shown. (B) MP1-tetramer+CD19R+ T cells lyse primary B-lineage ALL blasts. 51Cr-labeled thawed ALL blasts, CD19+ Daudi cells, and CD19–K562 cells were incubated with MP1- and CD19-bispecific effector T cells. After a 4-hour incubation period, supernatants were harvested, and CPM was quantified. Mean ± SD specific lysis was calculated from triplicate wells. The ALL blasts (CD19+CD10+CD45–) represented 56% of the total population and 78% of the lymphoid-gated population of these specimens. (C) MP1-tetramer+CD19R+ T cells can be activated by MP1+ or CD19+ stimulator cells for Tc1 cytokine secretion. (top) IFN-γ and (bottom) TNF-α secreted by HLA A2+ MP1- and CD19-bispecific T cells incubated at 37°C with CD19–K562 cells, autologous Hy+ T-APCs, autologous HyMP1+ T-APCs, or CD19+ Daudi cells. The relative ratio of responder T cells to γ-irradiated stimulator cells is shown. After 48 hours, cytokine concentration was determined by CBA. (D) MP1-tetramer+CD19R+ T cells can lyse CD19+ target cells after previous exposure to MP1+ or CD19+ target cells, or both. HLA A2+ MP1- and CD19-bispecific effector T cells were incubated at 37°C in media (□) or at a 1:1 ratio with γ-irradiated autologous Hy+ T-APCs (▴), MP1+ T-APCs (▾), CD19+ Daudi cells (♦), or 1:1 mixture of MP1+ T-APCs and CD19+ Daudi cells (•). After 5 days, 51Cr-labeled Daudi cells were added, and mean ± SD specific lysis was calculated after 4 hours. Lysis of CD19–K562 cells under these conditions at an E/T ratio of 25:1 was 6% to 13% (data not shown).
The ability of the MP1-tetramer+CD19R+ effector T cells to secrete cytokines in response to both MP1/HLA-A2 and CD19 antigens was investigated by culturing these effector cells with stimulator cells expressing CD19 or MP1 antigen. After 48 hours, a 5- to 8-fold increase in TNF-α and IFN-γ was detectable in cultures stimulated with CD19+ Daudi lymphoma tumor cells, and a 7- to 12-fold increase in TNF-α and IFN-γ was detectable on coculture with MP1+ T-APCs, compared with effector cells stimulated with MP1– stimulators or media alone (Figure 4C). Only low background levels of cytokine release were detected by T-APCs themselves in the absence of MP1-tetramer+CD19R+ responders.
MP1-tetramer+CD19R+CD8+ T cells retain cytolytic activity toward CD19+ tumor after coculture with MP1+ T-APCs or CD19+ tumor stimulators
To demonstrate that MP1-tetramer+CD19R+ T cells can recycle their CAR-regulated lytic effector function after activation through the endogenous TcR, CAR, or both, MP1×CD19 bispecific CTLs were cocultured with HyMP1+ T-APCs, CD19+ tumor cells, or a mixture of T-APCs and tumor cells. After 5-day coculture, these effectors were harvested and subjected to 4-hour CRA against CD19+ tumor cells. The specific lysis of Daudi targets was equivalent, regardless of the prior antigenic stimulation (endogenous TcR, CAR, or combined TcR/CAR) (Figure 4D).
Adoptive transfer of irradiated HyMP1+ T-APCs augments the antilymphoma activity of MP1-tetramer+CD19R+CD8+ CTL effectors in vivo
The usefulness of adoptively transferred HyMP1+ T-APCs for enhancing the in vivo antitumor potency of bispecific MP1×CD19 CTLs was investigated. Using a noninvasive, biophotonic optical imaging system, we tracked the regression of xenografted CD19+ ffLuc+ Daudi lymphoma tumors.41 The in vitro ffLuc activity of Daudi cells was approximately 2700-fold greater than that of parental Daudi cells (data not shown). In this model system, ffLuc+ Daudi cells are seeded into the peritoneum of nonobese diabetic/severe combined immunodeficiency (NOD/scid) mice. Before adoptive therapy, tumor engraftment was verified by documenting 2 successive biophotonic measurements with increasing ffLuc-mediated biophotonic tumor signal. Compared with tumor-bearing control mice given rhIL-2 alone (group A), there was significant (P = .05) reduction of tumor ffLuc signal in mice given MP1×CD19 bispecific CTLs in conjunction with 3 doses of HyMP1+ T-APCs and rhIL-2 (group B) (Figures 5 and 6A). Animals receiving bispecific effectors without MP1+ T-APCs (group C, Hy+MP1–T-APCs/rhIL-2; group D, bispecific CTLs/rhIL-2) had intermediate responses (Figures 5 and 6A). The reduction of biophotonic signal seen in mouse group B correlated with a lower cumulative biophotonic tumor flux for this group (Figure 6A), higher rates of biophotonic complete response rates (Figure 6B), and enhanced long-term progression-free survival (Figure 6C) compared with mouse groups A, C, and D.
Biophotonic imaging of ffLuc+ Daudi cells before and after adoptive T-cell therapy. Scatter graphs of tumor flux versus time and pseudocolor images of selected mice (red lines) representing light intensity from ffLuc+ Daudi cells in the peritoneum of NOD/scid mice serially imaged in ventral position. On day 0, NOD/scid mice were given 5 × 106 ffLuc+ Daudi cells by intraperitoneal injection. (ffLuc+ Daudi in vitro ffLuc activity was 141 ± 8 CPM/cell (mean ± SD), compared with 0.05 CPM/cell background ffLuc activity in parental Daudi cells.) The mice with progressive disease, documented by 2 concurrent measurements demonstrating increase in tumor flux (measured on days 2 and 6), were among 4 treatment groups. The 5 mice from group A received no further cellular therapy. On day 7, the 5 mice in groups B, C, and D received 20 × 106 MP1-tetramer+CD19R+CD8+ T cells by intraperitoneal injection. Mice from group D received additional injections of 20 × 106 MP1-tetramer+CD19R+CD8+ effector T cells on days 9 and 12. On days 7, 9, 12, 21, 23, and 25, the mice in groups B and C received separate intraperitoneal injections of 5 × 106 thawed and γ-irradiated autologous hygromycin B-resistant T-APC that had been genetically modified with HyMP1-pMG (group B) or pMGP̂ac (group C) coding for HyMP1 and Hy genes, respectively. All mice received rhIL-2 (25 000 U/mouse) by separate intraperitoneal injection on days 7, 9, 12, 21, 23, and 25. Each mouse was imaged at the same relative time point after D-luciferin administration, which was within 19 minutes of injection. Data are presented as photon flux for a region of interest (ROI) encompassing the whole mouse. Similar data were obtained in repeated experiments (data not shown).
Biophotonic imaging of ffLuc+ Daudi cells before and after adoptive T-cell therapy. Scatter graphs of tumor flux versus time and pseudocolor images of selected mice (red lines) representing light intensity from ffLuc+ Daudi cells in the peritoneum of NOD/scid mice serially imaged in ventral position. On day 0, NOD/scid mice were given 5 × 106 ffLuc+ Daudi cells by intraperitoneal injection. (ffLuc+ Daudi in vitro ffLuc activity was 141 ± 8 CPM/cell (mean ± SD), compared with 0.05 CPM/cell background ffLuc activity in parental Daudi cells.) The mice with progressive disease, documented by 2 concurrent measurements demonstrating increase in tumor flux (measured on days 2 and 6), were among 4 treatment groups. The 5 mice from group A received no further cellular therapy. On day 7, the 5 mice in groups B, C, and D received 20 × 106 MP1-tetramer+CD19R+CD8+ T cells by intraperitoneal injection. Mice from group D received additional injections of 20 × 106 MP1-tetramer+CD19R+CD8+ effector T cells on days 9 and 12. On days 7, 9, 12, 21, 23, and 25, the mice in groups B and C received separate intraperitoneal injections of 5 × 106 thawed and γ-irradiated autologous hygromycin B-resistant T-APC that had been genetically modified with HyMP1-pMG (group B) or pMGP̂ac (group C) coding for HyMP1 and Hy genes, respectively. All mice received rhIL-2 (25 000 U/mouse) by separate intraperitoneal injection on days 7, 9, 12, 21, 23, and 25. Each mouse was imaged at the same relative time point after D-luciferin administration, which was within 19 minutes of injection. Data are presented as photon flux for a region of interest (ROI) encompassing the whole mouse. Similar data were obtained in repeated experiments (data not shown).
Adoptive immunotherapy of CD19+ tumor using MP1- and CD19-bispecific T cells and T-APCs. (A) Serial measurement of flux from tumor. Trend lines for each group were derived by smoothing the tumor flux over all mice within a group. Background flux measurements, simultaneously measured from mice without ffLuc+ tumor that received D-luciferin, was between 106 and 107 p/s per cm2/sr, as discussed in “Statistical methods to analyze biophotonic data.” Tumor flux was measured periodically, as discussed in “Biophotonic tumor imaging.” Mouse treatment groups are as described in the legend to Figure 5. Group A is represented by the solid blue line; group B, red dashed line; group C, orange dotted line; group D, green dashed and dotted line. (B) Percentage of tumor-bearing mice that achieved complete remission. Time to complete remission was calculated from the beginning of the experiment until the first date when a mouse's tumor flux measurement fell below the detection threshold. Complete remission was defined as measurable flux beneath the minimum threshold of tumor detection, estimated as 3.4 × 106 p/s per cm2/sr, as described in “Statistical methods to analyze biophotonic data.” Mouse treatment groups are as described in the legend to Figure 5. (C) Progression-free survival. The tumor was defined as progressive when tumor flux was 10 times the value recorded on day 2. Mouse treatment groups are as described in the legend to Figure 5.
Adoptive immunotherapy of CD19+ tumor using MP1- and CD19-bispecific T cells and T-APCs. (A) Serial measurement of flux from tumor. Trend lines for each group were derived by smoothing the tumor flux over all mice within a group. Background flux measurements, simultaneously measured from mice without ffLuc+ tumor that received D-luciferin, was between 106 and 107 p/s per cm2/sr, as discussed in “Statistical methods to analyze biophotonic data.” Tumor flux was measured periodically, as discussed in “Biophotonic tumor imaging.” Mouse treatment groups are as described in the legend to Figure 5. Group A is represented by the solid blue line; group B, red dashed line; group C, orange dotted line; group D, green dashed and dotted line. (B) Percentage of tumor-bearing mice that achieved complete remission. Time to complete remission was calculated from the beginning of the experiment until the first date when a mouse's tumor flux measurement fell below the detection threshold. Complete remission was defined as measurable flux beneath the minimum threshold of tumor detection, estimated as 3.4 × 106 p/s per cm2/sr, as described in “Statistical methods to analyze biophotonic data.” Mouse treatment groups are as described in the legend to Figure 5. (C) Progression-free survival. The tumor was defined as progressive when tumor flux was 10 times the value recorded on day 2. Mouse treatment groups are as described in the legend to Figure 5.
To help understand the mechanism for the enhanced in vivo antitumor effect of combining T-APC with bispecific MP1×CD19 CTLs, we generated a panel of HLA A2+ artificial antigen-presenting cells (aAPCs) from U251T, which were genetically modified to express the genes Hy, HyMP1, or tCD19 or both tCD19 and HyMP1. These cells served as a platform to test the ability of the bispecific MP1×CD19 T cells to respond to CD19, HyMP1, or both. We demonstrate that MP1-tetramer+CD19R+CD8+ T cells can be activated for augmented proliferation and cytokine secretion after the endogenous and chimeric immunoreceptors contact their respective antigens compared with when the TcR or CD19R contacts MP1 or CD19 antigen, respectively (Figure 7). We also observed an augmentation of proliferation and cytokine release when CD19+ Daudi and autologous MP1+ T-APCs were mixed with bispecific MP1×CD19 CTLs compared with when these stimulator cells were incubated separately with the responding T cells (data not shown). This demonstrates that increased activation of bispecific T cells can occur when 2 different cells present the targets. These data support the hypothesis that exposure of the bispecific T cells to MP1 and CD19 antigens results in augmented T-cell activation, which, if it occurs in vivo, may lead to enhanced tumor control.
Stimulation of CD19- and MP1-bispecific T cells through introduced and endogenous immunoreceptor results in augmented activation. (A) To measure proliferation, 106 HLA A2+ MP1-tetramer+CD19R+CD8+ T cells were cultured for 96 hours at a 1:1 ratio with irradiated HLA A2+ aAPCs. For the last 18 hours, the cells were pulsed with 3H-thymidine, and the incorporated thymidine was determined using a scintillation counter. Data are presented as mean ± SD. (B) To measure IFN-γ cytokine production, bispecific MP1×CD19 CTLs were cocultured with the aAPCs, and the conditioned tissue-culture supernatant was collected after 48 hours and analyzed using a CBA. The U251T aAPCs were genetically modified to durably express tCD19, HyMP1, tCD19 and HyMP1, or Hy genes.
Stimulation of CD19- and MP1-bispecific T cells through introduced and endogenous immunoreceptor results in augmented activation. (A) To measure proliferation, 106 HLA A2+ MP1-tetramer+CD19R+CD8+ T cells were cultured for 96 hours at a 1:1 ratio with irradiated HLA A2+ aAPCs. For the last 18 hours, the cells were pulsed with 3H-thymidine, and the incorporated thymidine was determined using a scintillation counter. Data are presented as mean ± SD. (B) To measure IFN-γ cytokine production, bispecific MP1×CD19 CTLs were cocultured with the aAPCs, and the conditioned tissue-culture supernatant was collected after 48 hours and analyzed using a CBA. The U251T aAPCs were genetically modified to durably express tCD19, HyMP1, tCD19 and HyMP1, or Hy genes.
Discussion
Augmentation of the in vivo antitumor activity of adoptively transferred T cells is predicted to result in enhanced disease control. One strategy to achieve enhanced potency is to activate T cells using a vaccine recognized by the endogenous αβTcR. This approach may be particularly relevant to potentially facilitating the persistence or antitumor activity of CTLs genetically modified to express CARs with redirected tumor specificity given that these T cells would otherwise be relegated to nonphysiologic recycling through the engagement of tumor cells likely to be deficient in key immunoregulatory ligands necessary for T-cell survival and expansion.42-44 Taking advantage of the observation that CAR-redirected T cells maintain intact signaling of their endogenous clonotypic TcR, the selective grafting of CARs onto viral antigen–specific effectors provides a potential platform for transfer and boosting by delivery of viral antigen vaccines.
Efficient in vivo activation of CAR-redirected, virus-specific CD8+ CTLs demands that viral antigens be delivered in a manner that provides for viral antigen CTL-epitope presentation in the context of HLA class 1–restricting molecules and that is stochastically feasible for restimulating a large fraction of bispecific CTLs after adoptive transfer. However, the overall in vivo efficiency of the T-APCs to stimulate the bispecific effector cells may be influenced by resident antigen-specific T cells, which might compete for the ability of the adoptively transferred T cells to be similarly activated. This might be overcome by coordinating the anatomic localization of the T-APCs with that of the transferred effectors, ideally colocalizing effectors and vaccine to the tumor microenvironment. Currently available inactivated influenza vaccines will likely fail to efficiently drive the in vivo expansion of transferred CTLs because of antibody neutralization and inefficient processing and presentation of these preparations by APCs for CD8+ CTL triggering.45 Live cold-adapted viral vaccines, such as the intranasal attenuated influenza virus preparation recently approved by the United States Food and Drug Administration (FDA), may overcome some of these issues, but safety must be considered before use in immunocompromised oncology patients.46
In this study, the capacity of T cells to function as APCs by their genetic modification to express viral antigen transgenes was explored in vitro and with the use of xenograft modeling in NOD/scid animals. This approach is predicated on the observation in humans that adoptive transfer of T cells expressing immunogenic transgenes can elicit robust antitransgene T-cell rejection responses47,48 and that rat and human T cells can function as T-APCs to directly activate responding CD4+ T cells.49,50 This approach capitalizes on parallel technologic platforms used to isolate, genetically modify, and expand CAR-redirected CTLs for adoptive transfer suitable for clinical trials. Furthermore, the ability to manufacture large numbers of T cells can provide investigators with large cryopreserved banks of HLA-restricted APCs that might be used for vaccine development and that may not be as feasible using other sources of APCs.
We used influenza A MP1 as a model viral antigen and engineered a hygromycin phosphotransferase–MP1 fusion protein that ensured uniform antigen expression in genetically modified, hygromycin-resistant T-cell lines. The MP1 protein expressed by hygromycin-resistant ex vivo–expanded T cells was processed and presented HLA class 1– and class 2–restricted epitopes to autologous HLA-restricted, MP1-specific precursors present in PBMCs. Given that the full-length MP1 gene was introduced into the T cells, we anticipated that T-APCs could be generated from individuals expressing HLA alleles in addition to HLA A2. The efficiency of this direct presentation was evidenced by the numerical expansion and proliferation of MP1/HLA-A2 tetramer+ CD8+ CTLs in response to irradiated MP1+ T-APCs in vitro. Consistent with our finding that T-APCs expressed multiple costimulatory ligands used by T cells for full activation, these T-APC–elicited MP1-specific responders were functional with respect to cytolytic killing, cytokine production, and proliferation. The ability to use CD4+ T cells as APCs is expected to be useful when the presence of antigen-specific CD8+ CTLs present in the culture target and prevent the outgrowth of autologous T-APCs. The MP1-specific lines could be modified to express a CD19-specific CAR. The derived modified CTLs display HLA-restricted MP1 and HLA-unrestricted CD19 bispecificity. In vitro, CD19-specific redirected lymphoma lysis was retained after activation by HyMP1+ T-APCs or CD19+ tumor stimulators. In vivo, transfer of MP1×CD19 bispecific effector/responder T cells, followed by irradiated HyMP1+ autologous T-APCs, resulted in the augmented clearance of established Burkitt lymphoma xenografts compared with control groups receiving Hy+MP1neg T-APCs. Although the mechanism responsible for this observation remains to be established, we demonstrated that bispecific MP1×CD19 CTLs can be activated for augmented proliferation and cytokine production when the endogenous and introduced chimeric immunoreceptors are engaged. Presumably, it is this activation that leads to enhanced tumor clearance when bispecific effector T cells and T-APCs accumulate in the tumor microenvironment. These data imply that combination therapy infusing T-APCs and bispecific T cells could be improved if both cell types could traffic to sites of tumor.
Whereas transgene-expressing T-APCs likely evoke antitransgene T-cell responses in immunocompetent syngeneic hosts by cross-priming through the uptake of antigen from apoptotic T-APCs by professional APCs, such as DCs and macrophages, our system clearly establishes that T-APCs themselves can directly stimulate antitransgene responses by processing and presenting transgene-derived epitopes to CD4+ and CD8+ T cells. The capacity of T-APCs to present epitopes to HLA class 2–restricted CD4+ TH from endogenously expressed transgenes is likely important for coordinating the reactivation of CAR+ CTL effectors with the stimulation of endogenous CD4 antiviral help necessary to support these bispecific effectors. The capacity of T cells to stimulate an in vitro primary response is under investigation. However, the T-APC approach may be uniquely suited for selectively boosting the magnitude of adoptively transferred T cells when administered systemically, in part because of the expectation that these autologous stimulators will not be rapidly neutralized by antibody responses, as might be predicted by the use of viral vectors or, as previously reported, the use of allogeneic PBMCs to drive allospecific, CAR-redirected effector cells.51 Preadoptive transfer lymphodepletion may also be an important variable for facilitating the selective boosting of transferred bispecific effectors over a numerically larger endogenous pool of viral-specific responders.
Although the simplicity of generating T-APCs by nonviral gene transfer, as demonstrated in this work, offers feasibility and safety advantages for boosting bispecific CTLs in vivo, we hypothesize that viral antigen transgene expression represents a first-generation system amenable to significant additional enhancements by genetic engineering. Therefore, T-APCs might be further engineered to express transgenes to augment their APC activity, such as by enforced expression of CD19, desired costimulator molecules, or secretion of desired cytokines for loco-regional uptake. T cells used as APCs also offer the potential for systemically targeting the APCs to defined anatomic locations beyond the peritoneal cavity described in the current mouse model. These strategies might use the expression of chemokine receptor and selectin transgenes that recapitulate those used in the metastatic homing of tumors and, conceivably, may augment the antitumor activity of transferred bispecific effectors through their restimulation in the tumor microenvironment. Our group is particularly interested in augmenting the lymph node and bone marrow homing of T-APCs to be used in conjunction with CD19-specific, CAR-expressing effector cells for immunotherapy of B-cell lymphomas and leukemias.
In conclusion, our studies demonstrate the usefulness of T cells to function as APCs through their genetic modification to express viral antigens. T-APCs have considerable potential to enhance the activation of adoptively transferred, tumor-redirected effector T cells with resultant improvements in antitumor responses. Applications of T-APCs in transfer and boost clinical experimentation are anticipated to follow our currently active phase 1 trials for B-cell lymphoma and leukemias using adoptive transfer of CD20– and CD19–CAR-redirected CTLs.
Prepublished online as Blood First Edition Paper, October 26, 2004; DOI 10.1182/blood-2004-03-1208.
Supported by the National Institutes of Health (grants CA30206-23, CA033572-21), the Abe and Estelle Sanders Foundations, the Alliance for Cancer Gene Therapy, the Altschul Foundation, the Amy Phillips Foundation, the Leukemia and Lymphoma Society, the Lymphoma Research Foundation, the National Foundation for Cancer Research, the Pediatric Cancer Research Foundation, and the Sidney Kimmel Foundation for Cancer Research
L.J.N.C. and Z.A.-K. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Michael Kalos (City of Hope) for critical reading of the manuscript, Dr David Senitzer (City of Hope) for assistance with HLA typing, Lucy Brown for assistance with flow cytometry sorting, the Animal Resource Center at City of Hope, under the direction of Dr Richard Ermel, and the Children's Oncology Group ALL cell bank for providing primary ALL samples.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal