Abstract
Maintenance of protective humoral immunity depends on the generation and survival of antibody-secreting cells. The bone marrow provides niches for long-term survival of plasma cells generated in the course of systemic immune responses in secondary lymphoid organs. Here, we have analyzed migratory human plasma blasts and plasma cells after secondary vaccination with tetanus toxin. On days 6 and 7 after immunization, CD19+/CD27high/intracellular immunoglobulin Ghigh (IgGhigh)/HLA-DRhigh/CD38high/CD20–/CD95+ tetanus toxin–specific antibody-secreting plasma blasts were released in large numbers from the secondary lymphoid organs into the blood. These cells show chemotactic responsiveness toward ligands for CXCR3 and CXCR4, probably guiding them to the bone marrow or inflamed tissue. At the same time, a population of CD19+/CD27high/intracellular IgGhigh/HLA-DRlow/CD38+/CD20–/CD95+ cells appeared in the blood in large numbers. These cells, with the phenotype of long-lived plasma cells, secreted antibodies of unknown specificity, not tetanus toxoid. The appearance of these plasma cells in the blood indicates successful competition for survival niches in the bone marrow between newly generated plasma blasts and resident plasma cells as a fundamental mechanism for the establishment of humoral memory and its plasticity.
Introduction
Protective humoral memory is conferred by stable titers of specific antibodies (Abs) and can last for years.1 Although primary contact with an antigen (Ag) leads to the formation of Ab-secreting plasma blasts (PBs) with a lifespan of less than 1 week in extrafollicular foci and results in short Ab responses,2 most Ab-secreting cells (ASCs) generated during secondary (memory) immune response leave the follicles of the secondary lymphoid tissues as PBs. Specific ASCs are later found in the bone marrow (BM),3-5 mucosa-associated tissues, chronically inflamed tissues,6 or, to a lesser extent, the red pulp of spleens,7 with the phenotype of mature plasma cells (PCs) and a potential lifespan of more than 18 months.3,8,9 Indeed, specific Ab titers, mostly of immunoglobulin G (IgG) and IgA subclasses, can be stable for years10 and are produced mainly by resident PCs of the BM.10,11
The survival of PCs within the bone marrow (BM) is not an intrinsic capability of these cells but, rather, is regulated by the local microenvironment,7 which provides a limited number of survival niches for PCs.12-14 Because Abs of the IgG subclass have only a half-life of 3 weeks,12-14 the survival of PCs in the BM is prerequisite for the maintenance of Ab titers over long time periods (ie, protective immunity and memory). Release from secondary lymphoid organs, migration of PBs to the BM, and competition for the apparently limited number of survival niches with resident PCs generated earlier control the establishment and persistence of protective humoral memory.
Chemokines and their receptors are crucial for the control of lymphocyte trafficking. In mouse, CX-chemokine-receptor-4 (CXCR4) and its cognate ligand CX-chemokine-receptor ligand 12 (CXCL12), also termed stroma cell–derived factor 1 (SDF-1), and, to a lesser extent, CXCR3 and its ligand CXCL9, also termed monokine induced by IFN-γ (Mig), have been shown to be likely candidates for the attraction of PCs to the BM or to inflamed tissue.15,16 For human PBs, the functional expression of chemokine receptors has not been studied so far. We report here that migratory PBs, generated in an immune response against tetanus Ag and detectable in the blood on days 6 to 8 after secondary immunization, expressed CXCR3 and CXCR4 and were attracted by the respective ligands.
Apart from newly generated CD27high/intracellular (ic) Ighigh PBs expressing low levels of CXCR3 and CXCR4 and high levels of major histocompatibility complex (MHC) class 2 on the cell surface, a second population of CD27high/ic Ighigh cells is detectable in the blood of immunized persons at high numbers on days 6 to 8 after immunization. These cells express high levels of CXCR3 and CXCR4 and low levels of MHC class 2, thus resembling mature PCs of the BM.17,18 The Abs of these cells are not specific for the immunizing Ag, thus resembling the cells that Bernasconi et al19,20 had identified earlier. The present results suggest that these cells are in all likelihood resident PCs mobilized from their survival niches in the BM or elsewhere, in competition with newly generated PBs. Such competition would allow the humoral memory to adapt to its antigenic environment and gradually to lower the titer of Abs to those Ags that an immune system has not encountered for a long time.
Materials and methods
Preparation of blood samples
Citrate or heparinized whole blood (30-120 mL) from 13 healthy donors (6 women, 7 men; ages (means plus or minus standard deviations), 31.2 ± 8.6 years and 28.0 ± 5.9 years, respectively) was collected before and after immunization with tetanus toxin (TT). Peripheral blood mononuclear cells (PBMCs) were prepared by density gradient centrifugation over Ficoll-Paque (Amersham Pharmacia Biotech, Uppsala, Sweden) as reported previously.21 Serum was collected and stored at –20°C.
In the present study, healthy donors (6 women, 10 men) were immunized with TT (40IE, TT vaccine Merieux) or a combined TT/diphtheria vaccine (both Aventis Pasteur MSD, Strasbourg, France) after informed consent had been obtained. The ethics committee of the Medical Faculty of the Humboldt University (Charité) approved the study.
Antigens
Recombinant C-fragment of TT (rTT.C) (Hoffmann-La Roche, Indianapolis, IN) was conjugated to digoxigenin (Dig) (Boehringer, Mannheim, Germany), as described.22 rTT.C fragment is identical to the tetanus toxin C fragment generated from the TT heavy chain by digestion with papain, except for the presence of an N-terminal methionine in the recombinant fragment. It contains the carboxy-terminal 451 amino acids and thus the major antigenic determinant of TT.23,24
TT used in the presented enzyme-linked immunosorbent assay (ELISA) and enzyme-linked immunospot assay (ELISPOT) was kindly provided by Behring Werke (Chiron Behring, Marburg, Germany). DNA (Serva, Heidelberg, Germany) and hepatitis B Ag (hep B Ag) for the corresponding ELISPOT assays was kindly provided by Miltenyi Biotech (Bergisch-Gladbach, Germany).
Cytometric analysis
Immunofluorescence staining of cells for cytometric analysis was performed by incubating PBMCs with mouse antihuman monoclonal antibody (mAb) in phosphate-buffered saline (PBS)/0.5% bovine serum albumin (BSA) at 4°C for 10 minutes. The following Abs were used: anti-CD19 phycoerythrin (PE) mAb (clone, HD37; DAKO, Hamburg, Germany); anti-CD27 Cy5 mAb (clone, 2E4; a kind gift from René van Lier, Academic Medical Center, University of Amsterdam, The Netherlands); biotinylated anti-CD19 mAb (clone, HIB19), anti-CD20 fluorescein isothiocyanate (FITC) mAb (clone, 2H7), anti-CD45 peridinin chlorophyll protein (PerCP) mAb (clone, 2D1), anti-CD72 FITC mAb (clone, J4-117), anti-CD38 FITC mAb (clone, HIT2), anti-IgG FITC mAb (clone, G18-145), anti-CXCR3 PE mAb (clone, 1C6/CXCR3 (all from PharMingen, San Diego, CA); and anti–HLA-DR FITC mAb (clone, R3O), anti-CXCR4 PE mAb (clone, 12G5), and anti-CCR9 PE mAb (clone, 112509) (all from R&D Systems, Minneapolis, MN).
Before incubation with anti-Dig FITC or anti-Dig PE (Boehringer, Ingelheim, Germany) and streptavidin (SA)–PerCP (PharMingen) in indirect staining procedures, the cells were washed twice.
Propidium iodide (PI) (0.1 μg/mL) (Sigma-Aldrich, Steinheim, Germany) or DAPI (2,4 diamidino-2-phenylindole) (Molecular Probes, Eugene, OR) was added immediately before cytometric analysis to exclude dead cells. Flow cytometric analysis was performed using a FACScalibur (Becton Dickinson, San Jose, CA) or LSR (Becton Dickinson). FCS Express software was used for data processing (De Novo Software, Thornhill, ON, Canada); 1 × 105 to 3 × 106 events were collected for each sample.
For intracellular staining, cells were fixed with 2% formaldehyde (Merck, Darmstadt, Germany) for 20 minutes at room temperature (RT). Formaldehyde-fixed cells were incubated in PBS/0.5% BSA, with or without 0.5% saponin (Sigma, Munich, Germany) and biotinylated anti-IgG (clone, G18-145), anti-κ immunoglobulin light chain (clone, G20-193), anti-λ immunoglobulin light chain (clone, JDC-12; all PharMingen), SA-PerCP, rTT.C-Dig, and anti-Dig FITC or anti-Dig PE for 10 minutes at 4°C and then were washed and stored in PBS/BSA.
Enumeration of HLA-DRhigh/low CD19+/CD27high PBMCs
Absolute counts for lymphocyte subsets were determined using BD TruCount tubes and flow cytometry. Briefly, 50 μL heparinized whole blood was stained with anti-CD27 Cy5, anti-CD19 PE, anti-CD45 PerCP, and anti-CD20 FITC for 15 minutes in BD TruCount tubes at RT. Erythrocytes were lysed with 450 μL lysing solution (diluted 1:10) (Becton Dickinson) for 15 minutes, and CD45+/CD19+ lymphocytes were enumerated by flow cytometry. Absolute numbers of the various CD45+/CD19+ lymphocyte subsets were then calculated based on comparison with the known number of true count beads in that sample tube.
Enumeration of ASCs by ELISPOT
Single-cell suspensions were washed and resuspended in RPMI 1640 medium (Life Technologies, Paisley, United Kingdom) supplemented with 10% fetal calf serum (FCS; Invitrogen, Carlsbad, CA) and with penicillin, streptomycin, and glutamine (complete medium). To detect and enumerate antibody-secreting cells (ASCs), an ELISPOT technique was used, as described.25 Briefly, EIA/RIA flat-bottom 96-well plates (protein high binding) (Costar, Corning, NY) were coated with the following Ags or Abs: TT (21,5LF/mL) (Chiron Behring, Marburg, Germany), rTT.C (5 μg/mL) (Hoffmann-La Roche, Indianapolis, IN), DNA (10 μg/mL) (Serva, Heidelberg, Germany), hepatitis B Ag, goat anti–human IgG (5 μg/mL), goat anti–human IgA (5 μg/mL), or goat anti–human IgM (5 μg/mL) (all Sigma-Aldrich, Steinheim, Germany) in PBS at 4°C. Before they were coated with DNA, the plates were incubated with methyl-BSA (Serva) (10 μg/mL in PBS, pH 7.2) for 3 hours at 37°C.
Free protein–binding sites were blocked by incubation with 3% BSA (Biomol, Hamburg, Germany) 1 hour before cells were added at various dilutions in complete medium. PBMCs (5 × 105-1 × 106/well) were incubated in RPMI 1640 (Gibco, Paisley, United Kingdom) for 3 hours at 37°C and 5%CO2. After PBMCs were removed by vigorous washing with 3% BSA in PBS/0.01% Tween 20 (BSA/PBS/Tween), biotinylated goat anti–human IgG (1 μg/mL), anti–human IgA (2 μg/mL), or anti–human IgM (2 μg/mL) (all Sigma-Aldrich, Steinheim, Germany) in PBS/BSA/Tween was added, and plates were incubated for 20 minutes at RT. Thereafter, SA-alkaline phosphatase (Hoffmann-La Roche, Indianapolis, IN) was added. To visualize spot formation, a 2 M 5-bromo-4-chloro-3-indolylphosphate solution (Sigma-Aldrich) was used and was dissolved in 2-AMP-buffer (95 mL 2-amino-2-methyl-1-propanol, 0.1 mL Triton X-405, 150 mg/mL MgCl2, 900 mL distilled water, pH 10.25). Between each step, plates were washed 4 × with BSA/PBS/Tween20. The solution was then mixed with melted agarose (type 1, low electroendosmosis; Sigma-Aldrich) to obtain a final agarose concentration of 0.6%. The mixture was kept at 65°C for 20 minutes. After the substrate was added, plates were cooled to RT for solidification of the agarose. The plates were allowed to develop for 2 hours at 37°C. Spots were counted under an inverted microscope. The frequency of ASCs was calculated based on the number of plated PBMCs.
Chemotaxis assay
To test the chemotactic responsiveness of ASCs toward the chemokine ligands CXCL12 and CXCL9, a chemotaxis assay was performed as described previously.16,26,27 Briefly, 24-well plates with transwell inserts (6.5-mm diameter, 5-μm pore size; Costar) and RPMI 1640 medium (Life Technologies) supplemented with 0.5% BSA (low endotoxin; Sigma-Aldrich) was used as assay medium. Inserts were coated with 50-μL human fibronectin solution (Invitrogen) at a concentration of 10 μg/mL in distilled water and were incubated for 1 hour at 37°C and 5% CO2. The solution was removed, and the inserts were dried for 2 hours at 37°C. PBMCs were washed and diluted in medium at a final concentration of 5 × 105 cells/mL. Before application to the chemotaxis assays, freshly harvested cells were kept in prewarmed medium (37°C). The lower transwell chamber was filled with 600 μL chemokine solution containing 10 nM CXCL12 or 100 nM CXCL9 (both R&D Systems), and 100 μL cell suspension was added to the upper chamber. Cells were allowed to migrate for 90 minutes at 37°C in a humid atmosphere (5% CO2). Finally, the cells were collected from the lower transwell compartment and from control samples containing total cells before migration. In both fractions, IgG- or TT-specific ASCs were enumerated by ELISPOT, as described earlier in this section, and the frequencies of migrated cells were calculated accordingly.
Statistical analysis
Data were analyzed using GraphPad Prism software (San Diego, CA). Frequencies of B-cell populations were calculated with CellQuest software (Becton Dickinson, Franklin Lakes, NJ) or FCS Express (De Novo Software).
Results
Enumeration and kinetics of TT-specific plasma blasts and memory B cells
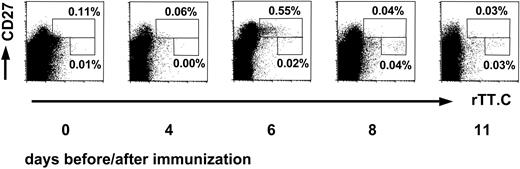
Using indirect labeling of PBMCs with Dig-conjugated rTT.C (rTT.C-Dig) and either anti-Dig FITC or anti-Dig Ab PE, the kinetics of appearance of rTT.C-specific B-memory lymphocytes and PBs in the blood of healthy subjects after TT booster immunization was analyzed (Figure 1). Those cells were then analyzed for their phenotype and migratory potential. The specificity of staining was confirmed by blocking with unconjugated rTT.C (data not shown).
Kinetics of rTT.C-specific CD19+ B cells after TT vaccination. The kinetics of the appearance of CD27high or CD27+ cells specific for rTT.C in blood, of a representative donor, demonstrates the sequential release of PBs and memory B cells. The rTT.C-specific CD19+ B cells at day 0 or days 4, 6, 8, and 11 after booster immunization with TT were identified by labeling of Ag-specific B cells with rTT.C-Dig and anti-Dig FITC. Staining of viable CD19+ PBMCs with anti-CD27 Cy5 mAb, rTT.C-Dig, and anti-Dig FITC is shown. Percentages of CD19+ B cells are indicated.
Kinetics of rTT.C-specific CD19+ B cells after TT vaccination. The kinetics of the appearance of CD27high or CD27+ cells specific for rTT.C in blood, of a representative donor, demonstrates the sequential release of PBs and memory B cells. The rTT.C-specific CD19+ B cells at day 0 or days 4, 6, 8, and 11 after booster immunization with TT were identified by labeling of Ag-specific B cells with rTT.C-Dig and anti-Dig FITC. Staining of viable CD19+ PBMCs with anti-CD27 Cy5 mAb, rTT.C-Dig, and anti-Dig FITC is shown. Percentages of CD19+ B cells are indicated.
On days 6 and 7 after booster vaccination with TT, the frequencies of rTT.C-binding CD19+/CD27high PBMCs peaked, and such cells could be detected in 13 of 13 (6 women, 7 men) healthy donors (Figure 1). By day 8 after vaccination, the frequencies of those cells had returned to normal. In earlier studies, it had been shown that the high expression of CD27 and the low expression of CD19 identifies peripheral PBs.21 Here, comparing the frequencies of IgG, IgM, and IgA ASCs, as determined by ELISPOT of 9 subjects, and rTT.C-specific CD19+ and CD27high cells, as determined cytometrically for 13 subjects, confirmed this finding for PBs generated in an intentional immune reaction. When the frequencies of rTT.C-binding CD19+/CD27high PBMCs of 15 measurements and of rTT.C-specific IgG ASCs were determined by flow cytometry and ELISPOT, respectively, there was a significant correlation (r = 0.8237; P < .0001), indicating that both methods provided comparable results and addressed the same cells.
Before immunization, the mean frequency of IgM, IgG, and IgA ASCs among PBMCs was 0.073% ± 0.040% (mean ± SD); 0.139% ± 0.079% of PBMCs were CD19+/CD27high. Six and 7 days after immunization, the frequency of ASCs among PBMCs increased to 0.101% ± 0.096%, and the frequency of CD19+/CD27high PBMCs increased to 0.201% ± 1.134%, a striking increase in the frequencies of ASCs and of CD19+/CD27high PBs in the blood.
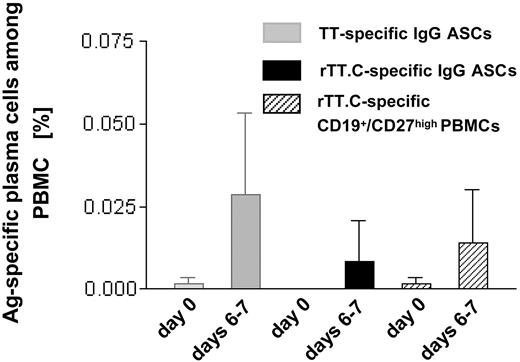
Parallel to the increase in the overall frequency of ASCs and CD19+/CD27high PBMCs, a drastic but variable increase in the frequencies of TT-specific, IgG-secreting ASCs, and rTT.C-specific PBMCs was observed (Figure 2). The frequencies of TT-specific ASCs, as determined by ELISPOT (0.0017% ± 0.005%; range, 0.005%-0.077%) and rTT.C-binding CD27high PBMCs, as determined by cytometry (0.001% ± 0.0015%; range, 0.001%-0.006%), increased to 0.029% ± 0.025% (range, 0.013%-0.188%) TT-specific ASCs and to 0.014% ± 0.016% (range, 0.001%-0.050%) rTT.C-specific, CD27high PBMCs at days 6 to 7 after immunization. It remains unclear whether any truly Ag-specific cells could be detected before vaccination because those numbers were close to the limit of sensitivity of the assays applied (day 0). On days 6 to 7, compared with day 0, there was at least a 17-fold average increase in frequencies of TT-specific ASCs and at least a 14-fold average increase in CD19+/CD27high PBMCs staining for rTT.C. It should be noted that on average, rTT.C-specific ASCs accounted for one third of the TT-specific ASCs (Figure 2).
Frequency of TT-specific IgG ASCs and rTT.C-specific PBMCs. At day 0 or days 6 to 7 after immunization with TT, the frequency of TT-specific IgG ASCs (▦), rTT.C-specific IgG ASCs (▪), or rTT.C-specific CD19+/CD27high PBMCs (▨) in the peripheral blood of 11 and 9 healthy donors, respectively, showed comparable results in ELISPOT and flow cytometry. The mean frequencies and SD are given.
Frequency of TT-specific IgG ASCs and rTT.C-specific PBMCs. At day 0 or days 6 to 7 after immunization with TT, the frequency of TT-specific IgG ASCs (▦), rTT.C-specific IgG ASCs (▪), or rTT.C-specific CD19+/CD27high PBMCs (▨) in the peripheral blood of 11 and 9 healthy donors, respectively, showed comparable results in ELISPOT and flow cytometry. The mean frequencies and SD are given.
rTT.C-specific memory B cells, generated by immunization with TT, appeared in the blood later than did specific PBs. In 3 of 6 subjects with frequencies of rTT.C-specific CD19+/CD27high PBs, rTT.C-specific CD19+/CD27+ memory B cells could be detected on days 8 and 11 but not yet on day 6 (Figure 1). The frequencies of these rTT.C-specific memory B cells ranged between 0.001% and 0.006% among PBMCs on day 8 and were approximately 10-fold lower than the frequencies of rTT.C-specific CD19+/CD27high PBs (average, 0.04%) of the same donors on days 6 to 7 after immunization. In the remaining cases, rTT.C-specific memory CD19+/CD27+ B cells could not be identified reliably at frequencies above the detection limit (among 10–5 PBMCs).
Although rTT.C-specific PBs were released from secondary lymphoid organs on day 6 after immunization and had already disappeared from the blood on day 8, rTT.C-specific memory B cells were released not before day 8, but they persisted in the blood over long time periods. In 1 donor of the present study, rTT.C-specific memory CD19+/CD27+ memory B cells were still detected in the blood 34 days after immunization.
rTT.C-specific PCs show the phenotype of recently generated plasma blasts
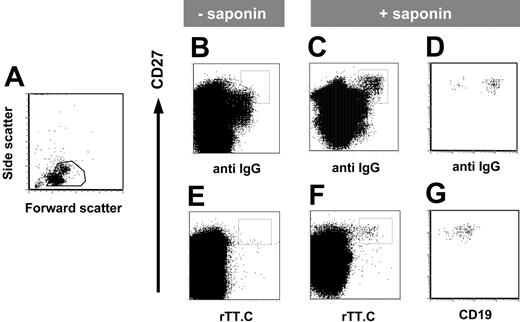
The expression of intracellular immunoglobulin is a hallmark of immunoglobulin-secreting PBs and PCs. Using saponin to permeabilize the plasma membrane, intracellular IgG specific for rTT.C could be demonstrated in the CD19+/CD27high PBMCs of a healthy person 6 days after TT boost (Figure 3B-C). This staining of CD27high PBMCs with rTT.C detected intracellular immunoglobulin because it was dependent on the presence of saponin (Figure 3F) and was not observed in the absence of saponin (Figure 3E). The CD27high PBMCs staining for rTT.C-specific Abs also stained for intracellular IgG and CD19low (Figure 3D, G).
rTT.C-specific CD27high PBMCs express intracellular IgG. Phenotype of rTT.C-specific PBMCs 6 days after TT booster immunization. Formaldehyde-fixed PBMCs were gated (A) and stained with anti-CD27 Cy5 mAb, biotinylated anti-CD19 mAb/SA PerCP, anti–human IgG-FITC, rTT.C-Dig, and anti-Dig PE in the presence (C-D, F-G) or absence (B, E) of saponin. Expression of intracellular IgG, surface CD27, and surface CD19 of rTT.C-specific PBMCs, gated as indicated in panels C and F, is shown in panels D and G, respectively.
rTT.C-specific CD27high PBMCs express intracellular IgG. Phenotype of rTT.C-specific PBMCs 6 days after TT booster immunization. Formaldehyde-fixed PBMCs were gated (A) and stained with anti-CD27 Cy5 mAb, biotinylated anti-CD19 mAb/SA PerCP, anti–human IgG-FITC, rTT.C-Dig, and anti-Dig PE in the presence (C-D, F-G) or absence (B, E) of saponin. Expression of intracellular IgG, surface CD27, and surface CD19 of rTT.C-specific PBMCs, gated as indicated in panels C and F, is shown in panels D and G, respectively.
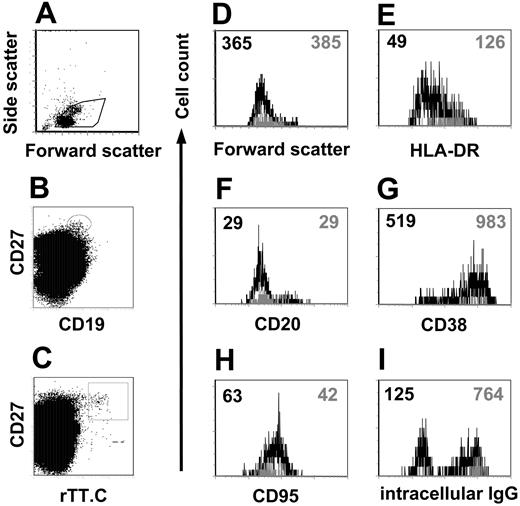
Although a major fraction stained, not all CD19+/CD27high/ic Ig+ PBMCs stained for rTT.C-specific Abs. When comparing the phenotype of the rTT.C-specific CD19+/CD27high cells with those CD19+/CD27high cells present at the same time in the blood but of different, undefined specificity, the rTT.C-specific cells expressed similarly low levels of CD20 (mean fluorescence intensity [MFI], 25-29 vs 23-29) but higher levels of CD38 (MFI, 983) than cells of unknown specificity (MFI, 519) (Figure 4F-G). CD95 expression was slightly lower in rTT.C-specific cells (MFI, 42) compared with all CD27high cells (MFI, 63) (Figure 4H). rTT.C-specific CD19low/CD27high PBMCs almost exclusively expressed intracellular IgG, whereas a considerable fraction of CD19+/CD27high PBMCs of unknown specificity showed no intracellular IgG (Figure 4I). It is likely that those intracellular IgG-negative cells expressed intracellular IgA because we routinely found such PBMCs (data not shown).
Phenotype of rTT.C-specific plasma blasts. Differential expression of various Ags on rTT.C-specific PBMCs from a representative donor 6 days after TT booster immunization. Formaldehyde-fixed PBMCs were gated (A) and stained with anti-CD27 Cy5 mAb, biotinylated anti-CD19 mAb/SA PerCP, rTT.C-Dig, and anti-Dig PE in the presence of saponin. rTT.C-specific CD19+/CD27high or CD19+/CD27high PBMCs with undefined specificity were identified by gating as indicated in panels B and C. Forward scatter, indicating the cell size, expression of HLA-DR, CD20, CD38, CD95, or intracellular IgG is shown for rTT.C-specific CD19+/CD27high (▦) or CD19+/CD27high PBMCs with undefined specificity (▪) (D-I). MFIs are indicated by the numbers in the top corners.
Phenotype of rTT.C-specific plasma blasts. Differential expression of various Ags on rTT.C-specific PBMCs from a representative donor 6 days after TT booster immunization. Formaldehyde-fixed PBMCs were gated (A) and stained with anti-CD27 Cy5 mAb, biotinylated anti-CD19 mAb/SA PerCP, rTT.C-Dig, and anti-Dig PE in the presence of saponin. rTT.C-specific CD19+/CD27high or CD19+/CD27high PBMCs with undefined specificity were identified by gating as indicated in panels B and C. Forward scatter, indicating the cell size, expression of HLA-DR, CD20, CD38, CD95, or intracellular IgG is shown for rTT.C-specific CD19+/CD27high (▦) or CD19+/CD27high PBMCs with undefined specificity (▪) (D-I). MFIs are indicated by the numbers in the top corners.
Expression of CXCR3 and CXCR4 on, and attraction of CXCL9 and CXCL12 to, 6-day-old rTT.C-specific plasma blasts
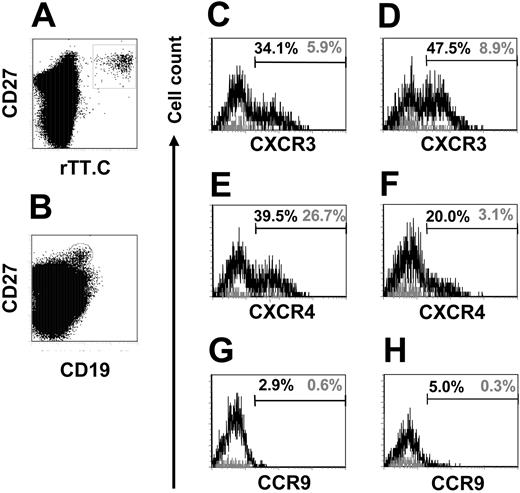
To address the homing potential of the migratory, rTT.C-specific CD19+/CD27high PBMCs, their expression of CXCR3, CXCR4, and CCR9 was analyzed. Twenty percent to 39.5% of CD19+/CD27high cells with undetermined specificity expressed CXCR4high PBMCs compared with 3.1% to 26.7% of rTT.C-specific CD19+/CD27high PBMCs from donors immunized with TT 6 to 7 days earlier (Figure 5E-F). In 2 donors analyzed, 5.9% and 8.9% of CD19+/CD27high PBMCs specific for rTT.C expressed CXCR3high, whereas 34.1% and 47.5% of the CD19+/CD27high PBMCs with unknown specificity were CXCR3high (Figure 5C-D). CCR9 was uniformly not detectably expressed on rTT.C-specific CD19+/CD27high PBMCs or on CD19+/CD27high PBMCs with unknown specificity (Figure 5G-H). The low cell surface expression of the chemokine receptors CXCR3 and CXCR4 further marked rTT.C-specific PBs as recently generated migratory cells. The up-regulation of CXCR3 and CXCR4 in ASCs has been shown in mice to be a hallmark of differentiation of migratory PBs into resident PCs of the BM and suggests that those CXCR3high and CXCR4high CD19+/CD27high PBMCs are mobilized resident PCs.16
CXCR3, CXCR4, and CCR9 expression on CD19+/CD27high PBMCs. Expression of CXCR3, CXCR4, and CCR9 on CD19+/CD27high PBMCs specific for rTT.C (black line) or of undefined specificity (gray line) of 1 representative donor, analyzed 7 days after immunization. rTT.C-specific PBs were identified by gating on formaldehyde-fixed CD19+/CD27high PBMCs (B) stained in the presence of saponin with rTT.C-Dig (A). Frequencies of CXCR3, CXCR4, and CCR9 expressing CD19+/CD27high PBMCs specific for rTT.C (▦) or of undefined specificity (▪) are shown (C-E). Donor 1, C, E, G; donor 2, D, F, H. Percentages indicate frequencies of cells that express the respective markers.
CXCR3, CXCR4, and CCR9 expression on CD19+/CD27high PBMCs. Expression of CXCR3, CXCR4, and CCR9 on CD19+/CD27high PBMCs specific for rTT.C (black line) or of undefined specificity (gray line) of 1 representative donor, analyzed 7 days after immunization. rTT.C-specific PBs were identified by gating on formaldehyde-fixed CD19+/CD27high PBMCs (B) stained in the presence of saponin with rTT.C-Dig (A). Frequencies of CXCR3, CXCR4, and CCR9 expressing CD19+/CD27high PBMCs specific for rTT.C (▦) or of undefined specificity (▪) are shown (C-E). Donor 1, C, E, G; donor 2, D, F, H. Percentages indicate frequencies of cells that express the respective markers.
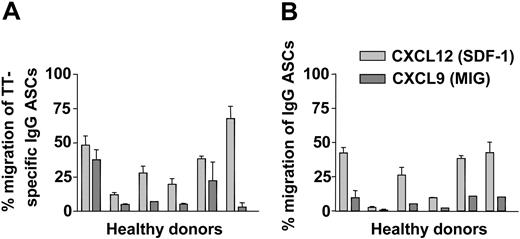
The migration of TT-specific IgG ASCs and IgG-secreting cells, in response to gradients of CXCL9 and CXCL12, was determined in transwell migration assays.16,27 Migrated compared with nonmigrated peripheral IgG ASCs and TT-specific IgG ASCs from 6 donors 6 or 7 days after TT immunization were enumerated by ELISPOT. Despite some variability among donors, a significant fraction of TT-specific IgG ASCs (35.8% ± 20.3%) (Figure 6A) and overall IgG ASCs (26.9% ± 17.3%) (Figure 6B) migrated in response to 10 nM CXCL12. In response to 100 nM CXCL9, 6.4% ± 4.4% of IgG ASCs (Figure 6B) and 13.5% ± 13.8% of TT-specific IgG ASCs crossed the transwell membrane (Figure 6A).
Migration of peripheral ASCs on day 6 after TT vaccination. On day 6 after immunization with TT, PBMCs of 6 different donors were tested for their chemotactic responsiveness to CXCL9 (dark gray bars) and CXCL12 (light gray bars). Frequencies of IgG ASCs and TT-specific IgG ASCs attracted by the chemokines were determined in transwell migration assays and were read out by ELISPOT. Frequencies of IgG ASCs (B) and TT-specific IgG ASCs (A) that migrated to the lower chambers of the transwells are shown in relation to input. Mean ± SD of duplicate measurements are indicated.
Migration of peripheral ASCs on day 6 after TT vaccination. On day 6 after immunization with TT, PBMCs of 6 different donors were tested for their chemotactic responsiveness to CXCL9 (dark gray bars) and CXCL12 (light gray bars). Frequencies of IgG ASCs and TT-specific IgG ASCs attracted by the chemokines were determined in transwell migration assays and were read out by ELISPOT. Frequencies of IgG ASCs (B) and TT-specific IgG ASCs (A) that migrated to the lower chambers of the transwells are shown in relation to input. Mean ± SD of duplicate measurements are indicated.
CD19+/CD27high/HLA-DRlow plasma cells are mobilized in an immune response
CD19+/CD27high PBMCs were heterogeneous not only with respect to expression of CXCR3 and CXCR4 (Figure 5) but also with respect to the expression of MHC class 2. In a bimodal distribution of expression, rTT.C-specific cells almost exclusively expressed high amounts of HLA-DR (MFI, 112-126), whereas CD19+/CD27high PBMCs expressed high or low levels of HLA-DR (MFI, 49-80) (Figure 4E). The high expression of CD38, the low expression of CXCR3 and CXCR4, and the high expression of MHC class 2 marked rTT.C-specific CD19+/CD27high PBMCs as recently generated PBs compared with resident PCs of the BM and those rare migratory PCs of the blood in healthy, uninfected donors with high expression of CXCR3 and CXCR4 and low expression of MHC class 2.4,16-18
Most interestingly, the numbers of HLA-DRlow PCs and HLA-DRhigh PBs among CD19+/CD27high cells in peripheral blood increased after vaccination in 3 donors tested (Figure 7), from an average of 512 (± 519) cells/mL on day 0 to 2470 (± 2470) cells/mL on day 7. At the same time, numbers of recently activated HLA-DRhigh PBs increased from 477 (± 282) cells/mL on day 0 to 2613 (± 900) cells/mL on day 7. In individual donors, the increase in cell numbers of HLA-DRlow and HLA-DRhigh cells correlated strikingly (Figure 7C). In all likelihood, this correlated mobilization of rTT.C-specific PBs and of resident PCs of undefined specificity reflected the successful competition of recently generated PBs with resident PCs of the BM or secondary lymphoid organs for habitation of PC survival niches.8
Mobilization of CD19+/CD27high/HLA-DRlow plasma cells after vaccination. In the course of TT/diphtheria vaccination, CD19+/CD27high PBMCs were analyzed for their expression levels of HLA-DR. Living PBMCs were identified according to light scatter properties and PI (gating not shown). The expression level of HLA-DR on CD19+/CD27high PBMCs, found in peripheral blood on day 0 (▦) and day7(▪) after immunization, is shown (A). According to the different expression level of HLA-DR found (A-B) CD19+/CD27high PBMCs were classified in HLA-DRlow/HLA-DRhigh (A). Recently generated PBs were identified as intracellular Ig+/HLA-DRhigh PBMCs specific for rTT.C (B). Based on the enumeration of CD19+ lymphocytes by TruCount and flow cytometry, the numbers of recently generated PBs CD19+/CD27high/HLA-DRlow (▪) and CD19+/CD27high/HLA-DRhigh PCs (▧) (as identified in panel A) from 3 healthy donors on days 0 and 7 after immunization were then calculated as shown in panel C.
Mobilization of CD19+/CD27high/HLA-DRlow plasma cells after vaccination. In the course of TT/diphtheria vaccination, CD19+/CD27high PBMCs were analyzed for their expression levels of HLA-DR. Living PBMCs were identified according to light scatter properties and PI (gating not shown). The expression level of HLA-DR on CD19+/CD27high PBMCs, found in peripheral blood on day 0 (▦) and day7(▪) after immunization, is shown (A). According to the different expression level of HLA-DR found (A-B) CD19+/CD27high PBMCs were classified in HLA-DRlow/HLA-DRhigh (A). Recently generated PBs were identified as intracellular Ig+/HLA-DRhigh PBMCs specific for rTT.C (B). Based on the enumeration of CD19+ lymphocytes by TruCount and flow cytometry, the numbers of recently generated PBs CD19+/CD27high/HLA-DRlow (▪) and CD19+/CD27high/HLA-DRhigh PCs (▧) (as identified in panel A) from 3 healthy donors on days 0 and 7 after immunization were then calculated as shown in panel C.
Specificity of peripheral ASCs after TT vaccination
The specificity of the mobilized CD19+/CD27high/HLA-DRlow PCs and the specificity of those CD19+/CD27high/HLA-DRhigh PBs not specific for rTT.C remained enigmatic. Recently, Bernasconi et al,20 using an immunization protocol similar to the one used here, demonstrated the appearance of ASCs reflecting the humoral memory repertoire in the periphery, after vaccination with TT. Here we determined the frequencies of hep B Ag-specific IgG ASCs and anti–dsDNA-ASC by ELISPOT on days 0 and 6 or 7 days after TT vaccination. All healthy donors had been previously immunized with hep B Ag. In 4 of the donors analyzed, no hep B Ag–specific ASCs could be detected in 106 PBMCs on days 0 and 6 or 7, whereas in 2 of 5 donors, 7 cells each were detected in 107 PBMCs on day 6 after immunization (data not shown). No dsDNA-specific IgG ASCs could be detected in 106 PBMCs of 10 donors each. Compared with frequencies of 1.3 to 1.9 × 10–4 for TT-specific PBs, hep B Ag–specific PCs thus appeared at least at 100 × lower frequency. Whether those CD19+/CD27high/HLA-DRhigh PBs not rTT.C specific recognized other fragments of TT or recognized the second Ag used in the vaccination—diphtheria—remains to be shown. rTT.C-specific ASCs make up one third of TT-specific ASCs. By extrapolation, and in accordance with the results of Bernasconi et al,20 most CD19+/CD27high/HLA-DRhigh PBs expressed Abs specific for the vaccine.
Discussion
Since their discovery in 1948, Ab-secreting PCs and their immediate precursors, the PCs, are known as the cellular correlate of humoral immunity. McMillan et al28 later identified the BM as the organ homing most Ab-secreting cells. PCs of the BM can survive for long time periods, up to the lifetime of the immune system, as has been shown experimentally for mice3,9 and as can be inferred indirectly for humans.29 They do not proliferate there. The survival of plasma cells in the BM is apparently dependent on a limited number of survival niches that provide a defined combination of survival signals.8,30 Several key features of the concept of long-lived PCs as the cellular correlate of humoral memory are addressed in the present study. By monitoring the release of Ag-specific PCs and memory B cells after vaccination of human volunteers, the present study determined when the cells were released from the secondary lymphoid organs and how long they stayed in the blood. The study showed which chemokine receptors enabled the cells to migrate to the BM. Most important, the study provided evidence that newly generated PBs apparently compete with resident PCs for survival niches.
It has been shown that when humans are vaccinated, ASCs appear in the blood, secreting Abs specific for the vaccinating Ag.31-33 These peripheral ASCs are believed to be on their way to the BM or to mucosa-associated lymphoid tissues.17,33-38 Here we characterized the phenotypic heterogeneity of peripheral ASCs and determined the phenotype of Ag-specific ASCs by cytometric immunofluorescence and their functional capacity to migrate toward chemokine gradients by monitoring the blood of healthy volunteers during a conventional TT booster immunization. Cytometrically, ASCs were identified according to high expression of intracellular immunoglobulin and of CD38 or CD27, or both, and according to low expression of CD19.21 Ab secretion was also analyzed by ELISPOT, with concordant results.
Migratory, Ag-specific plasma blasts leave the secondary lymphoid organs on day 7 after immunization
Six to 7 days after Ag stimulation in vivo (ie, after vaccination with TT or diphtheria/TT as in the experiments described here), a wave of Ag-specific ASCs appeared in the blood, as shown here cytometrically, and in the BM.9,39,40 rTT.C-specific CD19low/CD27high/CD138+/CD38high/CD95intermediate ASCs had the phenotype of recently generated PBs, in that they expressed MHC class 2high. A substantial fraction of the tonsillar IgG-secreting cells has been shown to express HLA-DR before and has been classified as recently generated PBs.3,41-44 As we had shown previously for recently generated murine PBs,16 the present study showed that human rTT.C-specific PBs migrated in response to CXCL9 and CXCL12, though they expressed CXCR3 and CXCR4, the corresponding chemokine receptors, only at low levels. Attraction by CXCL9 and CXCL12 would promote the immigration of recently generated PBs into the BM or into inflamed tissue.15,16,45,46 In accordance, most rTT.C-specific ASCs disappear from the blood after day 8.17 Although we could not show here that the human rTT.C-specific PBs homed to the BM, it is likely in view of what we know for murine PBs. Murine ASCs from chimeric mice reconstituted with an CXCR4-deficient immune system accumulate in peripheral lymphoid organs and in the blood, but they do not home to the BM.15 Like murine PBs, the human rTT.C-specific PBs also migrated in response to CXCL9, which is to say they expressed functional CXCR3. This response to proinflammatory chemokines would enable them to home to inflamed tissue, as murine PBs do.6 Interestingly, 1 week after vaccination rTT.C-specific ASCs, though they expressed CXCR3 and CXCR4 functionally, expressed these chemokine receptors phenotypically only at low levels. This is unlike the situation in a second population of ASCs present in the blood at the same time but also at other times.8,16,27,46,47 This second population most likely represents mobilized PCs, as is discussed in the next section.
Stoichiometric mobilization of MHC class 2low plasma cells accompanies migration of rTT.C-specific, MHC class 2high plasma blasts
Although rTT.C-specific, CXCR3low/CXCR4low/CD95intermediate MHC class 2high PBs appear in the blood on day 6 after vaccination, a second population of ASCs also appears that does not contain rTT.C-specific cells. These ASCs are CXCR3high/CXCR4high/CD95low and MHC class 2low. Their phenotype and behavior identifies them as mature PCs of BM.17,18 For mice, it has been shown that the corresponding cells, though expressing high amounts of CXCR4, occur late after immunization and no longer migrate toward CXCL12; rather, they respond by increased survival.16 Ratios of recently generated HLA-DRhigh PBs compared with HLA-DRlow PCs were similar on day 0 and days 6 to 7 after immunization, but their frequencies in the B-cell compartment and in their absolute numbers increased considerably from day 0 to days 6 to 7. This increase was variable in donors, ranging from 3- to 20-fold, but strictly correlated for PBs and PCs. The concomitant mobilization of recently generated MHC class 2high PBs, including the Ag-specific ASCs and mature MHC class 2low PCs, suggests that resident PCs from the BM are mobilized in the course of an immune response. Given that their numbers are roughly equivalent to those of the newly generated PBs, their appearance might reflect the expulsion of BM resident PCs from survival niches by newly generated PBs.8 Despite the reduced capacity of mobilized PCs to migrate toward CXCL12, as shown here, they are probably unable to home back to the BM. The stoichiometric mobilization of PCs, presumably polyclonal, in relation to the numbers of PBs generated in the response to a vaccinating Ag points to a remarkable capability of humoral memory, as conferred by long-lived BM PCs, to refresh, in dependence on the antigenic/pathogenic environment of the immune system.8,48
Although the present experiments do not enable determining the degree of self-renewal of humoral memory by polyclonal activation of memory B cells by cytokine and innate signals, as has been postulated recently by Bernasconi et al,20 they offer an alternative explanation. In the present vaccinations, a substantial fraction of the recently generated PBs is specific for fragment C of the vaccinating Ag TT, and, according to ELISPOT, these cells make up one third of the TT-specific ASCs. Nevertheless, it cannot be excluded that polyclonally activated PBs are generated on vaccination in a bystander reaction. It has been demonstrated ex vivo that human memory B cells can be activated by interleukin-15 (IL-15) and by anti–human immunoglobulin fragments but also by lipopolysaccharide (LPS) and bacterial unmethylated DNA (CpG). Polyclonal ASCs have been detected in the blood 1 week after Ag-specific immunization in vivo.25 However, in view of the present results, it remains to be shown whether these polyclonal ASCs were recently generated migratory PBs or were mobilized PCs, probably representing the humoral memory of the BM, with low migratory potential and thus a low chance to regain entrance to a BM niche providing signals for long-lived survival. If so, this would indicate a remarkable flexibility of the humoral memory in that new specificities would be added stoichiometrically at the expense of old ones.
Release of rTT.C-specific memory B cells into the periphery
Although Ag-specific ASCs appear in the blood 6 days after immunization and disappear a few days later, Ag-specific memory B cells cannot be detected cytometrically before day 8 after immunization. From then on, they remain constant in numbers for the time of observation (up to 2-3 weeks). Their frequencies and absolute numbers are considerably lower than those of ASCs. This puts technical constraints on their detection. rTT.C-specific memory B cells could be reliably identified in only 50% of the donors, with a threshold of frequency of 10–5 among memory CD19+/CD27+ B cells. The frequencies of rTT.C-specific CD19+/CD27+ memory B cells correlated with the frequencies of rTT.C-specific CD19+/CD27high PBs in donors but were approximately 10 × lower. In a previous analysis, we had enumerated rTT.C-specific, IgG+ peripheral memory B cells after magnetic enrichment with Ag.49 In this analysis, frequencies between 5 × 10–6 and 5 × 10–5 of rTT.C-specific, IgG+ B cells were detected among all CD19+ B cells. These frequencies did not correlate with the serum concentration of anti-TT IgG. However, if grouped according to recently challenged donors compared with unchallenged donors, the frequencies of Ag-specific memory B cells matched the concentration of Ag-specific serum IgG.25 This had been taken as indirect evidence for the maintenance of humoral memory by polyclonal activation of memory B cells. Here, we observed a negative correlation between preexisting titers of specific IgG and the generation of TT-specific ASCs on booster immunization (data not shown), in accordance with previous findings.48 By contrast, the numbers of ASCs generated and released into the blood on booster immunization did not correlate with the frequencies of preexisting memory B cells in the current study (data not shown).
The different kinetics of release of Ag-specific PBs and memory B cells into the periphery, and their distinct frequencies and persistence there, reflects their distinct roles for the maintenance of humoral memory.9,10,49 Although PBs provide short-lived peak responses and then either die or compete successfully for a survival niche in BM or inflamed tissue to provide long-lived humoral immunity, memory B cells circulate to provide reactive humoral immunity, in case of excessive Ag, or to provide polyclonal maintenance, as has been proposed.8,25,48 Although linked by Ag concentration, both compartments of humoral memory display proprietary characteristics of generation, providing unique options for interference to optimize vaccination protocols or treat Ab-mediated autoimmunity and allergy.
Prepublished online as Blood First Edition Paper, October 26, 2004; DOI 10.1182/blood-2004-07-2507.
Supported by grants from the Deutsche Forschungsgemeinschaft through Sonderforschungsbereich 421 (SFB) 421 and Do 491/5-3,4.
A.R. and T.D. contributed equally to this work
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Toralf Kaiser, Katharina Raba, and Karin Reiter for their skillful technical assistance.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal