Abstract
Idiopathic myelofibrosis (IM) is characterized by the constitutive mobilization of CD34+ cells. IM peripheral blood (PB) CD34+ cells had a reduced cloning efficiency and a lower frequency of cobblestone areas compared with normal granulocyte colony-stimulating factor (G-CSF)-mobilized PB CD34+ cells. IM CD34+ cells engrafted nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice, demonstrating that they contain bone marrow (BM)-repopulating cells. G-CSF-mobilized CD34+ cells produced multiple hematopoietic lineages within the NOD/SCID mice with a predominance of CD19+ cells. By contrast, IM CD34+ cells produced predominantly CD33+ cells, increased numbers of CD41+ cells, but fewer CD19+ cells. Transcriptional clonality assays of the engrafted human IM cells demonstrated their clonal origin. CD34+ cells from one patient isolated prior to leukemic transformation were capable of generating acute leukemia in NOD/SCID mice. The engrafted human cells exhibited the same abnormal karyotype as primary cells in a portion of the population. These findings demonstrate that BM-repopulating cells and more differentiated progenitor cells are constitutively mobilized into the PB in IM, and that their differentiation program is abnormal. In addition, the NOD/SCID model may be useful in gaining an understanding of the events occurring during the transition of IM to acute leukemia. (Blood. 2005;105:1699-1705)
Introduction
Chronic idiopathic myelofibrosis (IM) is a hematological malignancy characterized by splenomegaly, a leukoerythroblastic blood picture, teardrop poikilocytosis (dacrocytosis), varying degrees of bone marrow (BM) fibrosis, and extramedullary hematopoiesis.1-4 IM is thought to originate at the level of the multipotent hematopoietic stem cell (HSC).1-4 The HSC defect results in a profound hyperplasia of morphologically abnormal megakaryocytes and clonal populations of monocytes, which have been shown to locally release fibrogenic growth factors, leading to BM fibrosis.5-7
Rare CD34+ HSC/hematopoietic progenitor cells (HPCs) circulate in the peripheral blood (PB) of healthy individuals.8,9 Increased numbers of CD34+ cells can be mobilized into the PB following the administration of a variety of cytokines and/or chemotherapeutic agents.9-11 Barosi et al recently demonstrated that the PB of IM patients contained 360 times more CD34+ cells than normal controls and 18 to 30 times more CD34+ cells than patients with other Philadelphia chromosome-negative (Ph-) myeloproliferative disorders (MPDs).12 In addition, the PB CD34+ cell number was further shown to be related to disease progression and to serve as a biomarker for disease activity.12 The number of assayable HPCs present in the PB of IM patients have also been shown to be increased.13-15 Therefore, IM represents a unique situation in which the numbers of CD34+ cells appearing in the PB are frequently markedly increased in the absence of extrinsic stimuli.
Although the CD34 antigen is expressed by both the HSC and HPC, the functional potential of the CD34+ cells in the PB of IM patients has not been well defined. In this report we have further phenotyped the CD34+ cells of IM patients and examined the multilineage differentiation potential of these cells, as well as their ability to repopulate immunodeficient mice. In addition, we have demonstrated the utility of this in vivo model to analyze the cellular and molecular events that occur during the transition of IM to acute leukemia.
Patients, materials, and methods
Patients and healthy control subjects
All human tissue samples were obtained after informed consent following the guidelines of the institutional review board of the University of Illinois College of Medicine. PB samples were obtained from: (1) healthy donors in steady-state hematopoiesis; (2) healthy donors mobilized with granulocyte colony-stimulating factor (G-CSF; Amgen, Thousand Oaks, CA) at 5 μg/kg/d subcutaneously; and (3) patients with IM or polycythemia vera (PV) who met the World Health Organization (WHO) diagnostic criteria for IM and PV,1-4,16 as well as patients with secondary myelofibrosis associated with pulmonary hypertension.17 None of the patients were receiving cytotoxic agents at the time of study and none had evidence of transformation to acute leukemia. The specific clinical characteristics of the patients whose CD34+ cells were examined for BM-repopulating potential are described in Table 1.
Clinical characteristics of patients with IM
Patient no. (sex) . | Hgb, g/L . | WBC. × 109/L . | Platelet count, × 109/L . | Spleen size, cm below left costal margin . | % blasts in PB . | RBC transfusion requirement . | Severity of BM fibrosis . | Transition to acute leukemia . |
|---|---|---|---|---|---|---|---|---|
| IM-1 (F) | 106 | 24.2 | 275 | 4 | 0 | Yes | 3 | No |
| IM-2 (F) | 86 | 33.7 | 95 | 20 | 0 | Yes | 3 | No |
| IM-3 (F) | 75 | 18.0 | 26 | 16 | 0 | Yes | 3 | No |
| IM-4 (F) | 140 | 60.3 | 356 | NA* | 0 | No | 3 | Yes (5 wk) |
Patient no. (sex) . | Hgb, g/L . | WBC. × 109/L . | Platelet count, × 109/L . | Spleen size, cm below left costal margin . | % blasts in PB . | RBC transfusion requirement . | Severity of BM fibrosis . | Transition to acute leukemia . |
|---|---|---|---|---|---|---|---|---|
| IM-1 (F) | 106 | 24.2 | 275 | 4 | 0 | Yes | 3 | No |
| IM-2 (F) | 86 | 33.7 | 95 | 20 | 0 | Yes | 3 | No |
| IM-3 (F) | 75 | 18.0 | 26 | 16 | 0 | Yes | 3 | No |
| IM-4 (F) | 140 | 60.3 | 356 | NA* | 0 | No | 3 | Yes (5 wk) |
Not applicable; patient was splenctomized.
Purification of human PB CD34+ cells
The PB was layered onto Ficoll-Hypaque (1.077 g/mL; Amersham Biosciences, Piscataway, NJ), and low-density mononuclear cells (MNCs) separated after centrifugation. A CD34+ cell population was isolated using a magnetic activated cell sorting CD34+ Isolation Kit (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. The purity of the CD34+ cell population was analyzed using a FACSCaliber Flow Cytometer (Becton Dickinson, Mountain View, CA). Cell fractions showing a CD34+ cell purity of 85% or greater were used for subsequent experiments including HSC phenotyping, in vitro progenitor assays, and transplantation into nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice.
Phenotypic analysis of CD34+ cells
Separate aliquots of isolated CD34+ or MNCs were double stained with anti-CD34 and a panel of monoclonal antibodies (Abs) against c-kit, CD38, CD90, and CD33 (Becton Dickinson, San Jose, CA). The percentage of CD34+ cells expressing c-kit, CD90, and CD33, but not CD38, was then determined by flow cytometric analysis.
Cell-cycle analysis of PB CD34+ cells was performed by propidium iodide (PI; Sigma Chemical, St Louis, MO) staining as described previously with minor modifications.18 Briefly, cells were fixed in 70% ethanol; to avoid the formation of aggregates, the cell suspension was added dropwise onto 70% ethanol while vortexing and then kept on ice for 20 minutes. After twice washing with phosphate-buffered saline (PBS; Cambrex, Walkers-ville, MD), the cells were stained with a staining buffer containing 4 mM citrate buffer, 30 U of RNAase, 0.1% triton X-100, 5 μg/mL PI, and 0.138 mM NaCl at 4°C, and the DNA content analyzed with a FACSCalibur. Data files containing forward- and side-scatter peak signals as well as width and areas of the PI signal were collected. Doublet events were excluded by gating on the PI signal-width channel.
Colony-forming cell and cobblestone area-forming cell assays
CD34+ cells were incubated at a concentration of 500 cells/mL of culture mixture. One milliliter of culture mixture containing 500 cells, 0.9% methylcellulose, 30% fetal bovine serum (FBS), 1% bovine serum albumin (BSA), 0.05 mM 2-mercaptoethanol (2-ME; StemCell Technologies, Van-couver, BC, Canada) and a cytokine cocktail containing stem cell factor (SCF; 100 ng/mL), interleukin-3 (IL-3; 100 ng/mL), IL-6 (100 ng/mL), G-CSF (20 ng/mL), erythropoietin (EPO; 4 U/mL), and thrombopoietin (TPO; 100 ng/mL) (all from Amgen) was placed in 35-mm non-tissue culture dishes and incubated at 37°C in 5% CO2. All cultures were performed in triplicate and various colony types enumerated using an inverted microscope at days 12 to 14 of culture, according to previously described criteria.19
For the cobblestone area-forming cell (CAFC) assays, CD34+ cells were suspended in limiting dilution in Iscove modified Dulbecco medium (IMDM) + 10% FBS + cytokine cocktail (100 ng/mL SCF, 100 ng/mL leukemia inhibitory factor [LIF], 100 ng/mL IL-3, 100 ng/mL IL-6, and 50 ng/mL GM-CSF) and seeded onto pre-established feeder cell layers of an irradiated murine fibroblast cell line, M210B4.20 The cells were then incubated at 37°C in 5% CO2 with half of the incubating volume replaced with fresh media containing the same cytokine cocktail on a weekly basis. After 5 weeks of culture, the number of cobblestone areas was enumerated and the frequencies of CAFC calculated using L-CALC software.20
NOD/SCID repopulating assay
NOD/LtSz-scid/scid (NOD/SCID) mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and maintained in microisolators under specific pathogen-free conditions at the Biologic Resources Laboratory at the University of Illinois at Chicago. To optimize human cell engraftment, 7- to 8-week-old female NOD/SCID mice recipients received a series of pretreatments consisting of: (1) a sublethal dose of whole-body irradiation of 350 cGy with a cobalt radiation source; (2) intraperitoneal injections of 200 μg/mouse TM-β1 (BD Biosciences Pharmingen), a monoclonal antibody (mAb) directed against the murine IL-2Rβ to eliminate remaining natural killer (NK) activity21 after irradiation at 6 to 12 hours prior to transplantation; and (3) alternate-day intraperitoneal injections of 10 ng of recombinant human (Rhu) SCF, 6 μg of RhuGM-CSF, and 6 μg of RhuIL-3 per injection, for the first 10 days after transplantation.
Purified CD34+ cells were washed twice in PBS and injected into the tail veins of NOD/SCID mice at a dose of 0.5 to 3 × 106 cells/mouse in 0.5 mL PBS. In 2 cases, CD34- cells obtained from the flow-through of the CD34 selection columns were collected and injected intravenously at 15 to 30 × 106 cells/mouse. Nine to 16 weeks after human cell transplantation, the mice were killed and the PB, spleen, femur and tibia were harvested for analysis. From these organs, cell suspensions were prepared, the red blood cells (RBCs) were treated with a lysing buffer (Sigma Chemical), and cell numbers and viability determined.
For analysis of human cells in murine tissues, cell suspensions were preincubated for 30 minutes at 4°C in PBS containing 0.1% BSA, 20% mouse serum, and 20% human serum. Separate cell aliquots were then incubated for 30 minutes at 4°C with a panel of mAbs against the following human markers conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), or allophycocyanin (APC): CD34, CD45, CD33, CD19, CD41a, immunoglobulin M (IgM), CD38, CD56, and CD3 (Becton Dickinson, San Jose, CA). A separate cell aliquot was stained with isotype-matched mAbs labeled with the same fluorochromes to establish the levels of non-specific immunofluorescence. After washing, 2 μg/ml PI was then added to eliminate dead (PI+) cells from the analysis. The samples were then analyzed flow cytometrically using a FACSVantage. Routinely, 30 000 events were acquired per sample. The lack of cross-reactivity of human-specific antibodies with mouse cells was confirmed in every experiment by staining BM cells from an irradiated control mouse that did not receive a transplant. The percentage of human cells in the recipient was calculated as the number of human CD45+ cells/the number of PI- cells × 100. Engraftment of human cells was defined by the presence of at least 0.1% nucleated cells expressing CD45 over the background fluorescence.
Immunohistochemical staining
Formalin-fixed and paraffin-embedded sections of tibias from the recipient mice were stained with May-Grunwald-Giemsa. For immunochemical staining, the tissue sections were deparaffinized in xylene and hydrated in graded alcohol. The sections were then stained with a monoclonal mouse anti-human CD34 antibody (CD34 QBend 10; DakoCytomation, Carpenteria, CA). Staining was performed on a DakoCytomation Histostainer using a streptavidin/horseradish peroxidase detection kit with the chromogen 3.3′-diaminobenzidine tetrahydrochloride (DBA). All staining procedures were performed utilizing an isotypic antibody as a negative control. The sections were examined under 200 × magnification using a Splan 20 objective lens with a numerical aperture of 0.46 on an Olympus BH-2 light microscope (Olympus America, Melville, NY). The photomicrographs were taken using an Olympus DPII digital camera. Microsoft PowerPoint (Redmond, WA) was utilized for all data acquisition.
Transcriptional clonality assays
The genotypes for 5 X-chromosome exonic polymorphisms (MPP1, IDS, G6PD, BTK and FHL1) of the 3 IM females were determined by real-time polymerase chain reaction (PCR) using commercial ABI TaqMan probes (Applied Biosystems, Foster City, CA).22 To examine the clonal origin of the human IM hematopoietic cells engrafted in NOD/SCID mice, RNA was extracted from human CD33+ cells purified from the femurs of these mice utilizing Miltenyi cell selection devices (Miltenyi Biotec). Transcriptional clonality assays were then performed using single-strand conformation polymorphism (SSCP) analysis as previously described.22
Cytogenetic analysis
Cytogenetic analysis was performed on the BM of patient IM-4 at the time of leukemic transformation (May 2003) and 1 year prior to that time. The human CD45+ cells isolated from the mice that received transplants of PB CD34+ cells obtained from patient IM-4 (5 weeks prior to the leukemia transformation) utilizing Milteneyi cell-selection devices were also analyzed. Cells were cultured for approximately 24 hours in the absence of mitogens. Chromosome preparation and banding were performed as previously described.23 An attempt was made to analyze at least 20 metaphases. The description of karyotypes follows the recommendations of the International System for Human Cytogenetic Nomenclature.24
Statistical analysis
Data points are expressed as the mean ± standard error of the mean (SEM). Differences between percentages of cells expressing a particular cellular phenotype were calculated using the Wilcoxon test, whereas differences between other variables were compared using either a Student t test or analysis of variance (ANOVA).
Results
Phenotypic analysis of CD34+ cells mobilized into the PB of IM
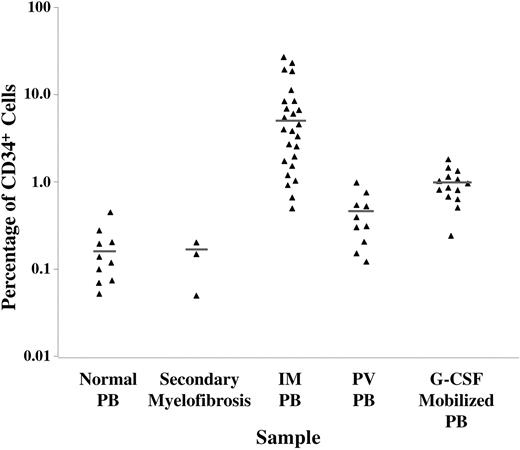
Previously, a greater number of CD34+ cells have been reported to circulate in the PB of patients with IM compared with healthy controls.12 We compared the percentage of CD34+ cells within the PB MNCs of patients with IM, healthy donors mobilized with G-CSF, healthy individuals, and patients with secondary myelofibrosis. As shown in Figure 1, the percentage of CD34+ cells within the PB MNC fraction from both patients with IM and healthy donors mobilized with G-CSF were dramatically elevated with a mean ± SEM of 8.34 ± 2.47% (P < .001), and 1.06 ± 0.42% (P < .01), respectively, compared with healthy volunteers (0.27 ± 0.20%). When 3 patients with secondary myelofibrosis associated with pulmonary hypertension were similarly studied, the percentage of CD34+ cells within the MNC fraction was not increased. However, a greater percentage of the cells within the MNC fraction in patients with PV were CD34+ than within that fraction in healthy controls (P < .05), albeit not to the same degree as that observed in IM. It is important to note the relative degree of CD34+ cell mobilization in IM was dramatically greater compared with G-CSF-mobilized donors (P < .01) and PV patients. These findings suggest IM is unique in that the CD34+ cells are preferentially mobilized into the PB.
The percentage of CD34+ cells in PB MNC fractions of healthy subjects (n = 10), secondary myelofibrosis (n = 3), IM (n = 25), PV (n = 10), and healthy donors mobilized with G-CSF (n = 14).
The percentage of CD34+ cells in PB MNC fractions of healthy subjects (n = 10), secondary myelofibrosis (n = 3), IM (n = 25), PV (n = 10), and healthy donors mobilized with G-CSF (n = 14).
CD34+ cells in IM PB were further characterized by mAb staining and flow cytometric analysis. Two-color staining and fluorescence-activated cell-sorting (FACS) analysis were performed on preisolated PB CD34+ cells from healthy volunteers or patients with IM. In some cases, IM PB MNCs were used directly for phenotypic analysis. As can be seen in Table 2, the fraction of IM PB CD34+ cells expressing CD117 (c-kit), CD38, CD90, and CD33 are significantly different than that of healthy volunteers. A greater percentage of IM PB than normal PB CD34+ cells were c-kit+, CD38-, and CD90+ (Table 2), suggesting that the CD34+ cells in the PB of IM patients might represent a more primitive population of HPCs than that which normally circulates in the PB. In addition, the expression of the myeloid lineage marker CD33 was shown to be significantly increased in IM PB CD34+ cells.
Phenotype of CD34+ cells in normal and IM PB
Cell surface antigens . | Mean normal PB ± SD, %* . | Mean IM PB ± SD, %† . |
|---|---|---|
| CD117+ (c-kit) | 16.0 ± 7.3 | 40.8 ± 15.1‡ |
| CD90+ (Thy-1) | 23.9 ± 4.6 | 43.5 ± 5.8‡ |
| CD38− (Negative) | 0.6 ± 0.4 | 4.7 ± 3.6‡ |
| CD33+ | 33.0 ± 5.8 | 57.2 ± 13.5‡ |
Cell surface antigens . | Mean normal PB ± SD, %* . | Mean IM PB ± SD, %† . |
|---|---|---|
| CD117+ (c-kit) | 16.0 ± 7.3 | 40.8 ± 15.1‡ |
| CD90+ (Thy-1) | 23.9 ± 4.6 | 43.5 ± 5.8‡ |
| CD38− (Negative) | 0.6 ± 0.4 | 4.7 ± 3.6‡ |
| CD33+ | 33.0 ± 5.8 | 57.2 ± 13.5‡ |
n = 5.
n = 8.
P < .05.
It has been reported that virtually all of the PB CD34+ cells mobilized by G-CSF are noncycling quiescent cells.22-24 We therefore performed cell-cycle analysis using flow cytometry to determine the percentage of quiescent IM PB CD34+ cells. Similar to normal PB CD34+ cells and G-CSF mobilized CD34+ cells, more than 98% of the IM CD34+ cells resided within the G0/G1 phase (data not shown).
IM PB CD34+ cells have decreased cloning efficiency in vitro
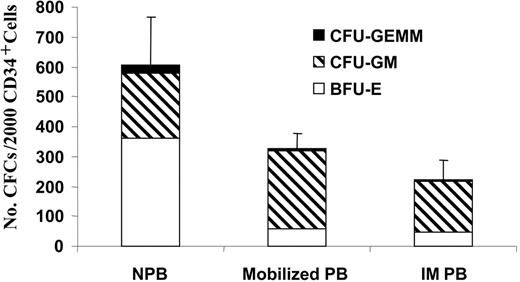
In order to examine the differentiation potential of IM HSCs/HPCs, the number and type of HPCs assayed from PB CD34+ cells isolated from 6 healthy volunteers, 4 healthy donors mobilized with G-CSF, and 7 patients with IM were enumerated. As shown in Figure 2, about 60% of the colonies formed by normal PB CD34+ cells were derived from burst-forming unit-erythroid (BFU-E), while both IM and G-CSF-mobilized CD34+ cells generated predominantly CFU-GM-derived colonies (∼ 80%). More importantly, the cloning efficiency of IM CD34+ cells was significantly lower than that of normal PB CD34+ cells (P < .01). The cloning efficiency of IM CD34+ cells and CD34+ cells isolated from G-CSF-mobilized PB were, however, similar (P > .05), as was the distribution of the colony types formed (Figure 3). Since there are greater numbers of CD34+ cells in the PB of IM patients, the absolute number of assayable HPCs are clearly increased in patients with IM compared with healthy individuals or even G-CSF-mobilized healthy volunteers.
Cloning efficiency of normal PB (NPB; n = 6), G-CSF-mobilized PB (n = 4) and IM PB (n = 7) CD34+ cells. The number of CFCs is the sum of the number of BFU-E (□)-, CFU-GM (▧)- and CFU-granulocyte erythrocyte monocyte macrophage (GEMM; ▪)-derived colonies cloned per 2000 CD34+ cells plated. Values are expressed as the mean ± SD. Difference in CFC formation between normal PB and IM PB was statistically significant (P < .005).
Cloning efficiency of normal PB (NPB; n = 6), G-CSF-mobilized PB (n = 4) and IM PB (n = 7) CD34+ cells. The number of CFCs is the sum of the number of BFU-E (□)-, CFU-GM (▧)- and CFU-granulocyte erythrocyte monocyte macrophage (GEMM; ▪)-derived colonies cloned per 2000 CD34+ cells plated. Values are expressed as the mean ± SD. Difference in CFC formation between normal PB and IM PB was statistically significant (P < .005).
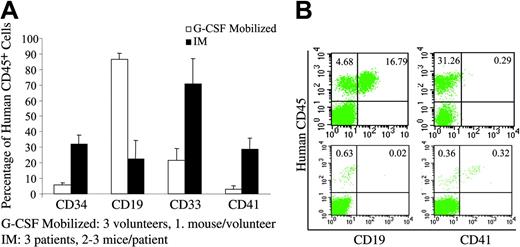
Multilineage engraftment of NOD/SCID mice. (A) Analysis of myelopoiesis, B lymphopoiesis, and megakaryocytopoiesis in the BM of the NOD/SCID mice that received transplants of either normal G-CSF-mobilized PB (□;n = 3) or IM PB (▪;n = 3) CD34+ cells. Overall distribution of myeloid (CD33+) lineages, B-lymphoid (CD19+) lineages, progenitor cells (CD34+), and megakaryocytes (CD41+) among human cells (CD45+). Values are shown as the mean ± SD. (B) Representative flow cytometric analysis of BM from mice that received transplants of PB CD34+ cells from a G-CSF-mobilized volunteer (top row) or a patient with IM (bottom row). BM cells were analyzed by flow cytometry at 9 to 15 weeks after transplantation.
Multilineage engraftment of NOD/SCID mice. (A) Analysis of myelopoiesis, B lymphopoiesis, and megakaryocytopoiesis in the BM of the NOD/SCID mice that received transplants of either normal G-CSF-mobilized PB (□;n = 3) or IM PB (▪;n = 3) CD34+ cells. Overall distribution of myeloid (CD33+) lineages, B-lymphoid (CD19+) lineages, progenitor cells (CD34+), and megakaryocytes (CD41+) among human cells (CD45+). Values are shown as the mean ± SD. (B) Representative flow cytometric analysis of BM from mice that received transplants of PB CD34+ cells from a G-CSF-mobilized volunteer (top row) or a patient with IM (bottom row). BM cells were analyzed by flow cytometry at 9 to 15 weeks after transplantation.
We next assessed the ability of IM PB MNCs and CD34+ cells to form CAFCs in a stromal cell-based culture system after 5 weeks of incubation. The frequency of CAFCs has been previously used as an in vitro surrogate assay for human HSCs.19 As can be seen in Table 3, the frequency of CAFCs in IM PB MNCs was shown to be higher than that observed in G-CSF-mobilized healthy donors, suggesting that IM is also associated with the mobilization of greater numbers of more primitive HPCs (CAFCs) than G-CSF mobilization. However, the frequency of CAFCs within the CD34+ cells was 3-fold lower in IM PB, compared with normal PB and G-CSF-mobilized PB (Table 4). These findings suggest that the preferential mobilization of HSCs/HPCs in IM is also accompanied by abnormalities in CD34+ cell function.
Frequency of CAFCs in PB MNCs from healthy G-CSF-mobilized donors and patients with IM
Source of PB MNCs . | No. studied . | CAFC frequency . |
|---|---|---|
| Mobilized PB | 2 | 1 in 1 386 |
| IM PB | 3 | 1 in 245 |
Source of PB MNCs . | No. studied . | CAFC frequency . |
|---|---|---|
| Mobilized PB | 2 | 1 in 1 386 |
| IM PB | 3 | 1 in 245 |
Frequency of CAFCs in PB CD34+ cells from healthy volunteers, healthy G-CSF-mobilized donors, and patients with IM
Source of CD34+ cells . | No. studied . | CAFC frequency (range of values) . |
|---|---|---|
| Normal PB | 2 | 1 in 206 (157-270) |
| Mobilized PB | 2 | 1 in 186 (162-212) |
| IM PB | 3 | 1 in 746 (477-955) |
Source of CD34+ cells . | No. studied . | CAFC frequency (range of values) . |
|---|---|---|
| Normal PB | 2 | 1 in 206 (157-270) |
| Mobilized PB | 2 | 1 in 186 (162-212) |
| IM PB | 3 | 1 in 746 (477-955) |
IM PB CD34+ cells contain NOD/SCID repopulating cells
Although there is a significant mobilization of CD34+ cells into the PB in IM, one cannot be certain, at present, if these PB CD34+ cells are composed exclusively of more differentiated progenitor cells or also contain HSCs. To address this question, the in vivo functional behavior of CD34+ cells isolated from the PB of IM patients and G-CSF-mobilized volunteers were studied by transplanting these purified CD34+ cells into NOD/SCID mice. A dose of 0.5 to 3 × 106 cells/mouse was transplanted. After 9 to 15 weeks, the mice were killed and the BM analyzed for the degree of human cell engraftment using flow cytometric analysis and monoclonal antibodies against CD45, CD34, CD19, CD33, IgM, CD56, CD41, and CD3.
As shown in Table 5, IM and G-CSF-mobilized PB CD34+ cells were capable of engrafting NOD/SCID mice and producing cells belonging to multiple hematopoietic lineages (Figure 3). By contrast, the CD34- cells from 2 IM patients did not possess the ability to repopulate the NOD/SCID mice. The differentiation program of IM CD34+ cells was remarkably different from that of G-CSF-mobilized healthy donors. Although both sources of CD34+ cells were capable of producing hematopoietic cells belonging to multiple hematopoietic lineages, CD19+ B cells represented the predominant cell population in the BM of mice that received transplants of G-CSF-mobilized PB CD34+ cells, while PB CD34+ cells from IM patients produced predominantly myeloid cells (CD33+ cells) as well as far greater numbers of CD41+ cells (megakaryocytes) and fewer CD19+ cells (Figure 3A-B). In addition, approximately 30% of the human cells in the BM of the NOD/SCID mice (at week 9) after transplantation with IM PB CD34+ cells were CD34+ compared with about 6% observed in the mice transplanted with G-CSF-mobilized PB CD34+ cells (Figure 3A). These data suggest that BM-repopulating cells exist within the PB of IM patients, and that they are restricted to the CD34+ cell population. In addition, the differentiation program of the IM CD34+ cells appears to differ from that of normal CD34+ cells mobilized into the PB by G-CSF as demonstrated by greater capacity to generate CD34+, CD41+, and CD33+ cells in vivo.
IM CD34+ cells contain SCID-repopulating cells
Donor no. . | No. of cells transplanted . | Frequency of engraftment . | Human CD45+ cells in BM, % . |
|---|---|---|---|
| G-CSF-mobilized PB CD34+ | |||
| N-1 | 1.9 × 106 | 1/1 | 12.4 |
| N-2 | 2.5 × 106 | 1/1 | 21.5 |
| N-3 | 0.5 × 106 | 1/1 | 0.3 |
| IM PB CD34+ | |||
| IM-1 | 3.0 × 106 | 6/6 | 1.9 |
| IM-2 | 1.2 × 106 | 2/2 | 0.7 |
| IM-3 | 0.5 × 106 | 2/2 | 1.5 |
| IM-4 | 3.0 × 106 | 3/3 | 83.0 |
Donor no. . | No. of cells transplanted . | Frequency of engraftment . | Human CD45+ cells in BM, % . |
|---|---|---|---|
| G-CSF-mobilized PB CD34+ | |||
| N-1 | 1.9 × 106 | 1/1 | 12.4 |
| N-2 | 2.5 × 106 | 1/1 | 21.5 |
| N-3 | 0.5 × 106 | 1/1 | 0.3 |
| IM PB CD34+ | |||
| IM-1 | 3.0 × 106 | 6/6 | 1.9 |
| IM-2 | 1.2 × 106 | 2/2 | 0.7 |
| IM-3 | 0.5 × 106 | 2/2 | 1.5 |
| IM-4 | 3.0 × 106 | 3/3 | 83.0 |
Engrafted human hematopoietic cells in NOD/SCID mice receiving IM CD34+ cells are clonal in origin
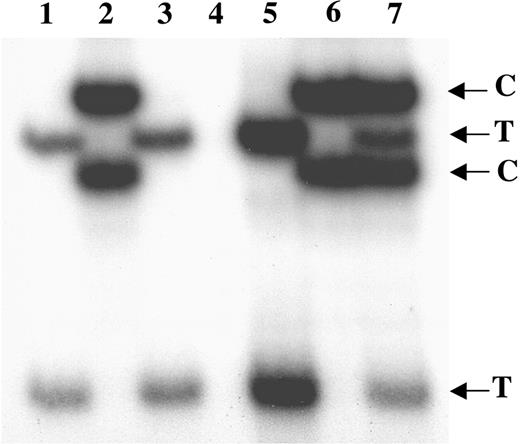
Using transcriptional clonality assays,22 we analyzed the clonal origin of those hematopoietic cells which engrafted into the NOD/SCID mice that received transplants of CD34+ cells from 3 female IM patients. Patient IM-1 was heterozygous for G6PD; IM-2 was heterozygous for MPP1, IDS, and G6PD; and IM-3 was heterozygous for MPP1 and G6PD. We analyzed the exonic allelic transcription of all X-chromosome polymorphic alleles in mice that received transplants of CD34+ cells from all 3 IM patients. CD33+ cells were isolated from the BM cells of each mouse and all 6 individual polymorphic alleles were examined; in each case the CD33+ cells expressed only a single allelic transcript of each X-chromosome polymorphism. As shown in the Figure 4 (only the G6PD assay shown), the CD33+ cells from all 3 mice only expressed either the T or C allele of G6PD, but not both. This finding demonstrated that the human hematopoiesis present in these mice was derived from the same clone as the human IM cells.
Transcriptional clonality assay of G6PD in human CD33+ cells isolated from mice that received transplants of IM PB CD34+ cells. Lanes 1-3 show clonal expression of G6PD in human CD33+ cells from 3 individual mice, each given transplants of CD34+ cells from a different female IM patient: IM-1 (lane 1), IM-2 (lane 2) and IM-3 (lane 3). Lane 4 shows negative control. Lane 5 shows positive control for clonal expression of G6PD on T allele. Lane 6 shows positive control for clonal expression of G6PD on C allele. Lane 7 shows positive control for polyclonal expression of G6PD on both T and C alleles.
Transcriptional clonality assay of G6PD in human CD33+ cells isolated from mice that received transplants of IM PB CD34+ cells. Lanes 1-3 show clonal expression of G6PD in human CD33+ cells from 3 individual mice, each given transplants of CD34+ cells from a different female IM patient: IM-1 (lane 1), IM-2 (lane 2) and IM-3 (lane 3). Lane 4 shows negative control. Lane 5 shows positive control for clonal expression of G6PD on T allele. Lane 6 shows positive control for clonal expression of G6PD on C allele. Lane 7 shows positive control for polyclonal expression of G6PD on both T and C alleles.
Development of leukemia in the mice receiving IM PB CD34+ cells
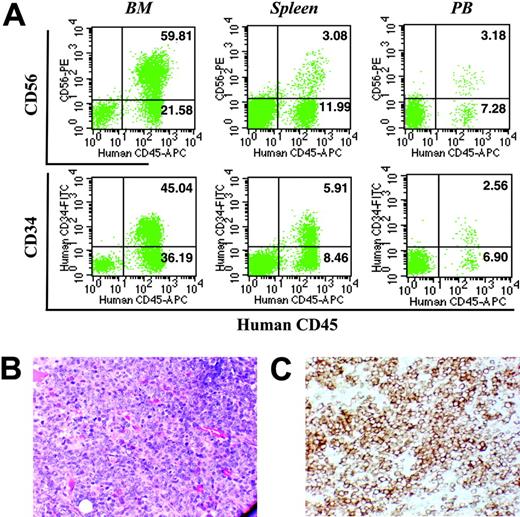
Patient IM-4 represented a unique opportunity to observe the evolution of IM to acute leukemia. Although the PB cells from this patient were harvested at a time when the patient's disease appeared stable, this individual's condition transformed into acute myeloid leukemia 5 weeks following blood collection. The patient developed an acute myeloid leukemia with blast cells that expressed CD34, CD45, CD33, and CD13. As can be seen in Figure 5A, the mice receiving PB CD34+ cells from this patient achieved an extremely high level of human cell engraftment, with approximately 83% human CD45+ cells in the BM, approximately 15% in the spleen, and approximately 11% in the PB. Flow cytometric analyses revealed that the CD45+ cells in these hematopoietic organs were predominantly CD34+ and CD56+ (Figure 5A) and CD33+ (data not shown), a phenotype similar to that observed during the leukemic phase of IM. Furthermore, the BM cavities of these NOD/SCID mice were heavily infiltrated with blastlike cells (Figure 4A), which were shown by immmunohistochemical staining to express the human CD34 antigen (Figure 5B). These data indicate that the leukemic transformation occurred in vivo in the NOD/SCID mice after transplantation, during a time interval virtually identical to that observed in the patient, and that these cells were present not only in the BM but also in the spleen and PB of these mice (Figure 5A).
Analysis of hematopoietic tissues obtained from NOD/SCID mice receiving transplants of CD34+ cells from patient IM-4. (A) Representative flow cytometric analysis of BM (left column), spleen (middle column), and PB (right column) from mice that received transplants of PB CD34+ cells isolated from patient IM-4. Cells were analyzed by flow cytometry at 12 weeks after transplantation. (B) May-Grunwald-Giemsa staining (× 200) and (C) anti-CD34 immunochemical staining (× 200) of a section of tibia from the mouse that received a transplant of cells isolated from patient IM-4.
Analysis of hematopoietic tissues obtained from NOD/SCID mice receiving transplants of CD34+ cells from patient IM-4. (A) Representative flow cytometric analysis of BM (left column), spleen (middle column), and PB (right column) from mice that received transplants of PB CD34+ cells isolated from patient IM-4. Cells were analyzed by flow cytometry at 12 weeks after transplantation. (B) May-Grunwald-Giemsa staining (× 200) and (C) anti-CD34 immunochemical staining (× 200) of a section of tibia from the mouse that received a transplant of cells isolated from patient IM-4.
In order to determine the origin of the human CD45+ leukemic cells isolated from the NOD/SCID mice, their karyotype was compared to that of the primary cells studied 1 year prior to leukemia transformation and at the time of the leukemic transformation (Table 6). A clone of cells containing the identical karyotypic abnormality (45, XX,?inv dup(1)(p21p33), der(3)t(3;16)(q11.2; p11.2), del(5)(q15q33), del(7)(q22),-10,-12, der(16)t(16;17)(p11.2; q11.2),-17, + 2mar) was detected in each of these cell samples indicating their common origin. Surprisingly, this clone represented the minority of human cells within the NOD/SCID mice. The majority of the donor derived cells, however, possessed a new abnormality (42∼44, XX, del(1)(p34.1),-4, add(6)(p2?3),-7, del(8)(q13),-10, der(11)t(?4;11)(q27;q?23), add(12)(q34.3), add(13) (q34),-17,?dup (17)(p11.2), + mar), which is likely a consequence of genomic instability characteristic of this secondary leukemia.
Cytogenetic abnormalities in cells from patient IM-4 engrafted in NOD/SCID mice
Source . | Distribution, % . | Karyotypes . |
|---|---|---|
| IM BM (May 2002) | ||
| 25 | 45, XX, ins(1)(p13), add(3)(q11.2), del(5)(q15q33), − 10, − 12, add(16)(p11.2), − 17, + 2mar* | |
| 20 | 46, XX, der(6)t(1;6)(q25;p23) | |
| 55 | 46, XX | |
| Leukemic transformation BM (May 2003) | ||
| 80 | 45, XX, ?inv dup(1)(p21p33), der(3)t(3;16)(q11.2;p11.2), del(5)(q15q33), del(7)(q22), − 10, − 12, der(16)t(16;17)(p11.2;q11.2), − 17, + 2mar* | |
| 5 | 46, XX, der(6)t(1;6)(q25;p23) | |
| 15 | 46, XX | |
| Engrafted human cells in NOD/SCID mice (December 2003) | ||
| 13 | 45, XX, ?inv dup(1)(p21p33), der(3)t(3;16)(q11.2;p11.2), del(5)(q15q33), del(7)(q22), − 10, − 12, der(16)t(16;17)(p11.2;q11.2), − 17, + 2mar* | |
| 87 | 42≈44, XX, del(1)(p34.1), − 4, add(6)(p2?3), − 7, del(8)(q13), − 10, der(11)t(?4;11)(q27;q?23), add(12)(q34.3), add(13) (q34), − 17, ?dup(17)(p11.2), + mar |
Source . | Distribution, % . | Karyotypes . |
|---|---|---|
| IM BM (May 2002) | ||
| 25 | 45, XX, ins(1)(p13), add(3)(q11.2), del(5)(q15q33), − 10, − 12, add(16)(p11.2), − 17, + 2mar* | |
| 20 | 46, XX, der(6)t(1;6)(q25;p23) | |
| 55 | 46, XX | |
| Leukemic transformation BM (May 2003) | ||
| 80 | 45, XX, ?inv dup(1)(p21p33), der(3)t(3;16)(q11.2;p11.2), del(5)(q15q33), del(7)(q22), − 10, − 12, der(16)t(16;17)(p11.2;q11.2), − 17, + 2mar* | |
| 5 | 46, XX, der(6)t(1;6)(q25;p23) | |
| 15 | 46, XX | |
| Engrafted human cells in NOD/SCID mice (December 2003) | ||
| 13 | 45, XX, ?inv dup(1)(p21p33), der(3)t(3;16)(q11.2;p11.2), del(5)(q15q33), del(7)(q22), − 10, − 12, der(16)t(16;17)(p11.2;q11.2), − 17, + 2mar* | |
| 87 | 42≈44, XX, del(1)(p34.1), − 4, add(6)(p2?3), − 7, del(8)(q13), − 10, der(11)t(?4;11)(q27;q?23), add(12)(q34.3), add(13) (q34), − 17, ?dup(17)(p11.2), + mar |
Apparent differences between these karyotypes, other than the deletion 7, are the result of differences in interpretation between 2 cytogenetic laboratories; the chromosomes are in fact the same. The dates represent the time of sample collection.
Discussion
The CD34 surface antigen has served as a means to identify and separate HSCs and HPCs from their more differentiated progeny. CD34 is present on approximately 0.05% of nucleated circulating cells.12,28 By quantitating the absolute number of CD34+ cells in the PB of a large, well-defined population of patients, Barosi et al have shown that the PB of IM patients contained 360 times more CD34+ cells than healthy controls, and 18 to 30 times more CD34+ cells than patients with other Ph- MPDs.12 The CD34+ cell number was further shown to be related to disease progression and to serve as a biomarker for disease activity.12
In this study, we characterized the IM PB CD34+ cells by an array of phenotypic and functional assays. We found that IM PB CD34+ cells contained a phenotypically more primitive population of HPCs than that of normal PB, with a greater percentage of the IM PB CD34+ cells exhibiting a c-kit+, CD90+, and CD38- phenotype. Most human primitive hematopoietic cells with high potential for self-renewal and multilineage differentiation have been reported to express high levels of c-kit or CD90, and low levels of CD38.28 In addition, Barosi et al have previously shown that only 66% (23%-99%) of CD34+ cells expressed CD38.12 Interestingly, despite containing a phenotypically more primitive population of HPCs, IM PB CD34+ cells displayed a significantly lower frequency of colony-forming cells (CFCs) and CAFCs compared with normal controls. This reduced cloning efficiency of IM CD34+ cells is either a consequence of the mobilization process or a function of a malignant transformation event that characterizes these cells. A resolution of this question will require additional studies using CD34+ cells isolated from larger numbers of IM patients.
These findings also suggest that the preferential mobilization of CD34+ cells in IM is accompanied by abnormalities in cell function. This discordance between phenotype and function is reminiscent of the behavior of CD34+ cells isolated from patients with myelodysplasia and acute myeloid leukemia, in which increased numbers of PB CD34+ cells have also been associated with reduced CD34+ cell cloning efficiency.29,30
The use of immunodeficient mice as hosts of human HSC transplantations has provided powerful models for both normal and abnormal human hematopoiesis.19,21,31-37 Subfractionation of the input cells coupled with time-course studies of the number and types of the mature progeny produced has revealed the presence of a hierarchy of primitive transplantable progenitors that produce different spectra of progeny for varying, but predictable periods.31-37 In this study, we have demonstrated for the first time that NOD/SCID BM-repopulating cells exist within the PB CD34+ cells of patients with IM. More important, the differentiation program of the IM PB CD34+ cells differ from that of normal CD34+ cells mobilized into the PB with G-CSF. These IM CD34+ cells have the unique ability to generate a greater fraction of CD34+, CD33+, and CD41+ cells. Similarly, Thanopoulou et al have reported that linage- cells from myelodysplasia patients repopulate NOD/SCID-β2m-/- mice and produce an abnormal differentiation pattern.33 Thus, our data demonstrate the potential of the NOD/SCID model for future investigations of IM.
IM is believed to originate at the level of the HSCs, resulting in a profound hyperplasia of morphologically abnormal megakaryocytes and clonal populations of monocytes.1-4 It would therefore be important to know if the engrafted human cells in the NOD/SCID mice are derived from the malignant clone. Using both transcriptional clonality assays and cytogenetic studies, we have demonstrated that the engrafted human hematopoietic cells in the mice given transplants of PB CD34+ cells from each IM patient were clonal in origin, indicating that they are derived from the abnormal clone of the patient. Although it has been reported that NOD/SCID mice have been repopulated with both normal and leukemic human cells after transplantation of cells from patients with chronic myeloid leukemia, the vast majority (> 90%) of human progenitors present in the BM of the NOD/SCID mice were Ph+ by cytogenetic analysis.34 Preliminary studies using marker chromosome abnormalities or restriction fragment length polymorphisms have documented that about 80% of circulating CD34+ cells in a patient with IM are clonal.40
One of the hallmarks of IM is the inevitable progression of the disease to acute leukemia.1-4 In this study, we also observed this leukemic transformation in vivo in NOD/SCID mice given transplants of stable-state IM PB CD34+ cells. The IM patient from whom the sample was derived eventually underwent acute leukemic transformation. This ability to observe the evolution of leukemia from IM in vivo has not to our knowledge been previously reported. Although such events would likely be rare, this model provides an in vivo system in which to analyze the events in individual patients that play a role in the progression of IM to acute leukemia. In addition, IM-engrafted NOD/SCID mice should offer new opportunities for developing and testing novel therapeutic agents for the treatment of IM.
Prepublished online as Blood First Edition Paper, October 7, 2004; DOI 10.1182/blood-2004-06-2485.
Supported by a grant from the Myeloproliferative Disorder Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Manuel B. Borse for his assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal