Abstract
Accumulating evidence indicates that platelets play a critical role in the pathogenesis of experimental severe malaria (ESM) elicited by infection with Plasmodium berghei. Mice injected on day 1 of P berghei infection (early) with either anti-CD41 or anti-CD61 monoclonal antibodies (mAbs) exhibited significantly (P < .001) increased survival from ESM compared with infection controls, indicating that platelets function early in the disease. In contrast, groups of mice treated on days 4, 5, and 6 (late) with anti-CD41 mAb exhibited similar mortality as controls. Because platelet depletion by anti-CD41 mAb on day 4 of infection did not protect mice, and platelet adherence occurs on day 6, platelet adherence to endothelium is not required to mediate malarial pathogenesis. Few platelet microparticles were detected in the blood during the course of malaria, but large numbers of erythrocyte vesicles, microparticles, and debris were detected. The protective effect of early anti-CD41 mAb treatment was independent of the number of platelets, platelet microparticles, erythrocyte-platelet conjugates, and erythrocyte vesicles. Mice treated early with anti-CD41 mAb exhibited markedly altered cytokine production on day 4 of P berghei infection (increased interleukin 10 [IL-10], IL-1α, IL-6, interferon-γ [IFN-γ], and tumor necrosis factor α [TNF-α]; decreased IL-2) but no decline in coagulation factors compared with rat immunoglobulin G (IgG)–treated controls, indicating that platelets regulate the levels of pathogenic cytokines.
Introduction
One of the hallmarks of cerebral malaria, which is caused by infection with Plasmodium falciparum, is petechial hemorrhaging into the brain, indicating that platelets may play a crucial role in malarial pathogenesis.1 Clinical studies of patients with P falciparum indicate a marked procoagulant state with consumption of clotting factors, the presence of fibrin dimers, and decreased levels of the anticoagulants protein C and S2-4 ; the procoagulant state correlates with the development of severe malaria. Grau and colleagues5 have proposed that platelet activation and adherence contributes to the development of severe malaria. Patients with severe falciparum malaria exhibit profound thrombocytopenia, and platelet accumulation in the brains of children who have succumbed to severe falciparum malaria is significantly higher than platelet accumulation in the brains of patients who died from other coma complications.6-9
These clinical results in humans with malaria provide the impetus for parallel mechanistic studies in animal models. Extrapolation of results from mouse models of severe malaria must be cautious because the P falciparum species of the malarial parasite cannot be used in rodent models of severe malaria because they do not replicate in mouse erythrocytes, and there are also differences between rodents and humans. Nevertheless, direct evidence for the importance of platelets in malarial pathogenesis has come from the P berghei model of severe malaria.10,11
CD41/CD61 (αIIb/β3 integrin heterodimer, also known as gpIIb/IIIa) is an important molecule in platelet biology and its major function is contributing to expansion of clot formation and platelet adhesion to the microvasculature.12-14 Our previous studies indicate that platelet adhesion during experimental severe malaria (ESM) occurs primarily in venules within the pial microvasculature and that CD41 plays an important role in this platelet adhesion.15 We therefore determined whether treatment with anti-CD41 or anti-CD61 monoclonal antibody (mAb) functions early or late in mediating protection against ESM, and what effect anti-CD41 mAb has on (1) platelet numbers, (2) platelet microparticle and erythrocyte vesicle formation, (3) the developing immune response, and (4) malarial coagulopathy. Early treatment with the mAb occurs just after infection, whereas late treatment corresponds to parasitemia being readily detectable (0.5%-5%) in blood. The early treatment corresponds to prophylaxis and the late time points corresponds to when a patient infected with P falciparum might present at the clinic, up to the development of severe complications. We elected to perform our studies in the P berghei model of ESM rather than the P yoelii model because P berghei–infected mice exhibit (1) a marked systemic inflammatory response, (2) profound thrombocytopenia, and (3) obtundation.16,17
Materials and methods
Infection and treatment of mice
Plasmodium berghei ANKA was stored as a frozen stabilate and this stabilate was injected into source mice to generate an inoculum for the experiments. The experimental C57BL/6 female mice were obtained at 4 to 5 weeks of age from Harlan (Indiapolis, IN) or Jackson Laboratories (Bar Harbor, ME) and injected with 1 × 106P berghei parasitized erythrocytes when they were between 6 and 10 weeks of age. The animals were housed in microisolator cages and provided food and water ad libitum. Parasitemia was assessed by counting the number of infected erythrocytes among 200 and 1000 erythrocytes in Giemsa-stained thin blood films. The institutional animal use committees of Louisiana State University Health Sciences Center, Targeted Molecules Corporation, and La Jolla Bioengineering Institute approved all procedures. Anti-CD41 mAb (clone MwReg30), anti-CD61 (clone 2C9.G2), and anti-Ter119 (Ly-76; clone Ter119) were purchased from Becton Dickinson (San Diego, CA).
Flow cytometry
Flow cytometry and determination of cell number was performed essentially as described previously.18,19 Briefly, the tail was pricked with a lancet and 2 μL blood was immediately transferred to 40 μL of citrate solution (Sigma, St Louis, MO) to prevent coagulation. All incubations were performed at room temperature. Anti-CD41 mAb conjugated with fluorescein isothiocyanate (FITC) and anti-CD61 conjugated with phycoerythrin (PE) were added to the blood and incubated together for 15 minutes. In selected experiments, anti-Ter119 mAb conjugated with PE-cyanin5 (PE-Cy5) was added to label erythrocytes. Cells were resuspended in 1 mL phosphate-buffered saline (PBS), and then the cells were analyzed on a FACSCalibur (Becton Dickinson, San Diego, CA). Data acquisition was performed using the CellQuest program (Becton Dickinson) on at least 5000 cells with size (forward scatter) and granularity (side scatter) of platelets. The data were analyzed using the Attractors program (Becton Dickinson). Propidium iodide (5 μg/mL) was added to selected samples to exclude permeable cell debris. For the first 3 experiments, Tru-count beads and tubes were used from Becton Dickinson for cell counting.18 In other experiments, Spherotech beads (Libertyville, IL) were used. The beads are easily distinguished from cells based on their unique forward- and side-scatter and fluorescence in all 3 channels. They are indicated in the flow cytometry figures. The number of cells was calculated using the following formula: No. of cells/μL = No. of positive events × bead count/test no. of beads collected sample volume (μL).
Cytokine protein and coagulation factor analysis
Blood (400 μL) was obtained from the retro-orbital plexus from anesthetized mice via heparinized capillary tubes and bled into collection tubes containing EDTA (ethylenediaminetetraacetic acid; Becton Dickinson). The blood samples were centrifuged at 500g to obtain plasma, which was frozen and stored at –80°C. Plasma (25 μL; 3 replicates) was analyzed in a modified enzyme-linked immunosorbent assay (ELISA) using Luminex beads (Luminex, Austin, TX) for levels of selected cytokine and coagulation factor protein. The cytokines analyzed in this multiplex assay were: IFN-γ, TNF-α, IL-1α, -1β, -2, -4, -5, -6, -7, -10, -12p70, -17, and -18. The coagulation factors analyzed were: fibrinogen, factor VII (fVII), tissue factor, and von Willebrand factor (VWF).
Statistical analysis
Analysis of variance (ANOVA) with the Statview program (SAS Institute, Cary, NC) with Fisher post-hoc test was performed to statistically compare all measurements with a P value cut-off of .05. The mean and standard deviation of the results are reported in the text and figures. The survival times are compared with nonparametric log rank test with a P value cut-off of .05.
Results
P berghei infection of mice is a very reproducible model of cerebral malaria with C57BL/6 infected mice usually succumbing between day 6 and day 8 of infection. Moribund mice exhibit obtundation, marked weight loss, rapid breathing, and minimal responses to neurologic tests (such as righting and gripping responses). Mice exhibiting these clinical signs die hours later. Mice surviving beyond day 12 of P berghei infection develop hyperparasitemia and die after day 20 of infection from anemia, and they do not exhibit impaired neurologic tests. Thus, day 12 of infection is considered by most investigators to be the last time point when the mice will develop cerebral malaria and is the rationale for terminating our experiments assessing the pathogenic mechanisms of cerebral malaria at this time point.20
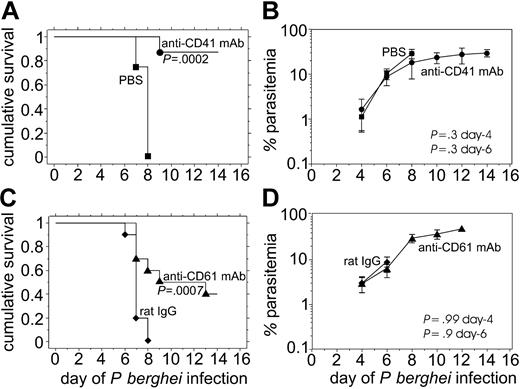
Either anti-CD41 or anti-CD61 mAb injected on day 1 of P berghei infection protects against the development of ESM
To determine whether either anti-CD41 mAb or anti-CD61 mAb treatment protects against the development of severe experimental malaria, we injected groups of 8 to 10 mice intraperitoneally with 106 parasitized erythrocytes and then injected 0.1 mg antibody intraperitoneally on day 1 of infection when parasites were not detectable in the Giemsa-stained thin blood films. Only 1 of 8 mice treated with anti-CD41 mAb on day 1 of infection succumbed between day 6 and day 12 of infection, whereas all control mice injected with PBS died by day 8 of infection (Figure 1A). The difference in survival between anti-CD41 mAb–treated mice and controls was significant (P = .0002).
Mice treated early during P berghei infection with 0.1 mg of either anti-CD41 mAb or anti-CD61 mAb are protected from severe P berghei malaria despite no detectable effects of the mAb treatment on parasitemia. The cumulative survival (A, C) and average P berghei parasitemia plus or minus standard deviation (SD) (B, D) in groups of 10 mice injected intraperitoneally on day 1 with 0.1 mg of either anti-CD41 mAb (A, B) or anti-CD61 mAb (C, D). • indicates anti-CD41 day 1; ▴, anti-CD61 day 1; ♦, rat IgG day 1; ▪, PBS day 1.
Mice treated early during P berghei infection with 0.1 mg of either anti-CD41 mAb or anti-CD61 mAb are protected from severe P berghei malaria despite no detectable effects of the mAb treatment on parasitemia. The cumulative survival (A, C) and average P berghei parasitemia plus or minus standard deviation (SD) (B, D) in groups of 10 mice injected intraperitoneally on day 1 with 0.1 mg of either anti-CD41 mAb (A, B) or anti-CD61 mAb (C, D). • indicates anti-CD41 day 1; ▴, anti-CD61 day 1; ♦, rat IgG day 1; ▪, PBS day 1.
Mice treated with anti-CD61 mAb on day 1 of P berghei infection exhibited a significantly (P = .0007) increased survival compared with rat IgG–injected controls (Figure 1C). In fact, 5 of 10 mice survived beyond day 12 of infection (Figure 1C). We have previously reported that there is no difference in parasitemia or survival between PBS-injected mice and rat IgG–injected mice, so both groups of mice are appropriate infection controls.21,22 The similar parasitemia in anti-CD41 mAb–injected mice and PBS-injected controls (Figure 1B) as well as in the anti-CD61 mAb–injected mice and rat IgG–injected controls indicates that protection against the development of severe experimental malaria by these mAbs cannot be attributed to altered replication of the parasite or inadequate infection (Figure 1B,D). Rather, the effects of the host response to the parasite determine whether the animal develops severe malaria.
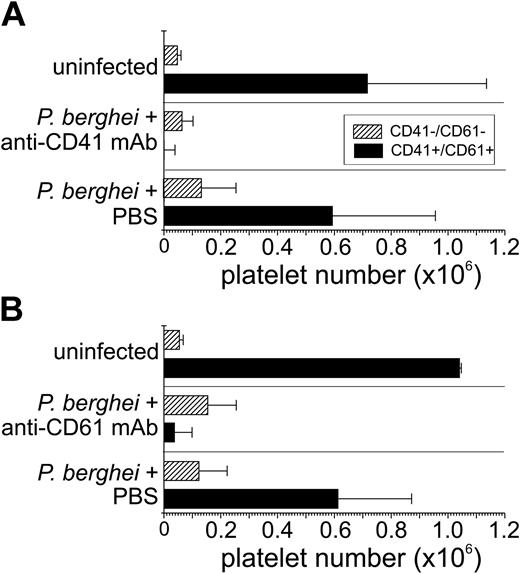
To determine the effects of anti-CD41 mAb and anti-CD61 mAb on platelet number, we assessed the number of platelets during the course of P berghei malaria in all mice by flow cytometry using only 2 μL blood from the tail vein. This low volume of blood meant that our assay method had a minimal effect on the course of the disease (stress or anemia). Platelets have a distinct forward scatter (measure of size) and side scatter (measure of granularity) being smaller and less granular than erythrocytes and leukocytes. However, some cell debris may also fall within this platelet region, which was excluded by propidium iodide (PI) labeling. The flow cytometric analysis encompassed only cells within the platelet region defined by forward and side scatter. The flow cytometric analysis of all groups of mice indicated there were few, if any, platelets that were single positive for CD41 or CD61 only (< 0.1%). In uninfected controls, the majority of cells within the platelet region were CD41+CD61+ (95% ± 1%) with few CD41–CD61– cells (5% ± 1%). Treatment with either anti-CD41 mAb or anti-CD61 mAb resulted in a marked decrease in the number of circulating CD41+CD61+ platelets on day 4 of P berghei infection compared with infection controls (Figure 2). In the platelet region, CD41–CD61– cells were detected in all groups of P berghei–infected mice (Figure 2), and the number of CD41–CD61– cells increased modestly with infection. On day 4 of P berghei infection, there were few if any detectable CD61+CD41– or CD41+CD61– cells (< 0.1%) in anti-CD41 mAb–, or anti-CD61 mAb–treated mice and infection control mice. Thus, antibody masking of its epitope cannot explain the lower platelet detection after treatment with either anti-CD41 or anti-CD61 mAb because the anti-CD41 and anti-CD61 mAbs recognize distinct epitopes and we detected few CD61+CD41– cells in the anti-CD41 mAb–treated mice and few CD41+CD61– cells in the anti-CD61 mAb–treated mice.
Both anti-CD41 mAb and anti-CD61 mAb deplete platelets from the circulation. Blood (2 μL) was obtained from mice treated with either anti-CD41 mAb on day 1 of P berghei infection or PBS (A), and the average number plus or minus SD of cells is shown on the x-axis. A similar analysis was performed with blood from mice injected with anti-CD61 mAb on day 1 of infection (B). The blood was analyzed by flow cytometry for levels of CD41 and CD61 on the surface of platelets in the groups of mice indicated on the y-axis. A platelet region was defined based on the distinct forward and side scatter of platelets and the number of cells with different expression patterns of CD41 and CD61 were calculated by using counting beads. ▪ indicates CD41+CD61+; ▨, CD41–CD61–. Few (< 0.1%) if any single positive cells (CD41+CD61– or CD41–CD61+) were detected.
Both anti-CD41 mAb and anti-CD61 mAb deplete platelets from the circulation. Blood (2 μL) was obtained from mice treated with either anti-CD41 mAb on day 1 of P berghei infection or PBS (A), and the average number plus or minus SD of cells is shown on the x-axis. A similar analysis was performed with blood from mice injected with anti-CD61 mAb on day 1 of infection (B). The blood was analyzed by flow cytometry for levels of CD41 and CD61 on the surface of platelets in the groups of mice indicated on the y-axis. A platelet region was defined based on the distinct forward and side scatter of platelets and the number of cells with different expression patterns of CD41 and CD61 were calculated by using counting beads. ▪ indicates CD41+CD61+; ▨, CD41–CD61–. Few (< 0.1%) if any single positive cells (CD41+CD61– or CD41–CD61+) were detected.
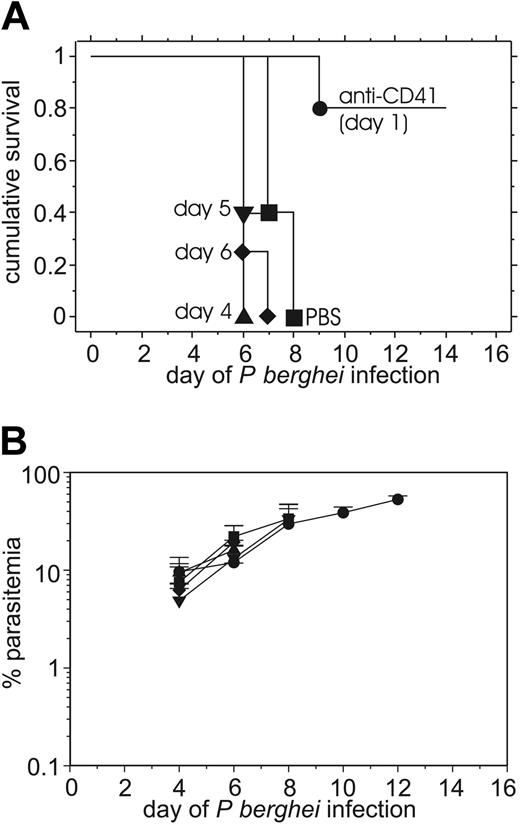
Late (therapeutic) treatment with anti-CD41 does not protect against the development of severe experimental malaria
Because early (prophylactic) treatment with anti-CD41 mAb protected mice against the severe malarial pathogenesis, we next determined whether late (therapeutic) anti-CD41 mAb treatment had a similar capacity to protect against malarial pathogenesis. Similar to the above results of the early treatment with anti-CD41 mAb (Figure 1), mice (n = 10) injected with anti-CD41 mAb on day 1 were protected (Figure 3A). In contrast, mice (n = 10) injected intraperitoneally with 0.1 mg anti-CD41 mAb on day 4, 5, or 6 of infection did not exhibit significant protection from severe malarial pathogenesis compared with infected controls. The late treatment (day 4 only) versus early treatment (day 1) with anti-CD41 mAb was repeated twice with similar results. We did not test late depletion with anti-CD61 mAb because both antibodies depleted platelets to a similar extent (Figure 2); thus, testing late administration of anti-CD61 was unlikely to provide additional information. Mice treated early or late with anti-CD41 mAb and control mice injected with rat IgG all exhibited similar levels of parasitemia (Figure 3B), indicating that differences in parasite replication or infection of mice were not causing the marked differences in survival.
Mice treated late with 0.1 mg of anti-CD41 mAb during P berghei infection are not protected from severe P berghei malaria, whereas early anti-CD41 mAb treatment does protect. The cumulative survival (A) and average P berghei parasitemia ± SD (B) in groups of 10 mice injected intraperitoneally on day 1 with 0.1 mg anti-CD41 mAb on day 1, 4, 5, or 6 of infection. • indicates anti-CD41 day 1; ▴, anti-CD41 day 4; ▾, anti-CD41 day 5; ♦, anti-CD41 day 6; ▪, PBS day 4.
Mice treated late with 0.1 mg of anti-CD41 mAb during P berghei infection are not protected from severe P berghei malaria, whereas early anti-CD41 mAb treatment does protect. The cumulative survival (A) and average P berghei parasitemia ± SD (B) in groups of 10 mice injected intraperitoneally on day 1 with 0.1 mg anti-CD41 mAb on day 1, 4, 5, or 6 of infection. • indicates anti-CD41 day 1; ▴, anti-CD41 day 4; ▾, anti-CD41 day 5; ♦, anti-CD41 day 6; ▪, PBS day 4.
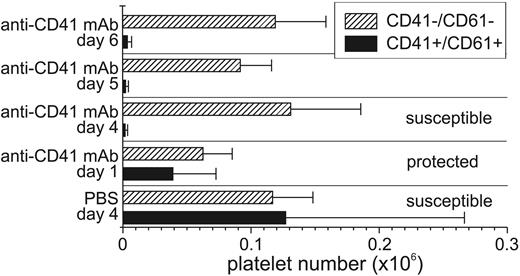
To verify that the intraperitoneal injections of anti-CD41 mAb on day 4, 5, and 6 of P berghei infection had similar effects on the circulating platelet count as injection on day 1, 2 μL blood was obtained from the tail vein of each of the mice in the groups of mice on day 6 of P berghei infection and then analyzed by flow cytometry. P berghei–infected control mice exhibited a profound thrombocytopenia on day 6 of infection compared with uninfected controls (Figure 4). The number of CD41–CD61– cells in the platelet region remained relatively the same across the different treatment groups and increased modestly during the course of P berghei infection. Mice injected with anti-CD41 mAb on day 1, 4, 5, or 6 of P berghei infection had few CD41+CD61+ platelets detectable by flow cytometry. A comparison of the numbers of cells in (1) protected mice treated with anti-CD41 mAb on day 1 of infection, (2) susceptible mice treated with anti-CD41 mAb on day 4, 5, or 6 of P berghei infection, and (3) susceptible controls showed similar numbers of CD41–CD61–; CD41+CD61–; or CD41–CD61+ cells in all groups of mice on day 6 of P berghei infection (Figure 4). The numbers of CD41+CD61+ platelets were slightly higher in protected mice treated with anti-CD41 mAb (39 734 ± 33 317) on day 1 of P berghei infection compared with susceptible mice treated with anti-CD41 mAb on day 4, 5, or 6 (day 4: 2042 ± 1580; day 5: 2391 ± 1836; day 6: 3803 ± 2976) but lower than the susceptible infection controls (127 274 ± 139 274).
Late depletion of platelets with anti-CD41 mAb injection does not protect against the development of severe malaria, whereas early treatment does. Blood (2 μL) was obtained from groups of mice injected with anti-CD41 mAb on either day 4, 5, or 6 of infection (not protected), from a group of mice injected with anti-CD41 mAb on day 1 (protected), and from a group of mice injected with PBS on day 4 (unprotected control). The number of CD41+CD61+ and CD41–CD61– platelets were determined by flow cytometry. The average number plus or minus SD of cells is shown (x axis). ▪ indicates CD41+CD61+; ▨, CD41–CD61–. Few (< 0.1%) if any single positive cells (CD41+CD61– or CD41–CD61+) were detected.
Late depletion of platelets with anti-CD41 mAb injection does not protect against the development of severe malaria, whereas early treatment does. Blood (2 μL) was obtained from groups of mice injected with anti-CD41 mAb on either day 4, 5, or 6 of infection (not protected), from a group of mice injected with anti-CD41 mAb on day 1 (protected), and from a group of mice injected with PBS on day 4 (unprotected control). The number of CD41+CD61+ and CD41–CD61– platelets were determined by flow cytometry. The average number plus or minus SD of cells is shown (x axis). ▪ indicates CD41+CD61+; ▨, CD41–CD61–. Few (< 0.1%) if any single positive cells (CD41+CD61– or CD41–CD61+) were detected.
Flow cytometric analysis of platelets during P berghei malaria
Because we had detected by flow cytometric analysis of blood from mice on day 6 of P berghei infection large numbers of CD41–CD61– PI– cells in the platelet region, we next determined whether these CD41–CD61– cells represented platelets, erythrocyte vesicles, or cell debris. To determine whether CD41–CD61– cells were viable cells, we labeled blood cells in a group of 10 mice on day 6 of P berghei infection with a vital dye fluorescein diacetate. The CD41–CD61– cells all labeled positively with fluorescein after 10 minutes of incubation (Figure 5A-B) but they did not label with propidium iodide. These data indicate CD41–CD61– cells contained active esterases in their cytosol to render the fluorescein diacetate fluorescent, but exhibited intact membranes to prevent propidium iodide from entering.
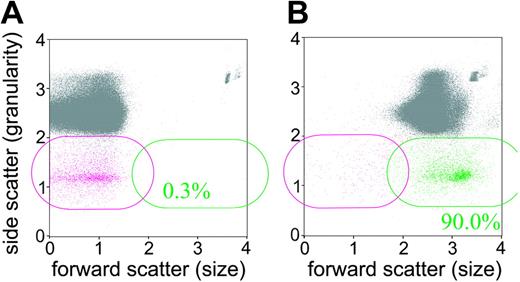
CD41–CD61–, PI– events in the anti-CD41 mAb–treated mice label with vital dye fluorescein diacetate. Blood cells were analyzed by flow cytometry for their expression of CD41 and CD61 (platelet) and then were fluorescence-labeled with the vital dye fluorescein diacetate. (A) Blood cell labeling prior to addition of fluorescein diacetate. (B) Blood cell labeling after 10 minutes of incubation with fluorescein diacetate. The CD41–CD61–, PI– cells are magenta and those cells labeling with fluorescein diacetate are green. The gray events comprise all cells that are not CD41–CD61–,PI– cells. The inset % is the percentage of CD41–CD61–,PI– cells that are labeled with fluorescein diacetate.
CD41–CD61–, PI– events in the anti-CD41 mAb–treated mice label with vital dye fluorescein diacetate. Blood cells were analyzed by flow cytometry for their expression of CD41 and CD61 (platelet) and then were fluorescence-labeled with the vital dye fluorescein diacetate. (A) Blood cell labeling prior to addition of fluorescein diacetate. (B) Blood cell labeling after 10 minutes of incubation with fluorescein diacetate. The CD41–CD61–, PI– cells are magenta and those cells labeling with fluorescein diacetate are green. The gray events comprise all cells that are not CD41–CD61–,PI– cells. The inset % is the percentage of CD41–CD61–,PI– cells that are labeled with fluorescein diacetate.
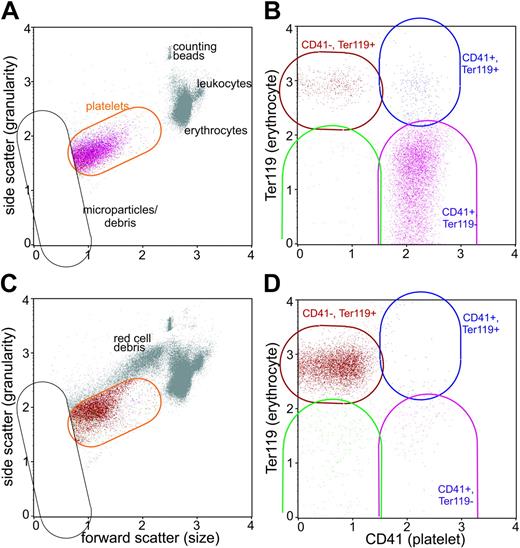
To determine whether CD41–CD61– cells represented erythrocyte ghosts that have formed vesicles, we analyzed the blood of mice by flow cytometry during the course of P berghei malaria in 5 infected mice and 5 uninfected controls by using anti-CD41 (platelets) and anti-Ter119 (erythrocyte) mAbs. The parasitemia on day 4 was 2.6% ± 0.9% and on day 6 was 8.8% ± 2.9%, and all 5 infected mice became moribund on day 7 of P berghei infection. The Attractor program was used to perform subset analysis. Cells with the distinct forward scatter and side scatter of platelets were within the orange platelet region (Figure 6A,C). The level of surface CD41 and Ter119 labeling on cells was used to define subsets within the platelet set (Figure 6B,D) and each subset was color coded so the forward and side scatter of these cell populations was apparent. Virtually all the CD41– cells in both the infected and uninfected mice labeled with anti-Ter119 mAb, indicating these cells within the platelet region were of erythrocyte origin (Figure 6A-B). Anti-CD41 mAb–treated mice infected with P berghei exhibited only the CD41–Ter119+ cell population (not shown). Pattanapanyasat et al23 have termed these Ter119+ events in the platelet region as erythrocyte vesicles, and these vesicles are larger and exhibit greater granularity than microparticles.
CD41– cells are of erythrocyte origin. Blood cells were analyzed by flow cytometry for their expression of CD41 (platelet) and Ter119 (erythrocyte) in uninfected mice (A, B) and in mice on day 6 of P berghei infection (C, D). The platelet set was defined by its forward scatter and side scatter (orange region in A, C) and microparticles and cell debris were excluded from the analysis (black set). The platelet subsets were color-coded in B and D based on their expression of CD41 and Ter119: the CD41+Ter119– platelet subset is purple, the CD41–Ter119+ subset is red, the CD41–Ter119– is green, and the CD41Ter199+ subset is blue. Note the colors identify the same platelet subsets in A and C. There were few orange events because virtually all cells within the platelet set were defined within the subsets.
CD41– cells are of erythrocyte origin. Blood cells were analyzed by flow cytometry for their expression of CD41 (platelet) and Ter119 (erythrocyte) in uninfected mice (A, B) and in mice on day 6 of P berghei infection (C, D). The platelet set was defined by its forward scatter and side scatter (orange region in A, C) and microparticles and cell debris were excluded from the analysis (black set). The platelet subsets were color-coded in B and D based on their expression of CD41 and Ter119: the CD41+Ter119– platelet subset is purple, the CD41–Ter119+ subset is red, the CD41–Ter119– is green, and the CD41Ter199+ subset is blue. Note the colors identify the same platelet subsets in A and C. There were few orange events because virtually all cells within the platelet set were defined within the subsets.
Platelet microparticles have been reported by Piguet et al24 to be present in high numbers in the platelet-rich plasma of mice infected with P berghei. In our analysis of whole blood from animals infected with P berghei, most (89% ± 2%) of the microparticles exhibited surface Ter119 protein whereas few (0.4% ± 0.2%) exhibited surface CD41 protein (Table 1). The ratio of CD41+ platelets to CD41+ microparticles when the animals were moribund from P berghei malaria was about 20:1 in our analysis compared with about 1:4 in Piguet's analysis, indicating a greater creation of platelet microparticles in platelet-rich plasma of infected mice than in whole blood.
Detailed flow cytometric analysis of platelets in blood of mice during the course of P berghei malaria
. | . | . | . | P . | . | |
|---|---|---|---|---|---|---|
. | Day 0 . | Day 4 . | Day 6 . | Day 0 vs day 4 . | Day 0 vs day 6 . | |
| % CD41+ cells forming intact RBC conjugates | 8.6 ± 2.5 | 10.0 ± 2.4 | 36.0 ± 5.0 | .7 | < .0001 | |
| % CD41+ cells forming microparticles | 8.8 ± 1.0 | 6.2 ± 0.9 | 3.2 ± 0.9 | < .0001 | < .0001 | |
| % CD41+ cells in platelet region | 81 ± 2 | 82 ± 2 | 57 ± 5 | .9 | < .0001 | |
| % microparticles that are CD41+ | 31.2 ± 14.2 | 18.1 ± 10.2 | 0.4 ± 0.2 | .1 | .0002 | |
| % microparticles that are Ter119+ | 55.0 ± 16.8 | 56.2 ± 7.4 | 89.1 ± 1.8 | .99 | .0003 | |
| No. CD41+ cells | 848 136 ± 475 698 | 264 841 ± 173 789 | 20 191 ± 3 593 | .03 | .002 | |
| No. CD41+ microparticles | 72 953 ± 39 499 | 16 472 ± 11 438 | 617 ± 82 | .009 | .0009 | |
| No. CD41+ cells in platelet region | 683 532 ± 372 774 | 219 228 ± 145 295 | 11 645 ± 3 231 | .02 | .001 | |
| No. CD41+ platelet: intact RBC conjugates | 79 851 ± 62 859 | 24 643 ± 16 642 | 7 157 ± 689 | .1 | .04 | |
. | . | . | . | P . | . | |
|---|---|---|---|---|---|---|
. | Day 0 . | Day 4 . | Day 6 . | Day 0 vs day 4 . | Day 0 vs day 6 . | |
| % CD41+ cells forming intact RBC conjugates | 8.6 ± 2.5 | 10.0 ± 2.4 | 36.0 ± 5.0 | .7 | < .0001 | |
| % CD41+ cells forming microparticles | 8.8 ± 1.0 | 6.2 ± 0.9 | 3.2 ± 0.9 | < .0001 | < .0001 | |
| % CD41+ cells in platelet region | 81 ± 2 | 82 ± 2 | 57 ± 5 | .9 | < .0001 | |
| % microparticles that are CD41+ | 31.2 ± 14.2 | 18.1 ± 10.2 | 0.4 ± 0.2 | .1 | .0002 | |
| % microparticles that are Ter119+ | 55.0 ± 16.8 | 56.2 ± 7.4 | 89.1 ± 1.8 | .99 | .0003 | |
| No. CD41+ cells | 848 136 ± 475 698 | 264 841 ± 173 789 | 20 191 ± 3 593 | .03 | .002 | |
| No. CD41+ microparticles | 72 953 ± 39 499 | 16 472 ± 11 438 | 617 ± 82 | .009 | .0009 | |
| No. CD41+ cells in platelet region | 683 532 ± 372 774 | 219 228 ± 145 295 | 11 645 ± 3 231 | .02 | .001 | |
| No. CD41+ platelet: intact RBC conjugates | 79 851 ± 62 859 | 24 643 ± 16 642 | 7 157 ± 689 | .1 | .04 | |
The percentages of CD41+ cells plus or minus SD forming platelet microparticles, intact platelets, and platelet-erythrocyte conjugates are provided, as is an analysis of microparticles and the number plus or minus SD of platelet microparticles, intact platelets, and platelet-erythrocyte conjugates. The day-0 values comprise all the uninfected animals (n = 20) and n = 5 for analysis on day 4 and day 6 of infection. RBC indicates red blood cell.
The platelet microparticles exhibited similar forward scatter during the course of P berghei malaria (uninfected: 2.9 ± 0.1; day 4: 3.0 ± 0.1; day 6: 2.8 ± 0.1) but levels of surface CD41 on the microparticles declined about 25% from 80 ± 6 mean fluorescence intensity (MFI) in uninfected mice to 60 ± 2 on day 6 of P berghei infection. In uninfected control animals, platelet microparticles exhibited significantly (P < .0001) less surface CD41 than intact platelets (0.5-fold: microparticles: 80 ± 2; platelets: 175 ± 21). The intact platelets increased significantly in forward scatter (size) during the course of P berghei malaria (uninfected: 16.2 ± 1.9; day 4: 19.2 ± 2.1; day 6: 40.6 ± 3.4; P = .04 for comparison of day 4 with uninfected and P < .0001 for comparison of day 6 with uninfected) but their levels of surface CD41 were constant.
To assess platelet erythrocyte conjugate formation during the course of P berghei infection, we assessed the number of CD41+ (platelet) and Ter119+ (erythrocyte) events by flow cytometry analysis on data obtained from the infected mice and uninfected controls described above (Figure 6). The percentage of platelets forming platelet and erythrocyte conjugates (CD41+Ter119+ cells) was similar in uninfected controls at each of the time points and to day 0 controls just prior to infection, indicating there was no significant day-to-day variability in the assay. However, the percentage of CD41+ cells forming erythrocyte-platelet conjugates increased significantly (P < .0001) from 8.6% ± 2.5% in uninfected controls (day 0) to 36% ± 5% in infected mice on day 6 of P berghei malaria (Table 1). In contrast to the increase in percentage of platelets forming conjugates with intact erythrocytes, the actual number of platelet-erythrocyte conjugates decreased significantly (P = .04) in infected mice on day 6 of P berghei malaria compared with uninfected controls (Table 1), and this decline in the number of conjugates reflects the profound decrease in the number of circulating platelets on day 6 of P berghei malaria.
Levels of blood cytokines but not coagulation factors are altered after anti-CD41 mAb treatment on day 1 of P berghei infection
Because the anti-CD41 mAb treatment was functioning early in P berghei infection to protect mice, we determined whether the presence or absence of platelets altered the inflammatory response or coagulopathy by assessing the levels of selected cytokines and coagulation factors in the blood of mice on day 4 of infection. The mice treated with anti-CD41 mAb on day 1 of P berghei infection exhibited similar parasitemia on day 4 of infection as the rat IgG–injected control mice (2.0% ± 0.4% and 1.3% ± 0.9%, respectively), indicating both groups of mice were appropriately infected. The number of CD41+ platelets was 10 074 ± 1949 per μL blood for anti-CD41 mAb–treated mice, 591 129 ± 376 921 for rat IgG–treated controls, and 731 254 ± 328 765 for uninfected mice, indicating that anti-CD41 mAb on day 1 had depleted platelets. The anti-inflammatory, protective cytokine IL-10 increased (2-fold) and the inflammatory, pathogenic cytokine IL-2 decreased (about 5-fold) in anti-CD41 mAb–treated mice compared with rat IgG–injected controls (Table 2). The inflammatory cytokines IFN-γ, IL-1α, TNF-α, and IL-6 were all increased in anti-CD41 mAb–treated mice compared with rat IgG–treated controls (Table 2). The cytokines IL-1β, -5, -7, -12p70, -17, and -18 were similar on day 4 of P berghei infection in rat IgG–injected mice, uninfected controls, and anti-CD41 mAb–treated mice. The coagulation factors fVII, tissue factor, and VWF were similar in all 3 groups of mice. The fibrinogen levels were significantly increased in the anti-CD41 mAb–treated mice compared with rat IgG–injected controls (Table 2).
Analysis of selected cytokines and coagulation factors in groups of mice (n = 5)
Cytokine/coagulation factor . | Uninfected . | Rat IgG, day 4 . | Anti-CD41, day 4 . | P, rat IgG vs α-CD41 . |
|---|---|---|---|---|
| IL-10, pg/mL | 135 ± 10 | 166 ± 26 | 386 ± 72 | .005 |
| IL-2, pg/mL | 1 ± 1 | 30 ± 8 | 5 ± 3 | .002 |
| IL-1α, pg/mL | 29 ± 4 | 53 ± 12 | 110 ± 27 | .03 |
| IFN-γ, pg/mL | 1 ± 1 | 25 ± 15 | 84 ± 18 | .01 |
| TNF-α, pg/mL | 0 ± 0 | 7 ± 7 | 62 ± 19 | .01 |
| IL-6, pg/mL | 0 ± 0 | 3 ± 3 | 33 ± 9 | .003 |
| Fibrinogen, μg/mL | 3156 ± 339 | 3363 ± 147 | 4560 ± 337 | .03 |
| fVII, ng/mL | 0.70 ± 0.05 | 0.60 ± 0.05 | 0.64 ± 0.06 | .6 |
| Tissue factor, ng/mL | 3.1 ± 0.06 | 3.3 ± 0.2 | 3.0 ± 0.2 | .5 |
| VWF, ng/mL | 444 ± 48 | 348 ± 152 | 344 ± 92 | .98 |
Cytokine/coagulation factor . | Uninfected . | Rat IgG, day 4 . | Anti-CD41, day 4 . | P, rat IgG vs α-CD41 . |
|---|---|---|---|---|
| IL-10, pg/mL | 135 ± 10 | 166 ± 26 | 386 ± 72 | .005 |
| IL-2, pg/mL | 1 ± 1 | 30 ± 8 | 5 ± 3 | .002 |
| IL-1α, pg/mL | 29 ± 4 | 53 ± 12 | 110 ± 27 | .03 |
| IFN-γ, pg/mL | 1 ± 1 | 25 ± 15 | 84 ± 18 | .01 |
| TNF-α, pg/mL | 0 ± 0 | 7 ± 7 | 62 ± 19 | .01 |
| IL-6, pg/mL | 0 ± 0 | 3 ± 3 | 33 ± 9 | .003 |
| Fibrinogen, μg/mL | 3156 ± 339 | 3363 ± 147 | 4560 ± 337 | .03 |
| fVII, ng/mL | 0.70 ± 0.05 | 0.60 ± 0.05 | 0.64 ± 0.06 | .6 |
| Tissue factor, ng/mL | 3.1 ± 0.06 | 3.3 ± 0.2 | 3.0 ± 0.2 | .5 |
| VWF, ng/mL | 444 ± 48 | 348 ± 152 | 344 ± 92 | .98 |
Plasma was obtained from uninfected mice or on day 4 of P berghei infection after injection with either anti-CD41 mAb or rat IgG on day 1 of P berghei infection. Mean plus or minus the standard error (SE) is reported. P indicates statistical significance by ANOVA for anti-CD41 mAb-treated group and rat IgG-injected group.
Discussion
The platelet adhesion hypothesis proposes that platelets function late in the disease by adhering to activated endothelial cells in the brain and lung, which then signal the endothelial cells to loosen their intracellular junctions or become more sensitive to apoptosis signaling by TNF; this process leads to increased vascular leak and then hemorrhage.11 Radiolabeled platelet studies, immunohistochemical analysis with anti-CD41, and intravital microscopic analysis of fluorescence labeled platelets all indicate that platelets accumulate in the brain late in the disease when the animals become moribund.5,15 In contrast to stroke and atherosclerosis, platelets roll and adhere primarily late in the P berghei infection to pial venules but not to the arterioles. ICAM-1, P-selectin (both platelet and endothelial), and anti-CD41 are required for platelet adhesion to pial microvasculature during experimental malaria, and mice lacking ICAM-1 or P-selectin are significantly protected from malarial pathogenesis.15,25,26 Massberg et al27 and Frenette et al28 report that P-selectin is critical for platelet adhesion during ischemia/reperfusion injury, a condition that also causes inflammation and platelet adherence in venules; this observation suggests that platelet adhesion via P-selectin is a conserved and important platelet adhesion molecule during diseases that elicit an inflammatory response.
A prediction of the platelet adhesion hypothesis is that removal of the platelets (either early or late) should protect mice from severe P berghei malaria. Mice treated early in the infection with antiplatelet serum or with anti-CD41 and anti-CD61 mAbs are protected from developing severe experimental malaria.5,29 Both the anti-CD41 mAb and the anti-CD61 mAb rapidly depleted platelets from the circulation so that few circulating platelets are detected 30 minutes after injection of the antibodies. The level of protection conferred by anti-CD41 mAb appeared to be greater than that of the anti-CD61 mAb (Figure 1). One possible explanation is interexperimental variation caused by using different parasite inocula or mice. However, we had included a group of 5 anti-CD41 mAb–treated mice in this anti-CD61 mAb experiment and only 1 of 5 mice injected with anti-CD41 mAb became moribund. A more likely explanation is that the level of platelet depletion on day 4 of P berghei infection is lower in the anti-CD61 mAb–injected mice compared with anti-CD41 mAb mice, suggesting that maintaining low levels of platelets early in the infection is critical for protection to occur. It is also possible that the effect of anti-CD61 mAb on endothelial β3 integrin is detrimental and reverses some of the protective effects of the platelet depletion by this mAb.
Although early treatment with anti-CD41 mAb is protective, which supports the platelet adhesion hypothesis, our data also show that mice treated late (day 4, 5, or 6 of P berghei infection) with anti-CD41 mAb are not protected from severe malaria, which contrasts with this hypothesis. It is unlikely that mice treated late with anti-CD41 mAb exhibit platelet adhesion to the brain microvasculature for the following reasons. Platelet adhesion during experimental malaria only occurs on day 6 of infection, just before the animals become moribund.15 Anti-CD41 mAb injection on day 4 or day 5 of infection when mice exhibit no overt clinical symptoms removes virtually all circulating platelets within 30 minutes. Thus, it is unlikely that any circulating platelets remain in the blood to adhere days later to the inflamed brain microvasculature when these unprotected animals start developing the disease. Further, our intravital microscopic analysis indicates that anti-CD41 mAb inhibits platelet adhesion to the inflamed pial endothelium on day 6 of P berghei infection.15 Finally, there is no apparent relation between the low numbers of circulating platelets and malaria morbidity (Figures 3 and 4) as is predicted by the platelet adhesion hypothesis. Mice protected from malarial pathogenesis by anti-CD41 mAb on day 1 of P berghei infection had fewer platelets than PBS-injected controls but more platelets than mice treated with anti-CD41 mAb on day 4, 5, or 6 of infection; both the PBS controls and mice treated late with anti-CD41 mAb all succumbed to severe experimental malaria.
Our finding that early but not late anti-CD41 mAb treatment protects against development of ESM suggests that platelets function as initiators of a pathogenic response rather than as effectors. Indeed, the removal of platelets by anti-CD41 mAb has a marked effect on the immune response elicited by infection, indicating that the coagulation system can control the immune system. It is well established that anti-inflammatory cytokines (IL-4 and IL-10) are not required for the development of ESM, whereas inflammatory cytokines (IL-1, IL-2, IFN-γ, and TNF-α) are all required.22,30,31 Differences in resistance and susceptibility to ESM in different strains of mice correlate with the development of inflammatory versus anti-inflammatory cytokines.20,32 Because administration of IL-10 actually protects against the development of ESM,33 we propose that the increased levels of anti-inflammatory IL-10 and lower levels of IL-2 in the protected anti-CD41 mAb–treated mice may account for the increased survival by counteracting the detrimental effect of increased proinflammatory cytokines.
Because coagulation factors are consumed during malaria and platelets assemble coagulation protease complexes,2-4,34 it is possible that depletion of platelets may ameliorate consumption of coagulation factors during malaria that in turn reduces hemorrhage and vascular leak. Senaldi et al observed no significant depletion of fibrinogen during P berghei malaria whereas injection of either TNF or lipopolysaccharide (LPS; which elicits another TNF-mediated systemic inflammatory response) depletes fibrinogen.35 Fibrinogen depletion by ancrod has no effect on P berghei–, TNF-, or LPS-induced mortality.35 Indeed, we also observe no fibrinogen depletion on day 4 of P berghei infection, but anti-CD41 mAb treatment leads to increased levels of fibrinogen. The similar levels of tissue factor, VWF, and fVII on day 4 of P berghei infection after treatment with anti-CD41 mAb and in rat IgG–injected controls indicates that the protective effect is not likely due to early changes in coagulation factor levels.
In inflammatory diseases such as atherosclerosis and stroke, there is adhesion of leukocytes via platelet P-selectin, followed by apoptosis of the platelets and formation of pro-coagulant microparticles; these events are critical for pathogenesis.36-38 In contrast, platelet-leukocyte conjugates are not detected by flow cytometry during experimental malaria, and leukocyte adhesion to brain microvasculature is not affected by the absence of P-selectin, indicating that P-selectin (platelet or endothelium) is not required for leukocyte adhesion to the activated endothelium during malaria.26,39 Our flow cytometric analysis of blood CD41+ cells obtained directly from the venous circulation indicate there are only about 600 detectable platelet microparticles/μL blood when the animals are moribund from P berghei malaria compared with 60 000 in blood of uninfected controls, indicating it is unlikely that platelet microparticles are being generated during experimental malaria. Indeed, mice with experimental malaria exhibit lower blood pressure and shear stress and increased shear stress is required to generate platelet microparticles, which makes it unlikely that platelet microparticles are formed during ESM.12,21,40 Platelet microparticles are therefore unlikely to contribute to the pro-coagulant state observed during malaria and removal of platelet microparticles by early anti-CD41 mAb treatment does not explain the protective effect of this treatment.
Platelet-erythrocyte conjugates or mini-thrombi are postulated to play an important role in the pathogenesis of septic shock, and clinical isolates of P falciparum often form in vitro conjugates with platelets, which is associated with the development of severe malaria.41 Our flow cytometric analysis of platelet-erythrocyte conjugates reveal that a greater percentage of platelets are coupled with erythrocytes. The actual number of these conjugates is low because of the profound thrombocytopenia observed during P berghei malaria. This low number may be due to adhesion of the platelet-erythrocytes conjugates to inflamed microvasculature during malaria or the rapid clearance of these conjugates by the spleen. Alternatively, few conjugates are formed during experimental malaria because platelets are removed from the circulation. Thus, prevention of mini-thrombi formation does not explain the protective effect of early anti-CD41 mAb treatment. Because the number of erythrocyte vesicles (CD41–CD61– cells in Figure 4) is similar in protected mice treated early with anti-CD41 mAb and in unprotected mice treated late with anti-CD41 mAb, prevention of erythrocyte vesicle formation cannot explain the protective effect of early treatment with anti-CD41 mAb.
Collectively, our data indicate that the role of platelets in malarial pathogenesis is very complex and they challenge our current understanding of the role of platelets in the development of severe malaria. These results indicate that malarial pathogenesis cannot be described by a domino effect in which removal of a domino prevents the cascade from reaching its conclusion. Rather, several parallel and interacting processes may best describe malarial pathogenesis with platelet activation representing one important process. These results also indicate that the major role of platelets in malarial pathogenesis is early in the infection. Platelet adhesion, which occurs just prior to death, is not required for the development of disease. In addition, there is no detectable development during experimental malaria of pro-coagulant microparticles, leukocyte-platelet or erythrocyte-platelet conjugates, all of which have been implicated in the pathogenesis of other inflammatory diseases. Our results indicate an unrecognized regulation of the immune response during malaria by platelets and this regulation is critical for the development of the pathogenic immune response leading to the development of ESM.
Prepublished online as Blood First Edition Paper, October 19, 2004; DOI 10.1182/blood-2004-06-2206.
Supported by National Institutes of Health grant AI40667 (H.C.v.d.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Clara Polizzi, Zaid Yusufi, John Nipkur, and Dennis Young for their technical assistance and Drs Elias Lazarides, John Frangos, and Barry Coller for their helpful discussions of the data. Dr Herman Sahlin and Mr Peter Sobolewski kindly reviewed the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal