Abstract
Patients with antiphospholipid antibodies (APLAs) are at increased risk for arterial and venous thrombosis. Many APLAs associated with these events react with β2 glycoprotein I (β2GPI), and endothelial cell reactive antibodies that activate endothelial cells in a β2GPI-dependent manner occur commonly in these patients. We previously reported that β2GPI binds with high affinity to annexin A2 on the endothelial surface, though the relevance of this interaction to APLA/anti-β2GPI antibody–induced endothelial activation has not been determined. In this report, we confirm that anti-β2GPI antibodies activate endothelial cells in the presence of β2GPI, and demonstrate that anti–annexin A2 antibodies directly cause endothelial cell activation of a similar magnitude and with a similar time course. Moreover, bivalent anti–annexin A2 F(ab′)2 fragments also caused endothelial cell activation, whereas monomeric Fab fragments not only did not cause activation, but blocked activation induced by anti–annexin A2 antibodies and F(ab′)2 fragments, as well as that caused by anti-β2GPI antibodies in the presence of β2GPI. These observations suggest a novel pathway for endothelial activation induced by APLA/anti-β2GPI antibodies that is initiated by cross-linking or clustering of annexin A2 on the endothelial surface.
Introduction
Antiphospholipid antibodies (APLAs) are associated with an increased risk of arterial and venous thrombosis, as well as recurrent fetal loss,1-4 and the presence of APLAs in patients who experience these events defines the antiphospholipid antibody syndrome (APS).5 Although once believed to directly recognize anionic phospholipids, over the last decade it has become apparent that most of these antibodies instead recognize phospholipid binding proteins, such as β2 glycoprotein I (β2GPI)6,7 and prothrombin,8,9 as well as additional targets such as oxidized phospholipid.10-12 β2GPI has emerged as a particularly common antigen for these antibodies,13,14 and clinical assays for direct measurement of anti-β2GPI antibodies are available.
Previous studies have demonstrated that plasma or serum from patients with APLAs frequently contains antibodies reactive with endothelial cells.15 Many of these antibodies induce endothelial cell activation, as determined by measurement of the expression of endothelial cell adhesion molecules, secretion of inflammatory cytokines, or expression of procoagulant activity.16-21 Moreover, the ability of these antibodies to induce endothelial cell activation requires β2GPI17,22 and may be mediated through a pathway involving nuclear factor (NF)–κB.23,24 These observations support the hypothesis that binding of β2GPI to an endothelial cell receptor, with receptor cross-linking or clustering occurring as a result of the binding of anti-β2GPI antibodies to receptor-bound β2GPI, may lead to activation of endothelial cell signaling responses and cellular activation. We previously reported that unstimulated endothelial cells bound β2GPI largely through a high-affinity interaction with annexin A2 expressed on the endothelial cell surface.25 However, annexin A2 is not a transmembrane protein, and how binding of β2GPI to annexin A2 might facilitate antiphospholipid/anti-β2GPI antibody–mediated endothelial cell activation is uncertain. In this manuscript, we confirm that anti-β2GPI antibodies activate endothelial cells in a β2GPI-dependent manner, and demonstrate an essential role for cell surface annexin A2 in this process.
Materials and methods
Materials
EH7A hybridoma cells26 were obtained from the American Type Culture Collection (Manassas, VA). Monoclonal anti–annexin I/II antibodies were purified from the conditioned medium of these cells using protein G sepharose, and detected only a single band of approximately 36 kDa, consistent with annexin A2, in endothelial cell extracts. An anti–human E-selectin (CD62E) monoclonal antibody (mAb) was obtained from Endogen (Woburn, MA), and a goat anti–human E-selectin antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Horseradish peroxidase (HRP)–conjugated rabbit antimouse and rabbit antigoat secondary antibodies were from Sigma (St Louis, MO), and control rabbit immunoglobulin G (IgG) and murine IgG1 (MOPC-21) were from Zymed Laboratories (South San Francisco, CA). Anti-β2GPI antibodies from patients with the antiphospholipid syndrome (APS; all had lupus anticoagulants and a history of venous thrombosis) and from β2GPI-immunized rabbits were affinity purified using a column of Affigel HZ to which purified human β2GPI was coupled (Bio-Rad Laboratories, Hercules, CA), as previously described.25 On immunoblots of human plasma, the affinity-purified human and rabbit antibodies recognized a single band of approximately 50 kDa.
β2GPI was isolated from outdated, fresh frozen human plasma using perchloric acid precipitation, heparin-ultraflow (Sterogene, Seattle, WA), and S-sepharose (Amersham-Pharmacia, Piscataway, NJ) chromatography, as previously described.25 The purified protein migrated as a single band of approximately 50 kDa under nonreducing conditions, with an apparent increase to approximately 62 kDa after reduction, and was recognized on immunoblotting by anti-β2GPI antibodies. All materials contained less than 0.000 25 ng endotoxin/μg protein, as determined by the Limulus Amebocyte lysate test performed at Associates of Cape Cod (Falmouth, MA).
Preparation of Fab and F(ab′)2 fragments
Antibody fragmentation was performed using immobilized Ficin (Pierce, Rockford, IL), per the manufacturer's instructions. Briefly, a column of immobilized Ficin was equilibrated with ImmunoPure (Pierce) IgG1 digestion buffer containing 0.2 mg/mL or 2 mg/mL cysteine · HCl for preparation of F(ab′)2 or Fab fragments, respectively. One milliliter of a 1 mg/mL solution of anti–annexin A2 antibody, or control murine IgG1, was added to the Ficin column, and incubated at 37°C for 5 hours (for preparation of Fab fragments) or 20 hours (for preparation of F(ab′)2 fragments). The digested antibody fragments were eluted after washing the column with 4 mL ImmunoPure binding buffer, and the eluate then passed through an ImmunoPure-protein A column to remove Fc fragments and intact IgG. The Fab and F(ab′)2 fragments were collected and dialyzed against phosphate-buffered saline (PBS) prior to analysis using 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE).
Cells
Human umbilical vein endothelial cells (HUVECs) were isolated as previously described.25 Cells were maintained in Medium 199 containing 10% fetal bovine serum (Hyclone, Logan, UT), penicillin-streptomycin, and endothelial cell growth supplement, isolated as described by Maciag et al.27 All experiments were performed using HUVECs of passage 3 or lower. Mono Mac 6 (MM6) cells were a kind gift from Dr H. Zeigler-Heitbrock,28 and were maintained in RPMI 1640 containing 10% serum.
Assessment of endothelial cell activation
Two assays, both of which measure the expression of cell adhesion molecules (CAMs) on the endothelial cell surface, were used to assess endothelial cell activation. First, we used a previously described assay that measured the CAM-dependent adhesion of MM6 cells to endothelial cells.22 Adhesion of MM6 cells in this assay is dependent on expression of endothelial cell E-selectin, vascular cell adhesion molecule-1 (VCAM-1), and intracellular adhesion molecule 1 (ICAM-1).22 Briefly, endothelial cells were seeded in 96-well microplates (NUNC, Rochester, NY) and allowed to achieve confluence. Prior to the assay, cells were washed with warm, serum-free Medium 199 and incubated for an additional 4 hours in Medium 199 containing 1% bovine serum albumin (BSA). Cells were then incubated with test materials (ie, β2GPI in the presence or absence of anti-β2GPI antibodies, anti–annexin A2 antibodies or antibody fragments, or control proteins as dictated by the specific experiment) for 4 hours, after which they were washed with warm, serum-free Medium 199 and incubated for an additional 10 minutes with 100 μL of a suspension of MM6 cells (1 × 106 cells/mL) prelabeled with 5-chloromethylfluorescein diacetate (CMFDA; Cell Tracker Green; Molecular Probes, Eugene, OR). Nonadherent MM6 cells were removed by gently washing the monolayers with warm medium, and cells adherent to the endothelial cell monolayer were quantitated by measuring the relative fluorescence (excitation wavelength 492 nm/emission wavelength 535 nm) of each well using a Perkin Elmer HTS 7000 fluorescent plate reader (Perkin Elmer, Norwalk, CT). Lipopolysaccharide (LPS; Sigma) or tumor necrosis factor alpha (TNFα) was used as a positive control in all assays of endothelial cell activation.
As a second method to assess endothelial cell activation, we directly measured the expression of endothelial cell E-selectin by enzyme-linked immunosorbent assay (ELISA). Briefly, confluent monolayers of HUVECs were prepared in 96-well microplates as described in “Cells.”After incubation with test materials for 4 hours, cells were washed and then fixed by exposure to 0.1% glutaraldehyde for 10 minutes. Cells were then incubated with PBS containing 5% nonfat milk (to block nonspecific antibody binding), washed with Tris-buffered saline containing 0.01% Tween 20 (TBS-T), and incubated for an additional hour with 1 μg/mL goat anti–human E-selectin antibodies. After washing, bound antibodies were detected by incubating cells with a 1:6000 dilution of peroxidase-conjugated rabbit anti–goat IgG, then for an additional 10 minutes with the peroxidase substrate, Turbo-TMB (Pierce). The reaction was stopped by adding an equal volume of 1.0 M H2SO4, and relative amounts of endothelial cell bound E-selectin antibodies were assessed by measuring A450.
Statistics
Data points are expressed as the mean plus or minus standard error. All experimental points were determined in quadruplicate, and all assays repeated at least 3 times. Differences between control and experimental conditions were assessed using the Student 2 tailed t test for paired samples.
Results
Activation of endothelial cells by β2GPI and anti-β2GPI antibodies
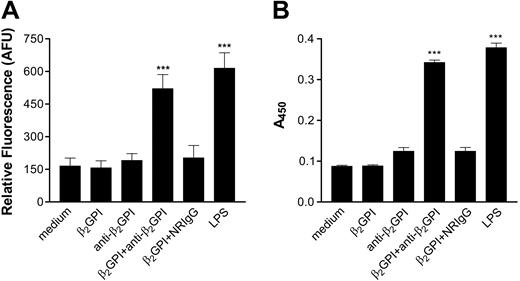
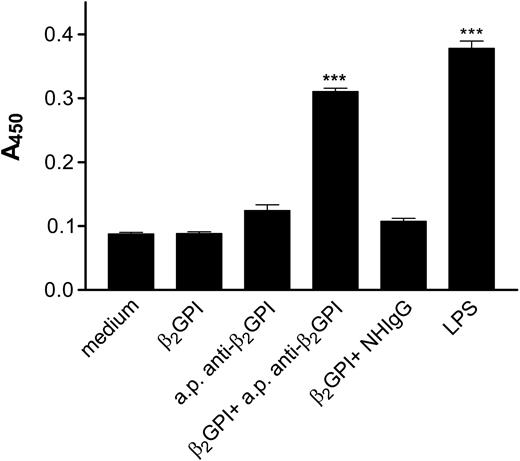
In initial studies, we confirmed that incubation of endothelial cells with β2GPI and anti-β2GPI antibodies induced endothelial cell activation, as assessed by the increased expression of cell adhesion molecules. Incubation of endothelial cells with β2GPI and rabbit anti-β2GPI antibodies resulted in significant increases in MM6 cell adhesion (Figure 1A), as well as cell surface E-selectin expression (Figure 1B). Both β2GPI and anti-β2GPI antibodies were required for activation, as cells incubated with β2GPI or anti-β2GPI antibodies alone were not activated. Identical responses were seen when affinity-purified human anti-β2GPI antibodies from 3 patients with the antiphospholipid syndrome were incubated with endothelial cells in the presence of β2GPI (Figure 2). In each case, the extent of activation was similar to that occurring in response to 1 μg/mL LPS.
Activation of endothelial cells by rabbit anti-β2GPI antibodies. Endothelial cells were cultured in 96-well microplates and prepared as described in “Materials and methods.” Cells were then incubated for 4 hours with either medium alone, human β2GPI (100 nM), rabbit anti–human β2GPI antibodies (600 nM), β2GPI (100 nM) and rabbit anti–human β2GPI antibodies (600 nM), β2GPI (100 nM) and normal rabbit IgG (NRIgG; 600 nM) or LPS (1 μg/mL). (A) Adhesion of CMFDA-labeled Mono Mac 6 cells, measured in arbitrary fluorescence units (AFUs). (B) Expression of endothelial cell surface E-selectin, measured by ELISA. Error bars represent the mean plus or minus SEM of quadruplicate points. ***P < .0001 versus medium alone. This experiment is representative of 4 so performed.
Activation of endothelial cells by rabbit anti-β2GPI antibodies. Endothelial cells were cultured in 96-well microplates and prepared as described in “Materials and methods.” Cells were then incubated for 4 hours with either medium alone, human β2GPI (100 nM), rabbit anti–human β2GPI antibodies (600 nM), β2GPI (100 nM) and rabbit anti–human β2GPI antibodies (600 nM), β2GPI (100 nM) and normal rabbit IgG (NRIgG; 600 nM) or LPS (1 μg/mL). (A) Adhesion of CMFDA-labeled Mono Mac 6 cells, measured in arbitrary fluorescence units (AFUs). (B) Expression of endothelial cell surface E-selectin, measured by ELISA. Error bars represent the mean plus or minus SEM of quadruplicate points. ***P < .0001 versus medium alone. This experiment is representative of 4 so performed.
Activation of endothelial cells by affinity-purified human anti-β2GPI IgG. Endothelial cells were cultured in 96-well plates and prepared as described in “Materials and methods.” Human anti-β2GPI IgG was affinity purified from patients with antiphospholipid antibodies using Affigel HZ conjugated to β2GPI. Endothelial cells were incubated for 4 hours with medium, β2GPI (100 nM), affinity-purified (a.p.) human anti-β2GPI IgG (600 nM), β2GPI (100 nM) and affinity-purified human anti-β2GPI IgG (600 nM), β2GPI (100 nM) and nonimmune human IgG (NHIgG) (600 nM), or LPS (1 μg/mL). Endothelial cell surface E-selectin expression was then measured by ELISA. Error bars represent the mean plus or minus SEM of quadruplicate points. ***P < .0001 versus medium alone. This experiment is representative of 3 so performed using affinity-purified anti-β2GPI from a single patient. Anti-β2GPI IgG from 2 additional patients yielded similar results.
Activation of endothelial cells by affinity-purified human anti-β2GPI IgG. Endothelial cells were cultured in 96-well plates and prepared as described in “Materials and methods.” Human anti-β2GPI IgG was affinity purified from patients with antiphospholipid antibodies using Affigel HZ conjugated to β2GPI. Endothelial cells were incubated for 4 hours with medium, β2GPI (100 nM), affinity-purified (a.p.) human anti-β2GPI IgG (600 nM), β2GPI (100 nM) and affinity-purified human anti-β2GPI IgG (600 nM), β2GPI (100 nM) and nonimmune human IgG (NHIgG) (600 nM), or LPS (1 μg/mL). Endothelial cell surface E-selectin expression was then measured by ELISA. Error bars represent the mean plus or minus SEM of quadruplicate points. ***P < .0001 versus medium alone. This experiment is representative of 3 so performed using affinity-purified anti-β2GPI from a single patient. Anti-β2GPI IgG from 2 additional patients yielded similar results.
Activation of endothelial cells by anti–annexin A2 antibodies and anti–annexin A2–derived fragments
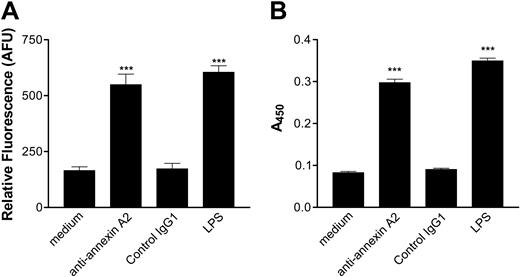
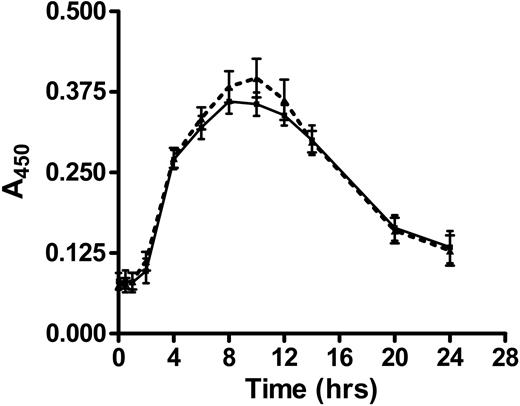
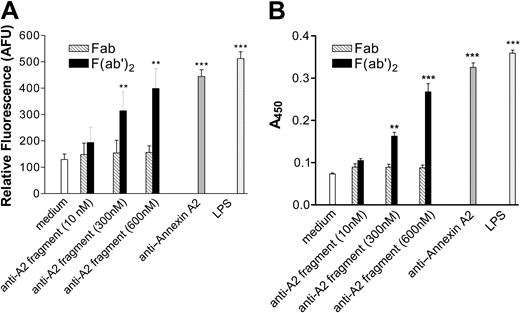
Based on the results depicted in Figures 1 and 2, we hypothesized that cross-linking of annexin A2–bound β2GPI by anti-β2GPI antibodies induced endothelial cell activation. If this were the case, we expected that direct cross-linking of endothelial cell surface annexin A2 by anti–annexin A2 antibodies might induce a similar activation response. To test this hypothesis, we incubated endothelial cells with monoclonal anti–annexin A2 antibodies under the same conditions described in the studies depicted in Figures 1 and 2, and assessed both adhesion of MM6 cells (Figure 3A) and E-selectin expression (Figure 3B). Anti–annexin A2 mAb induced endothelial cell activation of a similar magnitude as that caused by β2GPI and anti-β2GPI antibodies, whereas control murine IgG1 (MOPC-21) did not induce activation. Activation occurred in a concentration-dependent manner, with maximal responses occurring at an anti–annexin A2 antibody concentration of approximately 600 nM (not shown). Moreover, the time course of endothelial cell activation in response to anti–annexin A2 antibodies was identical to that observed when cells were incubated with β2GPI and anti-β2GPI antibodies (Figure 4).
Anti–annexin A2 antibodies directly activate endothelial cells. Endothelial cells were cultured in 96-well plates, prepared as described in “Materials and methods,” and then incubated with medium alone, anti–annexin A2 mAb (600 nM, purified from EH7A hybridoma cells), control murine IgG1 (600 nM), or LPS (1 μg/mL). (A) Adhesion of CMFDA-labeled Mono Mac 6 cells, measured in arbitrary fluorescence units (AFUs). (B) Expression of endothelial cell surface E-selectin, measured by ELISA. Error bars represent the mean plus or minus SEM of quadruplicate points. ***P < .0001 versus medium alone. This experiment is representative of 4 so performed.
Anti–annexin A2 antibodies directly activate endothelial cells. Endothelial cells were cultured in 96-well plates, prepared as described in “Materials and methods,” and then incubated with medium alone, anti–annexin A2 mAb (600 nM, purified from EH7A hybridoma cells), control murine IgG1 (600 nM), or LPS (1 μg/mL). (A) Adhesion of CMFDA-labeled Mono Mac 6 cells, measured in arbitrary fluorescence units (AFUs). (B) Expression of endothelial cell surface E-selectin, measured by ELISA. Error bars represent the mean plus or minus SEM of quadruplicate points. ***P < .0001 versus medium alone. This experiment is representative of 4 so performed.
Time course of endothelial cell E-selectin expression induced by anti–annexin A2 mAb or β2GPI and anti-β2GPI antibodies. Endothelial cells were cultured in 96-well microplates and prepared as described in “Materials and methods,” then incubated for increasing time intervals with either β2GPI (100 nM) and rabbit anti-β2GPI antibodies (600 nM; ▪ and solid line), or anti–annexin A2 mAb alone (600 nM; ▴ and dotted line). Cell surface E-selectin expression was then determined by ELISA. Error bars represent the mean plus or minus SEM of quadruplicate points. This experiment is representative of 2 so performed.
Time course of endothelial cell E-selectin expression induced by anti–annexin A2 mAb or β2GPI and anti-β2GPI antibodies. Endothelial cells were cultured in 96-well microplates and prepared as described in “Materials and methods,” then incubated for increasing time intervals with either β2GPI (100 nM) and rabbit anti-β2GPI antibodies (600 nM; ▪ and solid line), or anti–annexin A2 mAb alone (600 nM; ▴ and dotted line). Cell surface E-selectin expression was then determined by ELISA. Error bars represent the mean plus or minus SEM of quadruplicate points. This experiment is representative of 2 so performed.
To further address the hypothesis that annexin A2 cross-linking stimulated endothelial cell activation, we assessed the ability of anti–annexin A2 F(ab′)2 and Fab fragments to induce this response. We hypothesized that if annexin A2 cross-linking was required, then only the bivalent F(ab′)2 fragments would induce activation. As predicted, anti–annexin A2 F(ab′)2 fragments induced endothelial cell activation in a concentration-dependent manner, whereas Fab fragments at identical concentrations did not (Figure 5). The extent of endothelial cell activation that occurred in response to anti–annexin A2 F(ab′)2 fragments at a concentration of 600 nM was similar to that caused by intact anti–annexin A2 antibodies at the same concentration. These studies confirm that the mechanism by which anti–annexin A2 antibodies induce activation of endothelial cells involves annexin A2 cross-linking.
Activation of endothelial cells by anti–annexin A2 mAb-derived F(ab′)2 fragments. Endothelial cells were cultured in 96-well microplates and prepared as described in “Materials and methods,” and then incubated for 4 hours with medium, anti–annexin A2 mAb–derived fragments at the designated concentration (▧, Fab fragments; ▪, F(ab′)2 fragments), intact annexin A2 mAb (600 nM), or LPS (1 μg/mL). (A) Adhesion of CMFDA-labeled Mono Mac 6 cells, measured in arbitrary fluorescence units (AFUs). (B) Expression of endothelial cell surface E-selectin, measured by ELISA. Error bars represent the mean plus or minus SEM of quadruplicate points. **P < .001, ***P < .0001 versus medium alone. This experiment is representative of 3 so performed.
Activation of endothelial cells by anti–annexin A2 mAb-derived F(ab′)2 fragments. Endothelial cells were cultured in 96-well microplates and prepared as described in “Materials and methods,” and then incubated for 4 hours with medium, anti–annexin A2 mAb–derived fragments at the designated concentration (▧, Fab fragments; ▪, F(ab′)2 fragments), intact annexin A2 mAb (600 nM), or LPS (1 μg/mL). (A) Adhesion of CMFDA-labeled Mono Mac 6 cells, measured in arbitrary fluorescence units (AFUs). (B) Expression of endothelial cell surface E-selectin, measured by ELISA. Error bars represent the mean plus or minus SEM of quadruplicate points. **P < .001, ***P < .0001 versus medium alone. This experiment is representative of 3 so performed.
Anti–annexin A2 F(ab′) fragments block endothelial cell activation induced by intact anti–annexin A2 antibodies, or β2GPI and anti-β2GPI antibodies
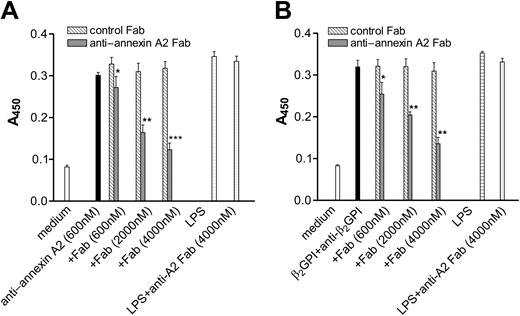
The results described above demonstrate that cross-linking of annexin A2 molecules on the endothelial cell surface by either intact anti–annexin A2 antibodies or anti–annexin A2 antibody–derived F(ab′)2 fragments induces endothelial cell activation. If so, then an agent that inhibits annexin A2 cross-linking by competing with bivalent ligands for binding of annexin A2 (or annexin A2–bound β2GPI) should block such activation responses. To address this hypothesis, we assessed the ability of monomeric Fab fragments to block endothelial cell activation caused by the ligands used in the studies above. Anti–annexin A2 Fab fragments blocked endothelial cell activation caused by anti–annexin A2 mAb (Figure 6A) and anti–annexin A2 F(ab′)2 fragments (not shown), as well as that caused by β2GPI and rabbit anti-β2GPI antibodies (Figure 6B) in a concentration-dependent manner. Anti–annexin A2 Fab fragments also blocked the activation of endothelial cells caused by β2GPI and affinity-purified anti-β2GPI antibodies from 2 of 2 patients with the antiphospholipid syndrome (not shown), whereas isotype control murine IgG1 Fab fragments did not block activation caused by any of these stimuli. The lack of complete inhibition observed in these studies, even when using the Fab fragment at a concentration approximately 6-fold greater than that of the anti-β2GPI antibody, likely reflects decreased affinity of the univalent anti–annexin A2 Fab fragment versus the high-affinity binding of bivalent anti-β2GPI antibodies to β2GPI concentrated on endothelial cell surface annexin A2.29
Inhibition of endothelial cell activation caused by anti–annexin A2 mAb or β2GPI and anti-β2GPI antibodies by anti–annexin A2 mAb–derived Fab fragments. Endothelial cells were cultured in 96-well microplates and prepared as described in “Materials and methods.” In (A), cells were then incubated for 4 hours with medium alone, anti–annexin A2 mAb (600 nM), anti–annexin A2 mAb (600 nM) in the presence of Fab fragments derived from control murine IgG1 (▧, control Fab) or anti–annexin A2 mAb (▦) at the designated concentrations, LPS (1 μg/mL), or LPS (1 μg/mL) and 4000 nM anti–annexin A2 Fab fragments (anti–annexin A2 Fab). In (B), cells were incubated for 4 hours with medium, β2GPI (100 nM) and rabbit anti-β2GPI antibodies (600 nM), β2GPI (100 nM) and rabbit anti-β2GPI antibodies (600 nM) in the presence of Fab fragments derived from control murine IgG1 (▧, control Fab) or anti–annexin A2 mAb (▦) at the designated concentrations, LPS (1 μg/mL), or LPS (1 μg/mL) and 4000 nM anti–annexin A2 Fab fragments. Error bars represent the mean plus or minus SEM of quadruplicate points. *P < .05, **P < .001, ***P < .0001 versus anti–annexin A2 mAb (A) or β2GPI and anti-β2GPI antibodies alone (B). This experiment is representative of 3 so performed.
Inhibition of endothelial cell activation caused by anti–annexin A2 mAb or β2GPI and anti-β2GPI antibodies by anti–annexin A2 mAb–derived Fab fragments. Endothelial cells were cultured in 96-well microplates and prepared as described in “Materials and methods.” In (A), cells were then incubated for 4 hours with medium alone, anti–annexin A2 mAb (600 nM), anti–annexin A2 mAb (600 nM) in the presence of Fab fragments derived from control murine IgG1 (▧, control Fab) or anti–annexin A2 mAb (▦) at the designated concentrations, LPS (1 μg/mL), or LPS (1 μg/mL) and 4000 nM anti–annexin A2 Fab fragments (anti–annexin A2 Fab). In (B), cells were incubated for 4 hours with medium, β2GPI (100 nM) and rabbit anti-β2GPI antibodies (600 nM), β2GPI (100 nM) and rabbit anti-β2GPI antibodies (600 nM) in the presence of Fab fragments derived from control murine IgG1 (▧, control Fab) or anti–annexin A2 mAb (▦) at the designated concentrations, LPS (1 μg/mL), or LPS (1 μg/mL) and 4000 nM anti–annexin A2 Fab fragments. Error bars represent the mean plus or minus SEM of quadruplicate points. *P < .05, **P < .001, ***P < .0001 versus anti–annexin A2 mAb (A) or β2GPI and anti-β2GPI antibodies alone (B). This experiment is representative of 3 so performed.
Taken together, these results strongly suggest that annexin A2 antibodies, and anti-β2GPI antibodies in the presence of β2GPI, activate endothelial cells through the same mechanism: cross-linking of annexin A2 molecules on the endothelial cell surface. Since endothelial cells were activated by anti–annexin A2 F(ab′)2 fragments as well as intact annexin A2 antibodies, endothelial cell activation induced by these stimuli does not appear to be Fcγ receptor dependent.
Discussion
Our studies provide compelling evidence that antiphospholipid/anti-β2GPI antibodies activate endothelial cells through cross-linking or clustering of annexin A2–bound β2GPI. This conclusion is supported by the observations that (1) both intact anti–annexin A2 antibodies and anti–annexin A2–derived F(ab′)2 fragments induced endothelial cell activation, whereas Fab fragments did not; (2) the magnitude and time course of endothelial cell activation induced by anti–annexin A2 antibodies, as well as anti-β2GPI antibodies in the presence of β2GPI, was virtually identical; and (3) anti–annexin A2 antibody–derived Fab fragments blocked endothelial cell activation caused by anti–annexin A2 antibodies, as well as that caused by anti-β2GPI antibodies in the presence of β2GPI. The importance of cross-linking of endothelial cell–bound β2GPI by anti-β2GPI antibodies is further suggested by the fact that Fab fragments derived from anti-β2GPI antibodies also blocked endothelial cell activation caused by intact anti-β2GPI antibodies in the presence of β2GPI (not shown). Taken together, these studies provide direct evidence that cross-linking of annexin A2 stimulates activation of endothelial cells.
Antiphospholipid antibodies are associated with an increased incidence of venous and arterial thrombosis, as well as recurrent fetal loss.1-4 Rather than phospholipid per se, most of these antibodies recognize specific proteins, such as β2GPI, bound to phospholipid or other appropriate surfaces.29 β2GPI-dependent antiphospholipid antibodies are more closely associated with clinical manifestations of the antiphospholipid syndrome than those that recognize phospholipid directly.30 In a recent systematic review, Galli et al31 note that the majority of reports addressing the relationship between anti-β2GPI antibodies and thrombosis demonstrate an association of these antibodies with thrombotic events, primarily venous, although additional work is needed to more clearly define the importance of these antibodies in the diagnosis of the antiphospholipid syndrome.
Several mechanisms may contribute to the thrombotic manifestations of the antiphospholipid syndrome. Among these, activation of endothelial cells with attendant loss of anticoagulant and gain of procoagulant functions is likely to be important.32 Numerous reports have demonstrated that plasma from patients with the APS contains antibodies that bind and activate endothelial cells,15,33 and that endothelial cell activation may occur in a β2GPI-dependent manner.17,22 The clinical relevance of endothelial cell activation in patients with the APS is supported by reports demonstrating increased circulating levels of tissue factor34 and VCAM-1,35 as well as endothelial microparticles,36 in these individuals.
Despite these observations, and the β2GPI-dependency of endothelial cell–reactive antibodies in patients with the APS, little information is available concerning the mechanisms by which anti-β2GPI antibodies induce endothelial cell activation. β2GPI binds specifically and with high affinity to annexin A2 expressed on the surface of unactivated endothelial cells25 ; bound β2GPI remains on the cell surface and is not internalized, at least within the time course of the experiments depicted in this manuscript (data not shown). However, the relevance of this interaction to endothelial cell activation has not been demonstrated, raising questions as to its significance.15 In this study, however, we demonstrate that annexin A2 cross-linking induces signaling responses in endothelial cells that lead to endothelial cell activation and expression of endothelial cell adhesion molecules. These observations suggest that the interaction between β2GPI and annexin A2 may be pathophysiologically significant.
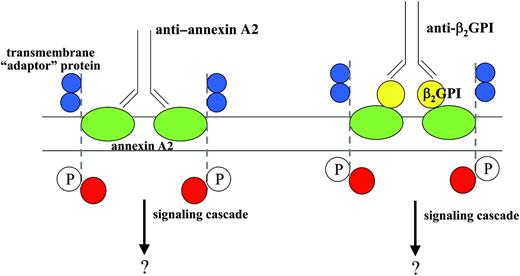
How annexin A2 cross-linking or clustering might mediate anti-β2GPI antibody–induced signal transduction remains unexplained, as annexin A2 is not a transmembrane protein.37 We have previously proposed that activation of signaling responses may require recruitment of other transmembrane “adaptor” proteins that associate with annexin A2 on the endothelial cell surface, as depicted in Figure 7. An example of such a mechanism is the enhancement of signal transduction through the glycophosphatidylinositol-linked urokinase receptor by integrins.38
Model for annexin A2–dependent endothelial cell activation induced by β2GPI and anti-β2GPI antibodies, or anti–annexin A2. In this model, annexin A2 (green) embedded in the outer plasma membrane of the endothelial cell may be directly cross-linked by anti–annexin A2 antibodies, or, alternatively, through the binding of anti-β2GPI antibodies bound to β2GPI (yellow) bound to annexin A2. We hypothesize that annexin A2 cross-linking may promote the aggregation of an annexin A2–associated transmembrane “adaptor” protein, perhaps with receptor tyrosine kinase activity (dashed line, blue extracellular domains), potentially leading to phosphorylation of this protein (P) and assembly of additional intracellular signaling proteins (red). Activation of endothelial cells by either scenario would be blocked by anti–annexin A2 Fab fragments.
Model for annexin A2–dependent endothelial cell activation induced by β2GPI and anti-β2GPI antibodies, or anti–annexin A2. In this model, annexin A2 (green) embedded in the outer plasma membrane of the endothelial cell may be directly cross-linked by anti–annexin A2 antibodies, or, alternatively, through the binding of anti-β2GPI antibodies bound to β2GPI (yellow) bound to annexin A2. We hypothesize that annexin A2 cross-linking may promote the aggregation of an annexin A2–associated transmembrane “adaptor” protein, perhaps with receptor tyrosine kinase activity (dashed line, blue extracellular domains), potentially leading to phosphorylation of this protein (P) and assembly of additional intracellular signaling proteins (red). Activation of endothelial cells by either scenario would be blocked by anti–annexin A2 Fab fragments.
Recently, Raschi et al24 have proposed that endothelial cell activation stimulated by anti-β2GPI antibodies may involve a MyD88-dependent signaling pathway leading to activation of NF-κB, which is stimulated through binding of β2GPI to toll-like receptors (TLRs). These investigators demonstrated that anti-β2GPI–induced endothelial cell activation could be inhibited in an immortalized endothelial cell line by dominant-negative constructs of MyD88 and TRAF6, suggesting involvement of a TLR-dependent intracellular signaling pathway. However, the role of this pathway in primary endothelial cells, as used in our studies, has not been investigated. Moreover, there is no evidence that β2GPI binds to any of the TLR family members. Nevertheless, our preliminary results also suggest that activation of NF-κB is a downstream effect of anti-β2GPI–mediated endothelial cell activation, and it is possible that TLR, in particular TLR4, might associate with annexin A2 in a multiprotein signaling complex on the cell surface.39 Alternatively, the MyD88 pathway might become activated through different proximal events. Further studies will be required to define the role, if any, of TLR in activation of endothelial cells by anti-β2GPI antibodies.
The significance of annexin A2 expression on endothelial cells has been thought to be primarily related to its ability to stimulate tissue-type plasminogen activator (t-PA)–dependent plasminogen activation by serving as a coreceptor for t-PA and plasminogen.40,41 Annexin A2 binds t-PA through its N-terminal tail region,42 whereas plasminogen has been reported to bind directly to a C-terminal lysine (Lys307) of annexin A2 exposed following annexin A2 proteolysis40 or to a C-terminal lysine of the p11 subunit of the annexin A2 heterotetramer (A22p112).43,44 Regardless, both annexin A245 and the heterotetramer44 stimulate t-PA–dependent plasminogen activation by lowering the Km and enhancing the catalytic efficiency of the reaction, though the heterotetramer may be more potent in this regard.46 This activity allows annexin A2 to contribute to the maintenance of blood fluidity, and enhance cellular invasiveness, the latter activity likely accounting largely for the decreased angiogenic potential in annexin A2–/– mice.47 However, whether migration might also be stimulated by signaling events initiated by binding of other annexin A2 ligands, such as matrix-associated tenascin-C,48 may also deserve consideration.
In summary, our studies provide compelling evidence that cross-linking of endothelial cell annexin A2 stimulates intracellular signaling pathways that lead to endothelial cell activation. This observation further justifies consideration of this protein as a cellular binding site for β2GPI that plays an important role in antiphospholipid/anti-β2GPI antibody–induced endothelial cell activation. Additional studies focused on delineation of the mechanisms by which annexin A2 cross-linking leads to transmembrane signaling responses, as well as the downstream pathways involved and their link to NF-κB, may lead to better understanding and treatment of antiphospholipid antibody–mediated thrombosis.
Prepublished online as Blood First Edition Paper, October 7, 2004; DOI 10.1182/blood-2004-05-1708.
Supported by an Established Investigator Grant (no. 9 940 234) from the American Heart Association (K.R.M.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal