Abstract
Immunoproliferative small intestinal disease (IPSID) was recently added to the growing list of infectious pathogen-associated human lymphomas. Molecular and immunohistochemical studies demonstrated an association with Campylobacter jejuni. IPSID is a variant of the B-cell lymphoma of mucosa-associated lymphoid tissue (MALT), which involves mainly the proximal small intestine resulting in malabsorption, diarrhea, and abdominal pain. Geographically, IPSID is most prevalent in the Middle East and Africa. IPSID lymphomas reveal excessive plasma cell differentiation and produce truncated α heavy chain proteins lacking the light chains as well as the first constant domain. The corresponding mRNA lacks the variable heavy chain (VH) and the constant heavy chain 1 (CH1) sequences and contains deletions as well as insertions of unknown origin. The encoding gene sequence reveals a deletion of V region and parts of CH1 domain. Cytogenetic studies demonstrated clonal rearrangements involving predominantly the heavy and light chain genes, including t(9;14) translocation involving the PAX5 gene. Early-stage IPSID responds to antibiotics (30%-70% complete remission). Most untreated IPSID patients progress to lymphoplasmacytic and immunoblastic lymphoma invading the intestinal wall and mesenteric lymph nodes, and may metastasize to a distant organ. IPSID lymphoma shares clinical, morphologic, and molecular features with MALT lymphoma, lymphoplasmacytic lymphoma, and plasma cell neoplasms.
Introduction
Malignant lymphomas of the small intestine are relatively common in certain geographic areas and they seem to be particularly so in developing countries, especially in the Middle East and North Africa.1 In the Iraqi tumor registry (1986-1988), for example, small intestinal lymphoma constituted about 19% of all non-Hodgkin lymphomas and 78% of all small intestinal malignant tumors. Generally, these lymphomas can be divided into 3 subtypes.2 Burkitt lymphoma, which is most common in children, usually involves the terminal ileum with extensive abdominal involvement. The second, relatively uncommon, is similar to the “Western” type of non-Hodgkin lymphomas most commonly large B-cell type involving various parts of the small intestine. The third type is the so-called Mediterranean lymphoma affecting mainly young adults with almost equal sex incidence and involves predominantly the proximal small intestine and is usually associated with chronic diarrhea and abdominal pain.3 In 1978, the World Health Organization recommended the term immunoproliferative small intestinal disease (IPSID) for the syndrome associated with Mediterranean lymphoma because at that time it was felt that the disease in its early stages “does not appear to be truly malignant lymphoma.”4 (p615)
Many of the patients with IPSID syndrome were found to have variable levels of abnormal immunoglobulin in the serum or other body fluids, which was later identified to be truncated α heavy chains (αHCs).5 Generally, at the present time IPSID is considered as a variant of mucosa-associated lymphoid tissue (MALT) lymphoma.6 In the recent WHO classification of hematopoietic and lymphoid tissue, IPSID is listed with the heavy chain diseases as a special variant of extranodal marginal zone B-cell (MALT) lymphoma.7 However, the WHO classification does not recognize the so-called “nonsecretory” IPSID as a specific entity. This variant, similar to MALT lymphomas of other mucosal sites, is characterized morphologically by the proliferation of small centrocyte-like lymphoid cells lacking the extreme plasmacytic differentiation observed in αHC secretory form. Both “secretory” and “nonsecretory” forms are common in the same geographic area, but have different local geographic distribution.8 For the purpose of this review, the terms IPSID and α heavy chain disease (αHCD) are used synonymously to refer to the secretory form, a relatively well-defined clinicopathologic entity. This is not withstanding the fact that there are rare patients with γ rather than α heavy chain IPSID9 and the rare colonic, gastric, or pulmonary αHCD.10-12 IPSID is a unique mature B-cell neoplasm regarding its epidemiology, clinical features, morphology, and molecular pathogenesis.13 It shares certain features with gastrointestinal MALT lymphomas, lymphoplasmacytic lymphoma, as well as plasma cell neoplasms. Although rare in many parts of the world, clinical experience and molecular investigation over the period of 4 decades have contributed not only to the diagnosis and management of IPSID, but also to the understanding of the pathogenesis and evolution of these B-cell neoplasms.
Epidemiology and clinical features
IPSID affects mainly older children and young adults (range, 10-35 years; mean, 25-30 years) of low socioeconomic status in developing countries. It is uncommon in young children and older adults. Geographically, the majority of cases reported were from the Middle East, North and South Africa, and the Far East.14-18 Sporadic cases have been reported from other countries and continents, especially in immigrants from the Middle East and North Africa.19 Clinically, intermittent diarrhea and colicky abdominal pain are the most frequent symptoms.20-22 Other symptoms and signs are mainly related to malabsorption. Intestinal obstructions, abdominal masses, and ascites are common in advanced stage (Table 1). The differential diagnosis usually includes chronic infections, parasitic infestations, sprue, tropical sprue, and lymphomas other than IPSID. Although the clinical, laboratory, and radiologic findings are pathognomonic, the final diagnosis is usually established by endoscopic biopsies and/or laparotomy. Upper gastrointestinal endoscopy shows abnormalities in the second, third, and fourth parts of the duodenum and upper jejunum in all patients except those with very early disease. Thickening, erythema, and nodularity of the mucosal folds are noted.16,17 As the disease progresses, tumors appear usually in the proximal small intestine and rarely in the stomach.
Clinical, laboratory, and radiologic findings in IPSID
Symptoms and signs . | Patients affected, % . |
|---|---|
| Abdominal pain | 80-100 |
| Diarrhea | 70-100 |
| Weight loss | 90-100 |
| Vomiting | |
| Early stage | 10 |
| Advanced stage | 30 |
| Low-grade fever | 30-50 |
| Abdominal masses | |
| Early stage | 20 |
| Advanced stage | 60 |
| Clubbing of fingers | 20-60 |
| Hepatomegaly, splenomegaly, or peripheral lymphadenopathy | 3-5* |
| Laboratory findings | |
| α heavy chain protein† | 40-100 |
| Low serum immunoglobulins and albumin | Common‡ |
| High alkaline phosphatase (intestinal isoenzyme) | Common‡ |
| Sugar and fat malabsorption | 60-80 |
| Hypocalcemia and hypomagnesemia | Common‡ |
| Mild to moderate anemia | 30-50 |
| Parasitic infestations, especially giardiasis | Very common‡ |
| Radiologic findings (barium) | |
| Malabsorption pattern, edema, and “postage-stamp appearance” of duodenal folds | Common early stage‡ |
| Multiple filling defects, ulceration, strictures, and enlarged mesenteric lymph nodes by CT scan | Common advanced stage‡ |
Symptoms and signs . | Patients affected, % . |
|---|---|
| Abdominal pain | 80-100 |
| Diarrhea | 70-100 |
| Weight loss | 90-100 |
| Vomiting | |
| Early stage | 10 |
| Advanced stage | 30 |
| Low-grade fever | 30-50 |
| Abdominal masses | |
| Early stage | 20 |
| Advanced stage | 60 |
| Clubbing of fingers | 20-60 |
| Hepatomegaly, splenomegaly, or peripheral lymphadenopathy | 3-5* |
| Laboratory findings | |
| α heavy chain protein† | 40-100 |
| Low serum immunoglobulins and albumin | Common‡ |
| High alkaline phosphatase (intestinal isoenzyme) | Common‡ |
| Sugar and fat malabsorption | 60-80 |
| Hypocalcemia and hypomagnesemia | Common‡ |
| Mild to moderate anemia | 30-50 |
| Parasitic infestations, especially giardiasis | Very common‡ |
| Radiologic findings (barium) | |
| Malabsorption pattern, edema, and “postage-stamp appearance” of duodenal folds | Common early stage‡ |
| Multiple filling defects, ulceration, strictures, and enlarged mesenteric lymph nodes by CT scan | Common advanced stage‡ |
Sources: Al-Saleem and Zardawi2 ; Ramot et al3 ; Bull4; Rambaud et al14 ; Fine and Stone18 ; Gilinsky et al20 ; Al-Bahranii et al22 ; Vassal et al23 ; Ramos et al.24
CT indicated computed tomography.
Rare except in very advanced disease
See “α Heavy chain protein: diagnosis, structure, synthesis, and secretion”
Exact numbers are not available
Pathology and evolution
The main pathologic feature of IPSID is the presence of dense mucosal infiltrate of “centrocyte-like” and many plasma cells involving long segments of the small bowel mucosa, predominantly the proximal parts.2,14 The overlying epithelial cells are usually intact, and the crypts are sparse (Figure 1C-D). Progression to higher grade large-cell lymphoplasmacytic and immunoblastic lymphoma is characterized by increased plasmacytic atypia with the formation of aggregates and later sheets of dystrophic plasma cells and immunoblasts invading into the submucosa and the muscularis propria. This large-cell component seems to evolve within the diffuse “low-grade” IPSID and is clonally related to it (Figure 1E-F).2,25 The rate of evolution of IPSID from low-grade to higher grade is not known. Most patients are diagnosed at the time of this transformation due to the severe symptoms of abdominal pain and obstruction. However, they usually have mild IPSID-related symptoms dating back up to 5 or even 10 years earlier. Patients with IPSID-associated large-cell lymphomas are 6 years older than those with pure IPSID, a statistically significant difference.2 Hepatic, splenic, or peripheral lymph node involvement are uncommon except in the late stages of disease. Bone marrow involvement and leukemic manifestations are rare.26,27
A hypothetical scheme for the pathogenesis and progression of immunoproliferative small intestinal disease and intestinal Burkitt lymphoma. Chronic C jejuni infection develops due to defective gut mucosal immunity. The cytolethal distending toxin of C jejuni may cause double-stranded DNA breaks in proliferating germinal center B cells that are even normally liable for mutations, deletions, and insertions. Truncated α heavy chain–producing plasma cells are selected due to the absence of variable determinants on the surface; they proliferate and progress resulting in IPSID. EBV infection in previously unexposed children may result in Burkitt lymphoma of the intestine. (A) Immunoselection, abnormal precipitation lines in patient's sera (1 and 2), and normal control showing precipitation around the trough (3). (B) Electron micrograph: IPSID neoplastic plasma cells with distended endoplasmic reticulum and intranuclear “inclusions” (Dutcher bodies). (C-D) Jejunal biopsies of early IPSID showing diffuse mucosal plasma cell proliferation with intact epithelium with sparse crypts (hematoxylin and eosin [H&E] stain). (E) Progressed IPSID mesenteric lymph node: lymphoplasmacytic and immunoblastic lymphoma (Giemsa stain). (F) Autopsy thickened intestinal wall along the whole length and enlarged mesenteric lymph nodes. The electron micrograph in panel B was taken with a Philips 100 electron microscope (Royal Philips Electronics, Eindhoven, Netherlands), original magnification × 2800. Light micrographs in panels C-E were taken with an Olympus BX50 microscope and an Olympus C-35AD4 camera (Olympus, Tokyo, Japan), at objectives of 10 ×/0.30 NA, 20 ×/0.50 NA, and 40 ×/0.75 NA (panels C-E, respectively). Images were processed with Adobe Photoshop (Adobe, San Diego, CA).
A hypothetical scheme for the pathogenesis and progression of immunoproliferative small intestinal disease and intestinal Burkitt lymphoma. Chronic C jejuni infection develops due to defective gut mucosal immunity. The cytolethal distending toxin of C jejuni may cause double-stranded DNA breaks in proliferating germinal center B cells that are even normally liable for mutations, deletions, and insertions. Truncated α heavy chain–producing plasma cells are selected due to the absence of variable determinants on the surface; they proliferate and progress resulting in IPSID. EBV infection in previously unexposed children may result in Burkitt lymphoma of the intestine. (A) Immunoselection, abnormal precipitation lines in patient's sera (1 and 2), and normal control showing precipitation around the trough (3). (B) Electron micrograph: IPSID neoplastic plasma cells with distended endoplasmic reticulum and intranuclear “inclusions” (Dutcher bodies). (C-D) Jejunal biopsies of early IPSID showing diffuse mucosal plasma cell proliferation with intact epithelium with sparse crypts (hematoxylin and eosin [H&E] stain). (E) Progressed IPSID mesenteric lymph node: lymphoplasmacytic and immunoblastic lymphoma (Giemsa stain). (F) Autopsy thickened intestinal wall along the whole length and enlarged mesenteric lymph nodes. The electron micrograph in panel B was taken with a Philips 100 electron microscope (Royal Philips Electronics, Eindhoven, Netherlands), original magnification × 2800. Light micrographs in panels C-E were taken with an Olympus BX50 microscope and an Olympus C-35AD4 camera (Olympus, Tokyo, Japan), at objectives of 10 ×/0.30 NA, 20 ×/0.50 NA, and 40 ×/0.75 NA (panels C-E, respectively). Images were processed with Adobe Photoshop (Adobe, San Diego, CA).
α Heavy chain protein: diagnosis, structure, synthesis, and secretion
The immunologic hallmark of IPSID is the presence of anomalous α heavy chain protein in the serum detected in 20% to 90% of patients.5,17,20,28-30 The higher detection rate reflects recent improvement in immunologic technique, especially the use of immunoselection.31,32 Immunoelectrophoresis into gel containing especially developed anti-Fab alpha serum provides the most sensitive and specific detection system for αHCD protein (Figure 1A). Alternatively, immunoselection is performed in 1% agarose gel incorporated with 30% vol/vol antikappa and antilambda antisera. Upon immunoelectrophoresis, normal immunoglobulin A (IgA) precipitates around the trough, while αHC protein migrates freely toward the anode producing various abnormal precipitation lines.31 In our own experience in Iraq, this latter immunoselection technique detected αHC protein in 42% of patients with a clinicopathologic picture of IPSID. This ratio was much higher (about 70%) if only the noninvasive low-grade–phase patients are considered (Table 2).32 In some apparently αHC-negative patients, the abnormality can be identified through immunohistochemical or immunofluorescence staining of the small bowel biopsies.33,34 These stains demonstrate positivity for αHC, while the light chain stains are negative.
Subtypes of 202 primary non-Hodgkin lymphomas of the small intestine in Iraq pathologically diagnosed by the senior author during a 13-year period (1973-1985): association with α heavy chains
Lymphoma type . | No. (%) . | No. α heavy chains +/no. tested (%)* . |
|---|---|---|
| IPSID | ||
| Early | 25 (12) | 13/19 (68) |
| Advanced† | 77 (38) | 17/52 (33) |
| Total | 102 (50) | 30/71 (42) |
| Non-IPSID | 25 (12) | 0/17 (0) |
| Burkitt | 73 (36) | 0/28 (0) |
| Intestinal T-cell | 2 (1) | 0/1 (0) |
Lymphoma type . | No. (%) . | No. α heavy chains +/no. tested (%)* . |
|---|---|---|
| IPSID | ||
| Early | 25 (12) | 13/19 (68) |
| Advanced† | 77 (38) | 17/52 (33) |
| Total | 102 (50) | 30/71 (42) |
| Non-IPSID | 25 (12) | 0/17 (0) |
| Burkitt | 73 (36) | 0/28 (0) |
| Intestinal T-cell | 2 (1) | 0/1 (0) |
Tested in the serum by immunoselection
IPSID-associated large-cell lymphoma
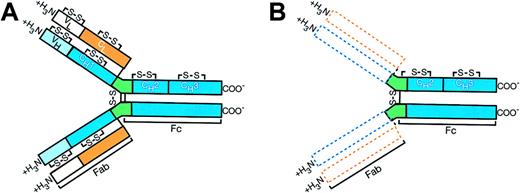
αHCD proteins are almost always α1 species and appear to consist largely of multiple polymers of different sizes.14 The molecular weight of the basic monomeric unit varies between 29 000 and 35 000. Allowance is usually made in these figures for carbohydrate, since the carbohydrate content of many of these αHCD proteins is unusually high. Thus, the length of the basic polypeptide subunit varies from patient to patient and in most instances it is between half and three fourths the size of its normal counterpart.35 Sequenced data showed that the αHCD protein lacked the variable heavy chain (VH) and the first constant domain. Normal sequence resumed at the beginning of the hinge region, with a valine residue corresponding to position 222 of a normal α1 chain. The carboxy terminal structure and conformational integrity remain intact.35,36 The αHCD protein has various deletions, insertions, and mutations similar to those observed in the much less common γ and μ heavy chain diseases (Figure 2).35
Alpha heavy chain protein molecular structure of IPSID (B) compared with normal IgA molecule (A). The dotted areas represent deleted variable and first constant regions of amino terminus of the heavy chain (blue) as well as missing entire light chain (orange). The normal structure of the immunoglobulin molecule resumes at the beginning of the hinge region (green). The carboxy terminal (COO-) portion of the polypeptide is intact. Joining chains, present in a majority of the IPSID α heavy chain proteins, are not shown in the diagram.16
Alpha heavy chain protein molecular structure of IPSID (B) compared with normal IgA molecule (A). The dotted areas represent deleted variable and first constant regions of amino terminus of the heavy chain (blue) as well as missing entire light chain (orange). The normal structure of the immunoglobulin molecule resumes at the beginning of the hinge region (green). The carboxy terminal (COO-) portion of the polypeptide is intact. Joining chains, present in a majority of the IPSID α heavy chain proteins, are not shown in the diagram.16
As expected, the αHCD messenger RNA lacks the VH and constant heavy chain 1 (CH1) sequences. It also contains an in-frame insert of unknown origin between the leader peptide and the normal CH2 and CH3 coding sequences.36,37 These inserts are of variable length (42 to 105 base pair [bp]), and they are unrelated to each other. Their structure suggests that they result from alternative splicing process. These sequences do not resemble any normal human genomic DNA. The absence of homology between these insertions could not support the hypothesis of infectious nonhuman DNA, either. They may represent highly altered sequences from human Ig locus. Since the amino acid sequence of αHCD proteins begins with a CH2 domain, it is most likely that the amino acid terminal sequence encoded by these insertions is cleaved intracellularly before secretion.38
The complete gene sequence encoding 3 αHCD proteins, MAL,37 YAO,39 and SEC has been determined.40 These 3 genes show a striking similarity in their position and extent of the 2 main deletions, which encompass sequences in the V/J and the switch/CH1 regions. In all cases, most, or all, of the V region is deleted as is the sequence starting in the switch region and extending through part, or all, of the CH1 domain.41 These findings are also similar to those present in the 2 γ heavy chain proteins gene sequenced (OMN42 and RIV43 ). Taken together, the analysis of γ and αHCD proteins and nucleic acids seems to show the emerging pattern of 2 large noncontiguous deletions in the heavy chain genes and the expression of low levels of light chain constant regions.42
Analysis of the DNA from IPSID tumors showed monoclonal heavy and light chain gene rearrangement even in the early stages of the disease.44 Southern blot analysis established that in all cases one or both κ genes were rearranged in tumor DNA, whereas the λ genes were in germ-line configuration. In some cases, the truncated mRNA was shorter than a normal kappa mRNA. This finding was interpreted as indicating the occurrence of genomic alterations in both heavy and light chain loci in HCD, as demonstrated by the analysis of the sequence of rearranged κ gene in a case of γ HCD.45 As a characteristic of α and γ HCD, there are no light chains detected in the serum or associated with the heavy chain fragments in most cases. Studies reported from various laboratories suggested that independent structural gene abnormalities are at least partially responsible for the uniform absence of detectable light chain production in HCD. In contrast to most normal and neoplastic Ig-producing cells, there is excess of heavy to light chain mRNA as well as protein. The elegant experiments by Teng et al41 demonstrated that this excess is a function of the cell independent of structural gene abnormality and is due to a low level of light chain transcription. Transcription can be increased by fusing the HCD cell line to murine myeloma cell line or transfecting the defective light chain gene into a murine plasma cell. Other findings suggested that the examined HCD cells either lack a transcription factor present in mature plasma cells or have a functional repressor of light chain transcription.41
The synthesis of αHCD protein by the proliferating cells has been demonstrated by immunofluorescence and/or immunohistochemical method and by biosynthesis studies.14,35 These studies and those of the membrane-bound Ig have shown that the immunoblastic cells in late-stage disease do not synthesize αHCD protein.46 In all cases studied, the αHCD protein was found in the jejunal juice when it was already present in the serum. αHC protein was found in the intestinal or gastric lumen in some cases, although it was undetectable in the sera of these patients in spite of the use of the most sensitive technique.14 The concentration of αHCD protein in urine is low and Bence Jones proteinuria has never been found. It was also noted that in rare cases the αHCD protein is absent from the serum, urine, and jejunal juice, but can be demonstrated by immunohistochemical staining of small bowel biopsies33 or that the Ig gene is rearranged by molecular studies.47 It has been demonstrated that gene deletions force “nonsecretory” αHCD plasma cells to produce membrane form αchain only.40 In the vast majority of αHCD, however, secretion of truncated αHC could be demonstrated by various techniques.
Normal mammalian α chains are 50 kDa and contain 1 variable and 3 constant region domains. Plasma cells in mucosal tissue assemble polymeric IgA intracellularly from monomeric IgA. Normal plasma IgA is monomeric, while mucosal IgA is dimeric or tetrameric. It contains joining (J) chains that help recognize the receptor (pIgR) expressed on basolateral surfaces of adjacent epithelial cells.48 Light chains have been shown to play a critical role in the Ig molecule secretions by the plasma cells.49 In αHCD mutations in both the heavy and the light chains seem to result in the secretions of the truncated α heavy chains by the neoplastic plasma cells in spite of the absence of light chains. J chains are present in the majority of αHCD proteins.16 However, the production of these truncated αHCD proteins probably outpace the synthesis of the secretory component by the enterocytes. This could be expected because in IPSID the crypts are atrophic and highly dispersed in contrast to celiac disease where they are hyperplastic (Figure 1C-D). Thus, large amounts of polymeric αHCD protein can usually be demonstrated in the serum and also in the jejunal and gastric fluids.14
Cytogenetics of IPSID
Although considered as a variant of MALT lymphoma, IPSID lacks the (11;18) chromosome translocation demonstrated in relatively high frequency in other MALT lymphomas, particularly those of the lung, stomach, conjunctiva, and orbit.50 However, other clonal cytogenetic abnormalities were demonstrated in the IPSID lymphoid cells of mesenteric lymph nodes, even in patients with early-stage disease limited to the mucosa. In one patient, rearrangement resulted from translocation (9;14) in 21 of 23 mitoses. The rearranged α1 gene fragment in that case was cloned and it was shown to contain chromosome 9 information by Southern blotting on sorted chromosome and by in situ hybridization.51 Further molecular studies identified this chromosomal translocation as involving the Pax5 gene, which was later demonstrated to be associated with some cases of lymphoplasmacytic lymphoma, too.50 Another chromosomal abnormality demonstrated in a single IPSID case was t(2;14) involving band p12 on chromosome 2 in the vicinity of the κ chain gene locus. Other clonal abnormalities demonstrated in αHCD were a case of t(5;9) and another case of 14q+ chromosome.52 In a patient with leukemic manifestation of αHCD, peripheral blood cytogenetics demonstrated an abnormal karyotype showing multiple reciprocal translocations including t(21;22) (q22;q11), which seems to be translocating the AML1 gene to the λ light chain locus.27 It is interesting also to note that the AML1 gene may be involved in some cases of multiple myeloma.53 Taken together, the relatively few cytogenetic studies in IPSID demonstrate clonal abnormalities involving the p32 heavy chain locus on chromosome 14 as well as the light chain loci on chromosomes 2 and 22, sharing features with both malignant lymphoma and immunoproliferative diseases.
Pathogenesis
The search for pathogenic factors was related to the well-known unique features of IPSID (its ethnic and geographic distribution as well its response to antibiotics) at least in the early stages. A striking association of IPSID with human leukocyte antigens (HLAs) AW19, A9, and B12, and the B blood group has been described.54,55 Genetic predisposition is further substantiated by the development of IPSID in relatives living apart55 and the presence of elevated intestinal isoenzyme alkaline phosphatase, a feature of IPSID in healthy family members of some patients with IPSID.54 Occult defects of the cellular and humeral immunities have been detected in first degree, but otherwise healthy, relatives of some patients with IPSID.16 It is difficult to decide whether these observations indicate a genetic predisposition or are due to shared environmental factors.
Recently, Lecuit et al26 demonstrated the presence of Campylobacter jejuni in the intestinal tissue obtained from a patient with IPSID who had a dramatic response to antibiotics. A follow-up retrospective analysis of archival intestinal biopsy specimen disclosed Campylobacter species in 4 of 6 additional patients with IPSID, using fluorescent in situ hybridization (FISH) and immunohistochemical techniques.13 However, this association has been challenged. C jejuni is an initiating factor in chronic autoimmune disease such as Guillan-Barre syndrome and reactive arthritis. It is not known to be a persistent colonizer of human gut,13 except in immunodeficient individuals.56 Besides, the temporal relation between C jejuni and IPSID is unknown.56
We believe that the association with C jejuni can fit into a plausible working hypothesis for the pathogenesis of IPSID (Figure 1). Patients with IPSID seem to have an acquired immune deficiency state in the humoral and cellular immunity. They usually have serum immunoglobulin levels lower than matched controls, more than what is expected from protein-losing enteropathy. They have impaired cellular immunity as demonstrated by low response to recall and sensitizing antigen, tuberculin, mumps, and dinitrochlorobenzene (DNCB).57 The proportion of T lymphocytes in IPSID patients is lower than normal, too.58 An in vitro study of the immunologic function of circulating mononuclear cells in 6 patients with IPSID who were in prolonged remission demonstrated varying degrees of defects in functions of B lymphocytes, T-suppressor cells, T-helper cells, and natural killer cells; 2 of these cases have been treated with tetracycline only.59 We have previously hypothesized that these immunologic defects may be attributed to the modulatory effects of Vibrio cholerae toxins acquired during the epidemic that swept the same IPSID geographic areas during the early 1960s.57 It is interesting to note that the reports on IPSID started to appear in these areas a few years after the Eltor cholera epidemic.
One can speculate, too, that in the presence of continuous antigenic stimulation within the gut and due to a presumed C jejuni infection, IgA-producing plasma cells proliferate in the lamina propria of the small intestine (Figure 1). Deletions and insertions develop at a high rate (approximately 6%) of somatic mutation introduced into rearranged VH region genes during normal development of germinal center B cells.60 Besides C jejuni, cytolethal distending toxin is known to induce double-stranded DNA breaks.61 Under these circumstances, occasional mutated B cells may develop and differentiate into aberrant plasma cells producing truncated αHC proteins. The absence of any variable region determinants on the surface of these mutated B cells may provide a selective advantage by eliminating idiotype and allowing the cells to escape the normal immune regulatory control.45 Continuing proliferation of these aberrant cells within the mucosa gives the clinicopathologic picture of αHCD. Although the initiating events are still not very clear, ongoing mutational events presumably involving Pax5 and/or other oncogenes may lead to a neoplastic progression into lymphoplasmacytic and immunoblastic lymphoma and to the full-blown lymphomatous phase of IPSID (Figure 1).
Treatment and outcome
Although spontaneous remissions occur in early stages, once established the untreated disease progresses relentlessly causing severe malabsorption and malnutrition. Early treatment is recommended to control the symptoms and hopefully slow or prevent progression of the disease.66 The Tunisian/French group published a small prospective study of 21 Tunisian patients with IPSID, all of whom underwent laparotomy and elaborate staging and investigative procedures (Table 3).30 Of these patients, 6 had early disease confined to the small bowel wall, 13 had advanced disease with tumor formation, and 2 were described as “intermediate.” The 6 patients with early-stage disease responded well to antibiotics (tetracycline or metronidazole and ampicillin/tetracycline), while the remaining 15 patients received anthracycline-based combination chemotherapy. The overall remission rate was 90 ± 12% at 2 years and 67 ± 25% at 3 years. All patients alive beyond 3.5 years were disease free. Another more recent small series from Turkey62 reported that tetracycline (1 g daily) alone in 7 early-stage patients yielded 71% complete remission rate and 43% 5-year disease-free survival (DFS) rate. The other 16 patients with intermediate or advanced disease received COPP (cyclophosphamide, vincristine, procarbazine, and prednisone) chemotherapy followed by tetracycline 1 g/d for 6 months. Of these 16 patients, 11 achieved complete remission (CR). The 5-year overall survival for the entire group was 70% and the 5-year disease-free survival for patients in CR was 75%. A treatment regimen was recommended by Rambaud and Halphen66 based on the review of about 100 well-documented cases from the literature including 18 of their own. They recommended first-line antibiotics, including tetracycline and metronidazole, for early-stage patients. Patients without marked improvement after a 6-month course of antibiotic or complete remission within 12 months should be given CHOP (cyclophosphamide, vincristine, adriamycin, and prednisone) chemotherapy. Chemotherapy was also recommended up front together with antibiotics for patients with advanced disease at presentation. The average overall CR rate using this regimen was around 50%, and the median survival was 67% at 3 years. This regimen also agrees with the clinical trials where anthracycline-containing regimen was found to be superior to nonanthracycline regimen.64 Relapses of the low-grade IPSID component may happen as expected and may be controlled by antibiotics alone.67 Whether maintenance antibiotic is necessary for a long period of time is not established. Life-long suppression of antigenic stimulus has been proposed.65 Response to antibiotic diminishes with increasing dysplasia of the lymphoplasmacytic cells and with tumorous infiltration of the bowel wall and the mesenteric lymph nodes. In addition to marked clinical improvement and histologic tumor regression by serial jejunal biopsies, response can be roughly quantitated by estimating the serum level of αHC protein. However, in some patients, large-cell transformation or relapse may be associated with a stable or even declining αHC protein levels.
Treatment options and response of IPSID
Stage of disease . | Treatment . | Overall response . |
|---|---|---|
| 1. Early bowel wall involvement; no visible tumor | 1. Antibiotics: tetracycline, 1 g/d for 6 mo16,17,19,22 | 30%-70% CR lasting months to several years |
| 2. Metronidazole plus ampicillin/tetracycline62 | 43% 5-y DFS | |
| 3. H pylori regimen for 7 d (1 patient)63 | 5+ mo | |
| 4. C jejuni treatment with H pylori regimen for 5 mo (1 patient)26 | 12+ mo | |
| 2. Advanced disease with bowel wall tumor formation with or without mesenteric node involvement | Anthracycline-based combination chemotherapy ± tetracycline30,64,65 | 50%-60% CR lasting months to years (60%-70% DFS at 3 y) |
| 3. Advanced bulky tumor with mechanical complications | Corrective surgery, palliative radiation therapy, combination chemotherapy16,19,22 | Partial response, few months to less than 1 y |
Stage of disease . | Treatment . | Overall response . |
|---|---|---|
| 1. Early bowel wall involvement; no visible tumor | 1. Antibiotics: tetracycline, 1 g/d for 6 mo16,17,19,22 | 30%-70% CR lasting months to several years |
| 2. Metronidazole plus ampicillin/tetracycline62 | 43% 5-y DFS | |
| 3. H pylori regimen for 7 d (1 patient)63 | 5+ mo | |
| 4. C jejuni treatment with H pylori regimen for 5 mo (1 patient)26 | 12+ mo | |
| 2. Advanced disease with bowel wall tumor formation with or without mesenteric node involvement | Anthracycline-based combination chemotherapy ± tetracycline30,64,65 | 50%-60% CR lasting months to years (60%-70% DFS at 3 y) |
| 3. Advanced bulky tumor with mechanical complications | Corrective surgery, palliative radiation therapy, combination chemotherapy16,19,22 | Partial response, few months to less than 1 y |
Surgery is usually reserved for a palliation of obstructing tumor masses or as a diagnostic and staging procedure. Total abdominal radiation has been recommended in bulky abdominal disease with some reported remissions.22 Unresponsive disease progresses relentlessly at a variable pace and most patients die of malnutrition, sepsis, intestinal obstruction, and other disabling complications secondary to massive involvement of the bowel and abdominal cavity by tumor. Intensive chemotherapy and autologous bone marrow transplantation was recommended for patients with advanced or refractory disease,14 but to our knowledge there are no reports in the literature demonstrating the utility of bone marrow or hematopoietic cell transplantation in IPSID.
Future investigations
We believe that the more recent discoveries in the molecular pathogenesis of IPSID raise some important questions that can be answered only by cooperative international studies. The real incidence and the geographic distribution of IPSID need to be better defined. Studies from the Middle East indicate a decline in the incidence of IPSID over the last 3 decades,68-70 a phenomenon that could not be totally explained by improved socioeconomic and hygienic conditions. International cancer registry comparative studies are needed to document this decline, and an attempt be made to explain it. The role of C jejuni and maybe other bacterial organisms including V cholerae needs more clinicopathologic and experimental studies. The contribution of double-strand DNA breaks caused by C jejuni toxin to carcinogenesis is an important topic for future studies, since that organism is relatively prevalent all over the world.
The frequent occurrence of deletions and duplications during somatic hypermutations occurring normally in the germinal center B cells has been implicated for oncogene translocations in Burkitt lymphoma as well as αHCD.60 Abdominal and intestinal Burkitt lymphomas are relatively common childhood tumors in the same geographic areas as IPSID. Malaria was eradicated in these areas for many years, thus the high frequency of Burkitt lymphoma cannot be blamed on endemic malaria as in Africa.71 More than 2 decades ago, we envisioned a common pathway for the pathogenesis of these 2 types of lymphoma with Burkitt lymphoma developing due to an Epstein-Barr virus (EBV) infection in children previously not exposed to EBV and IPSID developing due to another presumed infection in young adults immune to EBV (Figure 1). We speculate that chronic C jejuni infection can explain the high incidence of IPSID in young adults versus Burkitt lymphoma, which is EBV related in children. However, more studies are needed to clarify the mechanism(s) involved in IPSID and intestinal Burkitt lymphoma whether they are immunomodulatory and/or initiating events.
The role of Pax5 in the pathogenesis and progression of IPSID lymphoma is worth investigating, too. Pax5 gene encoding the transcription factor B-cell–specific activator protein (BSAP) is required for progression of B lymphopoiesis beyond the pro-B stage.72 Plasma cell differentiation involves repression of Pax5.73 Pax5 translocations and mutations are involved to a certain degree in the closely related lymphoplasmacytic lymphoma, as well as in multiple myeloma.50,74,75 Another interesting molecular feature of IPSID is the observation that the αHCD protein is restricted to α1 species. This can be attributed to the C jejuni antigenic drive. Another possibility could be related to the fact the α2 chains are quickly degraded resulting in the “nonsecretory” form αHCD.
New clinical trials are needed in light of the recent discoveries regarding C jejuni association with IPSID. Previous trials have not incorporated anti-CD20 (rituximab) in the management of IPSID. As expected, the centrocyte-like cells are CD20+, but the plasma cells are not.76,77 The role of rituximab in IPSID is worth investigating both in the early and the advanced large-cell IPSID. Besides, it would be interesting in light of the extreme plasma cell differentiation and the plasmacytic nature of the large-cell IPSID lymphoma to investigate a possible role for newer multiple myeloma therapies including proteasome inhibitors at least in refractory cases.78
We believe that further investigations of IPSID can yield additional information contributing to the understanding of the molecular biology as well as clinical aspects and managements of the mature B-cell neoplasms in general.
Prepublished online as Blood First Edition Paper, November 12, 2004; DOI 10.1182/blood-2004-07-2755.
The authors are grateful to Lori Schaffert for her skillful secretarial support.

![Figure 1. A hypothetical scheme for the pathogenesis and progression of immunoproliferative small intestinal disease and intestinal Burkitt lymphoma. Chronic C jejuni infection develops due to defective gut mucosal immunity. The cytolethal distending toxin of C jejuni may cause double-stranded DNA breaks in proliferating germinal center B cells that are even normally liable for mutations, deletions, and insertions. Truncated α heavy chain–producing plasma cells are selected due to the absence of variable determinants on the surface; they proliferate and progress resulting in IPSID. EBV infection in previously unexposed children may result in Burkitt lymphoma of the intestine. (A) Immunoselection, abnormal precipitation lines in patient's sera (1 and 2), and normal control showing precipitation around the trough (3). (B) Electron micrograph: IPSID neoplastic plasma cells with distended endoplasmic reticulum and intranuclear “inclusions” (Dutcher bodies). (C-D) Jejunal biopsies of early IPSID showing diffuse mucosal plasma cell proliferation with intact epithelium with sparse crypts (hematoxylin and eosin [H&E] stain). (E) Progressed IPSID mesenteric lymph node: lymphoplasmacytic and immunoblastic lymphoma (Giemsa stain). (F) Autopsy thickened intestinal wall along the whole length and enlarged mesenteric lymph nodes. The electron micrograph in panel B was taken with a Philips 100 electron microscope (Royal Philips Electronics, Eindhoven, Netherlands), original magnification × 2800. Light micrographs in panels C-E were taken with an Olympus BX50 microscope and an Olympus C-35AD4 camera (Olympus, Tokyo, Japan), at objectives of 10 ×/0.30 NA, 20 ×/0.50 NA, and 40 ×/0.75 NA (panels C-E, respectively). Images were processed with Adobe Photoshop (Adobe, San Diego, CA).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/6/10.1182_blood-2004-07-2755/6/m_zh80060575650001.jpeg?Expires=1764955994&Signature=zPNNVMgnUIbR7YKg5n6ITAHh1qluRFTxQ-2Fe4rkvjpY~lERXCFOe-W5yVKp4fVblUDInhV6K205uA4e2dLWKxSfRbY81czibkTs~CevsztM8M7znyaYYyLSC4dnDPwA4foRhV2AmFk8kpmuqD6W~rVP~H7Itmwi9gB7vi9oRBM-m6a~OWMIhrvrFaIE0pIG~OZS63ZAZhyPBDUGQQl8~RXTu8G8O6Y04buGQ4ccRkUh4KQc-oTmPefRcBZBmJK3~miROiJm4EuL0aE6iifAhF~qTkspHCBwjhpRmDCnqlOiEyQnByDDBxvTtpJwUTtrw~rhHwq-FDICjSa7Jzv1sQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal