Abstract
The tyrosine kinase inhibitor imatinib (imatinib, STI571, Glivec, and Gleevec) is increasingly used in patients undergoing allogeneic transplantation for leukemia. However, little is known regarding its potential immunoregulatory effects. Here, we investigate the effect of imatinib on T-cell receptor (TCR)–mediated activation of human T cells. Following stimulation with the anti-CD3 antibody 12F6, proliferation of activated T cells was almost completely inhibited by 10 μM imatinib. Furthermore, antigen-triggered expansion of CD8+ T cells in response to immunodominant cytomegalovirus (CMV) and Epstein-Barr virus (EBV) peptides was significantly reduced. Up-regulation of the activation markers CD25 and CD69 in response to TCR cross-linking was suppressed by imatinib at a mean inhibitory concentration 50% (IC50) of 5.4 μM and 7.3 μM, respectively; interleukin 2 (IL-2) production was also impaired. Analysis of the TCR-induced signaling cascade showed that imatinib substantially reduced tyrosine phosphorylation of ZAP70 and LAT in response to activation through the TCR. Sequence comparisons of all 90 tyrosine kinase genes in the human genome for homology in the adenosine triphosphate (ATP) binding pocket identified LCK, which is required for ZAP70 activation, as a likely target for imatinib. The IC50 for LCK inhibition by imatinib was 0.6 μM to 0.8 μM in an in vitro tyrosine kinase assay. In summary, imatinib can interfere with T-cell activation in vitro, and its impact on the frequency of opportunistic infections and graft-versus-host or graft-versus-leukemia reactions after transplantation should be investigated in clinical trials.
Introduction
Imatinib mesylate (imatinib, STI571, Glivec, and Gleevec; Novartis, Basel, Switzerland) is a potent selective inhibitor of the tyrosine kinases (TKs) ABL, ARG, PDGFR α and β, and c-KIT. It has proven clinical efficacy in the treatment of malignancies characterized by constitutive activation of these TKs: chronic myeloid leukemia (CML), Philadelphia chromosome–positive (Ph+) acute lymphocytic leukemia (ALL), myleoproliferative disorders due to chromosomal rearrangements in the PDGF-R locus and gastrointestinal stromal tumors (GIST) with mutations in c-KIT.1-5
Imatinib can induce reversible dose-dependent hematologic side effects, predominantly neutropenia and thrombocytopenia.3,6 In CML, this may in part be attributed to compromised normal hematopoiesis in addition to suppression of the BCR-ABL–positive clone that can dominate myelopoiesis. An additional mechanism may be inhibition of c-KIT in normal hematopoietic progenitor cells, which could account for the mild imatinib-induced myelosuppression observed in some patients with GIST.2 However, these mechanisms are unlikely to account fully for the observed lymphopenia and hypogammaglobulinemia in patients with CML on long-term imatinib therapy. In one study, 25% of patients with CML on 400 mg imatinib daily developed mild lymphopenia and a gradual reduction in serum immunoglobulin levels over 3 to 12 months of therapy.7 Another recent study found an inhibitory effect of imatinib on the development of progenitor cell–derived dendritic cells (DCs) and demonstrated that DCs exposed to imatinib were less potent at inducing primary cytotoxic T-cell reactions against tumor antigens and recall antigens in vitro.8 The molecular target of imatinib in DCs was not defined but a role for c-KIT inhibition appeared unlikely. This raises the possibility that imatinib could affect normal hematopoiesis and immune function through inhibition of additional TKs.
Effects of imatinib on T-cell activation and function have not been well defined. However, TKs play a prominent role in T-cell receptor (TCR) signal transduction and thus it is conceivable that imatinib may interfere with this process. Physiologic activation of T lymphocytes in response to antigen is controlled by the TCR.9,10 The TCR is comprised of α and β chains, the signaling subunits CD3 ϵ, γ, and δ chains, and TCR ζ. TCR binding to cognate foreign peptide bound to major histocompatibility complex (MHC) molecules on the surface of antigen-presenting cells (APCs) triggers a signaling cascade that includes activation of the TKs LCK and FYN.11 LCK phosphorylates the immunoreceptor tyrosine-based activation motifs (ITAMs) on the TCR subunits to which ZAP70 is recruited. LCK activates ZAP70, which in turn phosphorylates the adaptor protein linker of activated T cells (LAT) that serves as a key adaptor molecule controlling several important downstream activation events. ZAP70 is essential for TCR signal transduction, and ZAP70 deficiency has been shown to cause a severe immunodeficiency in humans and mice.12,13 The early TCR signaling events are enhanced by simultaneous engagement of the coreceptors CD8 and CD4 for MHC class I– and MHC class II–restricted antigens respectively, and, in some circumstances, by costimulatory molecules such as CD28 and integrin molecules. Functional consequences of T-cell activation include proliferation, cytokine production, degranulation, and up-regulation of activation markers such as CD25 and CD69.14
The aim of this study was to investigate the effects of imatinib on TCR-dependent T-cell activation. We show that imatinib reduces TCR-induced proliferation and activation at drug concentrations achieved in vivo, and provide evidence that imatinib inhibits the most proximal signal transduction events through an inhibitory effect on the SRC-family TK LCK.
Materials and methods
Imatinib
Imatinib mesylate was extracted from a commercially available 400 mg imatinib mesylate tablet (Gleevec; Novartis, Basel, Switzerland). Identity and purity were established by nuclear magnetic resonance spectroscopy, mass spectroscopy, and elemental analysis. The melting point found (214°C-215°C) was in accordance with the published melting point (217°C).15 Imatinib mesylate was dissolved in dimethyl sulfoxide (DMSO) at 10 mM stock and used at 1, 5, 10 or 25 μM. The biologic activity of extracted imatinib was tested in a cell death titration assay on BA/F3 bcr-abl and BA/F3 tel-abl cells.4
Cell culture, stimulation, and flow cytometry
Jurkat T cells and primary human T cells from healthy blood donors were cultured in RPMI 1640 (Life Technologies, Gaithersburg, MD) containing 10% fetal calf serum (FCS), 2 mM l-glutamine, 50 U/mL penicillin, and 50 μg/mL streptomycin (R10). Primary T cells were purified by Ficoll-Hypaque density gradient centrifugation, followed by negative selection enrichment using magnetic beads coated with an antibody mix of CD19, CD14, CD56, CD16, and CD15 (Miltenyi Biotec, Auburn, CA). CD3+ T cells were 86% plus or minus 8% pure, as determined by flow cytometry. After overnight rest, the cells were incubated for one hour with or without imatinib and then stimulated with 50 ng/mL anti–human CD3 antibody 12F6.16 Phytohemagglutinin (PHA) and staphylococcal enterotoxin B (SEB) were obtained from Sigma (St Louis, MO) and used at 5 μg/mL and 10 μg/mL, respectively. DMSO diluted 1:10 in R10 was included as a negative control in functional assays. The activation level of the T cells was evaluated by CD25–allophycocyanin (APC)/CD69-phycoerythrin (PE) expression analysis after 6 hours and 24 hours of stimulation with 50 ng/mL 12F6 with or without imatinib. Untreated T cells served as negative controls in all experiments. Peripheral blood mononuclear cells (PBMCs) were collected from healthy volunteer donors under the National Institutes of Health (NIH) Department of Transfusion Medicine approved protocol no. 99-CC-0168 after informed consent was obtained.
All antibodies for flow cytometry were obtained from BD Pharmingen (San Diego, CA). Stained samples were collected on a 4-color FACS Calibur flow cytometer (BD Immunocytometry Systems, San Diego, CA). List mode data files were analyzed using FlowJo software (Tree Star, San Carlos, CA). In all cases at least 150 000 events were collected for analysis. Gates were set on live cells and on CD3+ cells to evaluate proliferative response to 12F6, SEB, or PHA, and on CD8+ T cells to evaluate responses to cytomegalovirus (CMV) or Epstein-Barr virus (EBV) peptides. The percentage of dividing cells as well as the average number of cell divisions was calculated using proliferation platform of FlowJo.
Apoptosis assay
T cells (2 × 106/mL) were stimulated with 50 ng/mL 12F6 with or without 1, 5, 10 or 25 μM imatinib for 96 hours after 24 hours of rest in R10 media, harvested, and stained with annexin V–PE (incubation in the dark) and propidium iodide (PI). Apoptotic cells were defined by flow cytometry as PI-negative and annexin V–positive.
CFSE proliferation assay
Purified human T cells were suspended in phosphate-buffered saline (PBS; 1 × 106/mL) and labeled with the vital dye carboxyfluorescein diacetate succinimidyl ester (CFSE) at a final concentration of 0.25 μM (Molecular Probes, Eugene, OR) for 7 minutes at 37°C. After labeling, the cells were washed 3 times in FCS-rich medium and resuspended in R10 (2 × 106 cells/mL). After 24 hours of rest, CFSE-labeled cells were preincubated with imatinib for one hour as indicated, and then cultured for 96 hours in the presence of 50 ng/mL 12F6.
Activation of epitope-specific CD8+ T cells with CMV and EBV peptides
T cells from HLA A2+ CMV IgG seropositive healthy donors were MACS-sorted and CFSE-labeled as described above. Purified cells were stimulated with 2 μM of the HLA A2–restricted CMV peptide pp65495-503 with the amino acid sequence NLVPMVATV (Beckman Coulter, Miami, FL)17 or with 2 μM of the HLA A2–restricted EBV peptide BMLFI259-267 with the amino acid sequence GLCTLVAML over 4 days18,19 in the presence of 1 μg/mL antibodies against the costimulatory molecules CD28 and CD49d (BD Pharmingen). Proliferation of CMV- or EBV-specific CD8+ cells was determined after 4 or 8 days of culture by staining with CD8–peridinin chlorophyll protein (PerCP) antibody and HLA A2–CMV pp65495-503 peptide tetramer PE or HLA A2–EBV peptide BMLFI259-267 tetramer as described previously.20 CMV and EBV tetramers were produced as described.21
IL-2 ELISA
Purified T cells (2.5 × 105/well) were plated in 96-well plates with or without 10 μM imatinib for 24 hours, and stimulated with 100 ng/mL 12F6 in the presence or absence of antibodies against costimulatory molecules CD28 and CD49d. Interleukin 2 (IL-2) production was measured with a commercially available enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions (OptEIA Human IL-2; BD Biosciences).
Analysis of TCR signal transduction in Jurkat cells
Jurkat T cells (1 × 107/mL) were incubated for one hour with imatinib and stimulated for 5 minutes with 100 ng/mL 12F6 or the alternative anti-CD3 antibody OKT-3 (mouse ascites 1:250, a kind gift of B.E. Bierer, NHLBI).22 Cells were lysed in 1 mL lysis buffer containing 1% Triton X-100. Protein concentrations were adjusted by Bradford assay. For each sample, 20 μg protein was loaded on an acrylamide gel and transferred to nitrocellulose. For immunoprecipitation, 4 μg of a mouse monoclonal antibody to ZAP70 (clone 2F3.2; Upstate Biotechnology, Lake Placid, NY) was added to 500 μg protein and captured using protein G-beads. Western blots were incubated with indicated antibodies in PBS with 4% milk. Antibodies were as follows: to ZAP70 as above, rabbit polyclonal antihuman to phosphorylated LAT Tyr171 and Tyr191 mixed 1:1 (Cell Signaling Technology), to total LAT (clone 2E9; Upstate Biotechnology), mouse monoclonal IgG2b antibodies to phosphotyrosine clones PY20 and PY99 mixed 1:1 (Santa Cruz Biotechnology), and to γ-tubulin (GTU-88; Sigma). Blots were developed by chemiluminescence (Amersham, Piscataway, NJ).
LCK tyrosine kinase assay
The Kinase-Glo Luminescent Kinase Assay (Promega, Madison, WI) was used to determine the direct effects of imatinib on LCK tyrosine kinase activity. In this assay, the ATP concentration, which decreases with increasing tyrosine kinase activity, is measured as relative light units (RLUs). Serial 4-fold dilutions of imatinib, starting at 100 μM, were prepared in solid white 96-well plates. Reaction conditions were as follows: purified LCK kinase 4 mU/well (Upstate Biotechnology), rATP 3 μM/well and PTK peptide 2 substrate V288A 250 μM/well (Promega), in kinase reaction buffer (8 mM imidazole hydrochloride [pH 7.3], 8 mM β-glycerophosphate, 200 μM EGTA, 20 mM MgCl2, 1 mM MnCl2, and 0.1 mg/mL bovine serum albumin [BSA]).
Statistical analyses
Statistical significance was determined by Student t test, matched pairs Wilcoxon-Alrich test, or analysis of variance (ANOVA). The mean inhibitory concentration 50% (IC50) was calculated using Graph Pad Prism software (sigmoidal dose response, variable slope). Percentage of dividing cells and average number of cell divisions were calculated using the proliferation platform of FlowJo. Homology search for the ATP binding pocket was carried out using Blast search, and sequences were aligned using Multalin software (http://prodes.toulouse.inra.fr/multalin/multalin.html).23
Results
Imatinib inhibits T-cell proliferation induced by CD2 and TCR signaling
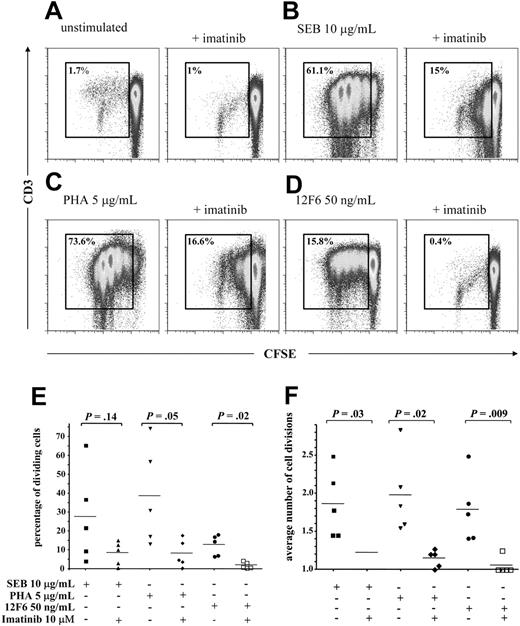
To investigate the effect of imatinib on T-cell proliferation, we cultured human peripheral blood T cells from healthy donors with phytohemagglutinin (PHA), which activates through the CD2 receptor, the TCR superantigen staphylococcal enterotoxin B (SEB), which induces a Vβ–restricted T-cell response, or the monoclonal antibody 12F6, which directly activates the CD3 component of the TCR complex. To visualize cell proliferation, we labeled the cells with the vital dye CFSE. This dye is retained in the cytoplasm and diluted out with each cell division, and thus allows tracking of cell divisions in cultured cells by flow cytometry. All mitogens induced proliferation in all donors, and a majority of cells underwent multiple cell divisions in the 4-day test period. Imatinib at 1 μM and 5 μM reduced proliferation in response to all mitogens (data not shown) and at 10 μM inhibited T-cell proliferation on average by more than 75% (Figure 1A-D); notably, 12F6-induced T-cell proliferation was almost completely inhibited at this latter concentration. Both the overall percentage of cells undergoing cell division (Figure 1E) and the average number of divisions completed (Figure 1F) were significantly reduced. We focused our subsequent analyses on the TCR pathway and used 12F6 because of its ability to initiate TCR signaling directly and reproducibly.
Imatinib inhibits T-cell proliferation. Purified CD3+ T cells were labeled with 0.25 μM CFSE, preincubated for one hour in 10 μM imatinib, then stimulated for 4 days. A representative experiment of 5 is shown in panels A-D; gates are set on the dividing cell population. (A) Effect of imatinib on unstimulated T cells, (B) on T cells stimulated through the TCR Vβ chain with 10 μg/mL SEB, (C) on T cells stimulated through the CD2 receptor with 5 μg/mL PHA, and (D) on T cells stimulated through the CD3/TCR complex with 50 ng/mL 12F6. The effect of imatinib at 10 μM on (E) the fraction of dividing T cells and on (F) the average number of cell divisions of stimulated T cells is shown for 5 independent experiments. Horizontal bars indicate means. Fraction of dividing T cells and average number of cell divisions were calculated using proliferation platform of FlowJo.
Imatinib inhibits T-cell proliferation. Purified CD3+ T cells were labeled with 0.25 μM CFSE, preincubated for one hour in 10 μM imatinib, then stimulated for 4 days. A representative experiment of 5 is shown in panels A-D; gates are set on the dividing cell population. (A) Effect of imatinib on unstimulated T cells, (B) on T cells stimulated through the TCR Vβ chain with 10 μg/mL SEB, (C) on T cells stimulated through the CD2 receptor with 5 μg/mL PHA, and (D) on T cells stimulated through the CD3/TCR complex with 50 ng/mL 12F6. The effect of imatinib at 10 μM on (E) the fraction of dividing T cells and on (F) the average number of cell divisions of stimulated T cells is shown for 5 independent experiments. Horizontal bars indicate means. Fraction of dividing T cells and average number of cell divisions were calculated using proliferation platform of FlowJo.
Imatinib does not induce apoptosis in T cells
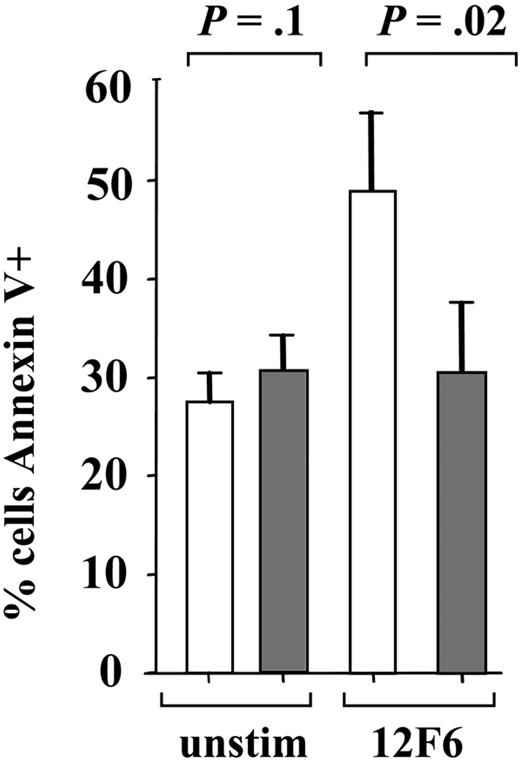
To investigate whether induction of apoptosis contributes to the reduced proliferation of imatinib-treated T cells, we measured apoptosis by staining with annexin V and propidium iodide (Figure 2). In unstimulated cells, imatinib at concentrations up to 25 μM did not increase apoptosis compared with untreated cells, but at this high concentration there was a clear increase in nonapoptotic cell death that was not evident at concentrations of 10 μM or below. Stimulation of normal human T cells in vitro is commonly associated with increased apoptosis, described as the phenomenon of activation-induced cell death (AICD). After stimulation with 12F6, we found that up to 50% (57.4% ± 33.8%) of primary T cells became annexin V–positive after 96 hours in culture as compared with 30% of the unstimulated cells (34.1% ± 12.1%). Consistent with an inhibitory effect on TCR signal transduction, imatinib at 10 μM abrogated 12F6-induced AICD (P = .02).
Imatinib does not increase apoptosis. Apoptosis was assessed by annexin V staining of T cells cultured for 4 days under the conditions indicated. 12F6 (50 ng/mL) stimulation (□) and imatinib (10 μM) pretreatment (▦) was performed as in Figure 1. Mean and standard deviation for 4 independent experiments are shown.
Imatinib does not increase apoptosis. Apoptosis was assessed by annexin V staining of T cells cultured for 4 days under the conditions indicated. 12F6 (50 ng/mL) stimulation (□) and imatinib (10 μM) pretreatment (▦) was performed as in Figure 1. Mean and standard deviation for 4 independent experiments are shown.
Imatinib decreases TCR-mediated T-cell activation
We next investigated whether imatinib influenced TCR-dependent T-cell activation. Upon activation, T cells up-regulate expression of the cell surface molecules CD69 and CD2514 and secrete cytokines, including IL-2. We analyzed expression of CD69 and CD25 by flow cytometry on purified human peripheral blood T cells at 6 hours and 24 hours following activation by 12F6 with or without imatinib treatment (Figure 3). Expression of both markers in resting T cells was low and was unaffected by imatinib. Up-regulation of the early T-cell activation marker CD69 was significantly inhibited by imatinib at concentrations as low as 1 μM (P = .0012) and completely abrogated at 25 μM (Figure 3); IC50 was 7.3 μM. The effect on CD69 was apparent 6 hours after stimulation and persisted at 24 hours. In contrast, 12F6-induced expression of CD25 was only seen in a subset of T cells, and became apparent only after 24 hours of incubation. However, the inhibitory effect of imatinib on CD25 expression was comparable to its effect on CD69 with significant inhibition of CD25 up-regulation detectable at 1 μM (P = .005) and an IC50 of 5.4μM. We also found comparable inhibition of CD69 up-regulation in 2 donors stimulated simultaneously through both CD3/TCR and the costimulatory molecules CD28 and CD49d (data not shown).
Imatinib inhibits 12F6-induced CD69 and CD25 up-regulation in a dose-dependent manner. Untreated T cells (□) and T cells preincubated with imatinib ( ) at 1, 5, 10, or 25 μM for one hour as indicated and then stimulated for 24 hours with 12F6 at 50 ng/mL. The mean values and standard deviations of 5 independent experiments are shown. (A) Percentage of cells expressing CD69 above isotype control and (B) percentage of cells expressing CD25 above isotype control.
) at 1, 5, 10, or 25 μM for one hour as indicated and then stimulated for 24 hours with 12F6 at 50 ng/mL. The mean values and standard deviations of 5 independent experiments are shown. (A) Percentage of cells expressing CD69 above isotype control and (B) percentage of cells expressing CD25 above isotype control.
Imatinib inhibits 12F6-induced CD69 and CD25 up-regulation in a dose-dependent manner. Untreated T cells (□) and T cells preincubated with imatinib ( ) at 1, 5, 10, or 25 μM for one hour as indicated and then stimulated for 24 hours with 12F6 at 50 ng/mL. The mean values and standard deviations of 5 independent experiments are shown. (A) Percentage of cells expressing CD69 above isotype control and (B) percentage of cells expressing CD25 above isotype control.
) at 1, 5, 10, or 25 μM for one hour as indicated and then stimulated for 24 hours with 12F6 at 50 ng/mL. The mean values and standard deviations of 5 independent experiments are shown. (A) Percentage of cells expressing CD69 above isotype control and (B) percentage of cells expressing CD25 above isotype control.
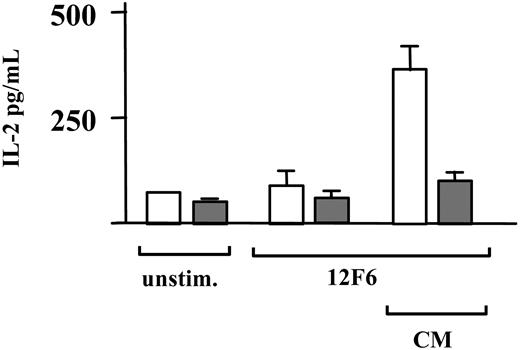
Induction of IL-2 secretion in naive T cells is dependent on signals mediated through both the TCR and costimulatory receptors such as CD28, the receptor for the B7 family of costimulatory molecules, and CD49d, the receptor for integrin alpha. Therefore, we stimulated T cells with 12F6 in the presence or absence of activating antibodies against CD28 and CD49d, and measured IL-2 in the cell culture supernatant 24 hours after induction. The IL-2 produced under these conditions was almost completely suppressed by imatinib at 10 μM (Figure 4).
Imatinib inhibits 12F6-induced IL-2 production. Untreated CD3+ T cells (□) and CD3+ T cells pretreated with 10 μM imatinib (▦) for one hour were incubated for 24 hours either unstimulated or stimulated with 12F6 (100 ng/mL), with or without activation of costimulatory receptors using antibodies against CD28 and CD49d at 1 μg/mL (CM). Means for T cells from one donor assayed in triplicate are shown with standard deviation. Comparable results were obtained in a second donor.
Imatinib inhibits 12F6-induced IL-2 production. Untreated CD3+ T cells (□) and CD3+ T cells pretreated with 10 μM imatinib (▦) for one hour were incubated for 24 hours either unstimulated or stimulated with 12F6 (100 ng/mL), with or without activation of costimulatory receptors using antibodies against CD28 and CD49d at 1 μg/mL (CM). Means for T cells from one donor assayed in triplicate are shown with standard deviation. Comparable results were obtained in a second donor.
CMV- and EBV-specific CD8+ T-cell responses are suppressed by imatinib
To test whether imatinib could also inhibit TCR stimulation by cognate antigen, we studied the responses of CD8+ T cells specific for the HLA A2–restricted peptides CMV pp65495-503 and EBV BMFLI259-267.17-19,24 These antigens are well characterized and clinically relevant; they are also typically immunodominant in the context of the respective infections. Initially, we used HLA-peptide tetramers, which allow direct visualization of antigen-specific T lymphocytes, to identify donors with a detectable CMV-specific CD8+ T-cell population. We then stimulated purified T cells from these donors with the CMV peptide pp65495-503 and followed proliferation with the vital dye CFSE over a period of 4 days (2 donors) and 8 days (6 donors). Specific CD8+ T cells from all 8 donors proliferated in response to the CMV peptide. In 4 of 6 donors we found that imatinib reduced the proliferative response in a dose-dependent manner over 8 days (Figure 5A). Similar results were obtained in assays conducted over 4 days in the other 2 donors; representative data are shown in Figure 5B. To confirm that this observation was generally applicable and not limited to the CD8+ T-cell response to CMV, we repeated the experiment in one donor with a CD8+ T-cell response to the EBV-derived peptide BMFLI259-267. We found comparable inhibition of specific CD8+ T-cell expansion in response to this antigen (Figure 5C). However, in the 2 donors with the largest CMV-specific CD8+ T-cell populations (1% and 2.9% of total CD8+ T cells as defined by tetramer staining), imatinib was unable to inhibit expansion. This is in contrast to donors with CMV-specific CD8+ T-cell populations in the range of 0.2% to 0.6%. The basis for this difference is unclear but might relate to increased cross-stimulation in these large populations or to the relative distribution of central memory and effector CD8+ T cells resulting in different thresholds for CD3/TCR-induced proliferation between these individuals.25
Imatinib inhibits virus-specific T-cell proliferation in response to cognate antigen. (A) T cells from healthy HLAA2+ human blood donors with tetramer-defined CD8+ T-cell responses to CMV pp65495-503 were purified, CFSE-labeled, and kept in culture for 8 days in the presence of CMV peptide and antibodies against the costimulatory molecules CD28 and CD49d without imatinib (dashed line) or with imatinib at 5 μM (dotted line) or 10 μM (solid line). Data presented are overlaid histograms representing percentage fluorescence intensity of CFSE-labeled HLA A2–CMV pp65495-503 tetramer-positive CD8+ T cells (donors 1-3 [d1-3]) and CMV tetramer-positive CD3+ T cells (donor 4 [d]). The y-axis is scaled to 100% defined by the highest value on the y-axis for each condition. (B) Effects of imatinib on expansion of CMV-specific CD8+ T cells over a 4-day period in response to cognate peptide. (C) Effects of imatinib on expansion of EBV-specific CD8+ T cells over a 4-day period in response to cognate peptide.
Imatinib inhibits virus-specific T-cell proliferation in response to cognate antigen. (A) T cells from healthy HLAA2+ human blood donors with tetramer-defined CD8+ T-cell responses to CMV pp65495-503 were purified, CFSE-labeled, and kept in culture for 8 days in the presence of CMV peptide and antibodies against the costimulatory molecules CD28 and CD49d without imatinib (dashed line) or with imatinib at 5 μM (dotted line) or 10 μM (solid line). Data presented are overlaid histograms representing percentage fluorescence intensity of CFSE-labeled HLA A2–CMV pp65495-503 tetramer-positive CD8+ T cells (donors 1-3 [d1-3]) and CMV tetramer-positive CD3+ T cells (donor 4 [d]). The y-axis is scaled to 100% defined by the highest value on the y-axis for each condition. (B) Effects of imatinib on expansion of CMV-specific CD8+ T cells over a 4-day period in response to cognate peptide. (C) Effects of imatinib on expansion of EBV-specific CD8+ T cells over a 4-day period in response to cognate peptide.
Imatinib inhibits proximal signal transduction components of the CD3-TCR complex and decreases ZAP70 and LAT phosphorylation
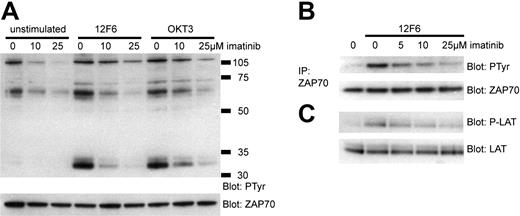
The effects of imatinib on T-cell proliferation and activation could be due to the inhibition of proximal signaling events mediated by the TCR, or due to a more global inhibition of cellular functions downstream of the initial signaling events. To investigate whether imatinib could inhibit TCR signal transduction we used the well-characterized Jurkat T-cell line. We stimulated Jurkat cells in the presence or absence of imatinib with 12F6 and OKT3, a second monoclonal antibody directed against CD3. Imatinib inhibited constitutive phosphorylation of proteins in the 120 kDa and 65 kDa range in the Jurkat T-cell line, especially at higher concentrations (Figure 6A). After 5 minutes of stimulation with 12F6 or OKT3, there was a rapid and strong induction of phosphotyrosine proteins, most notably in the 70 kDa and 35 kDa range (Figure 6A). We hypothesized that these proteins might correspond to ZAP70, the essential TK for TCR signal transduction, and to LAT. To determine the lowest imatinib concentration needed to inhibit activation of ZAP70 and LAT (linker for T-cell activation), we stimulated Jurkat cells exposed to increasing concentrations of imatinib with 12F6 and analyzed the amount of tyrosine phosphorylated ZAP70 and LAT by specific immunoprecipitation and immunoblotting (Figure 6B-C). We found a dose-dependent decrease in the activation of these key-signaling molecules with a major reduction of tyrosine phosphorylation already evident at 5 μM imatinib. Thus, the observed effects of imatinib on the proximal TCR signaling events correlate with the observed functional impairments of imatinib-treated normal human T cells.
Imatinib inhibits signal transduction through TCR. Jurkat T cells were stimulated with 12F6 (100 ng/mL) or OKT3 (mouse ascites 1:250) for 5 minutes in the presence or absence of imatinib. (A) Whole-cell lysates were analyzed by Western blotting for tyrosine phosphorylation (PTyr). To control for protein loading, the antiphosphotyrosine antibody was inactivated and the membrane was reprobed with ZAP70 antibody. (B) ZAP70 was immunoprecipitated from 500 μg whole-cell lysate. Immunoblotting was performed with anti-ZAP70 or antiphosphotyrosine antibodies. (C) Whole-cell lysates were analyzed for LAT activation by immunoblotting for phosphorylated LAT (Tyr 171 and Tyr 191) and for total LAT as indicated.
Imatinib inhibits signal transduction through TCR. Jurkat T cells were stimulated with 12F6 (100 ng/mL) or OKT3 (mouse ascites 1:250) for 5 minutes in the presence or absence of imatinib. (A) Whole-cell lysates were analyzed by Western blotting for tyrosine phosphorylation (PTyr). To control for protein loading, the antiphosphotyrosine antibody was inactivated and the membrane was reprobed with ZAP70 antibody. (B) ZAP70 was immunoprecipitated from 500 μg whole-cell lysate. Immunoblotting was performed with anti-ZAP70 or antiphosphotyrosine antibodies. (C) Whole-cell lysates were analyzed for LAT activation by immunoblotting for phosphorylated LAT (Tyr 171 and Tyr 191) and for total LAT as indicated.
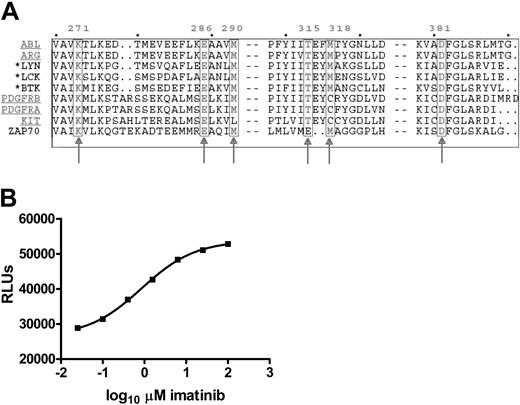
Some cytoplasmic TKs involved in immunoreceptor signaling share sequence homologies in the ATP binding pocket with known imatinib targets
Six amino acids in the ATP binding pocket of ABL have been identified as crucial for imatinib binding.26 These amino acid residues are highly conserved in the known imatinib-sensitive TKs. To identify additional TKs that might be sensitive to imatinib, we searched all 90 TKs in the human genome for homology in the ATP binding pocket with the 5 known imatinib targets. Thirteen TKs were identified, including the known imatinib targets. The additional TKs belong to the SRC (LCK, LYN, SRC, and FYN), FRK (FRK), and TEC (BTK, BMX, and TEC) families, which are phylogenetically related to ABL (Figure 7A). Interestingly, 4 of these potentially imatinib-sensitive TKs (ie, LCK, FYN, LYN, and BTK) are important for signaling by immunoreceptors both in T and B cells.
Homology of the ATP binding pocket of known imatinib-sensitive and selected TKs important for immunoreceptor signaling. (A) Protein sequences of the ATP binding pockets of the known imatinib-sensitive TKs (underlined), and related kinases important for immunoreceptor signaling were aligned using Multalin software. The 6 amino acid residues known to interact directly with imatinib are boxed and numbered as they appear in ABL (arrows). Three TKs important for immunoreceptor signaling that share homology in all residues, including the critical threonine 315, are highlighted by an asterisk. ZAP70 has been added for comparison. (B) LCK in vitro tyrosine kinase assay with serial 4-fold dilutions of imatinib from 100 μM to 0.025 μM, given on the x-axis in logarithmic scale. The ATP concentration, which decreases with increasing tyrosine kinase activity, is measured as relative light units (RLUs), shown on the y-axis. Values are mean of triplicates. IC50 for imatinib is 0.8 μM(R2 = 0.99).
Homology of the ATP binding pocket of known imatinib-sensitive and selected TKs important for immunoreceptor signaling. (A) Protein sequences of the ATP binding pockets of the known imatinib-sensitive TKs (underlined), and related kinases important for immunoreceptor signaling were aligned using Multalin software. The 6 amino acid residues known to interact directly with imatinib are boxed and numbered as they appear in ABL (arrows). Three TKs important for immunoreceptor signaling that share homology in all residues, including the critical threonine 315, are highlighted by an asterisk. ZAP70 has been added for comparison. (B) LCK in vitro tyrosine kinase assay with serial 4-fold dilutions of imatinib from 100 μM to 0.025 μM, given on the x-axis in logarithmic scale. The ATP concentration, which decreases with increasing tyrosine kinase activity, is measured as relative light units (RLUs), shown on the y-axis. Values are mean of triplicates. IC50 for imatinib is 0.8 μM(R2 = 0.99).
Imatinib inhibits LCK activity directly in vitro
LCK is the first tyrosine kinase activated by TCR signaling and, as such, assumes a pivotal role in the transduction of extracellular recognition events into cellular effector functions. To confirm the predictions based on our sequence homology search, we tested the direct effect of imatinib on LCK in an in vitro tyrosine kinase assay. Imatinib inhibited LCK activity with an IC50 of 0.6 μM to 0.8 μM in 2 independent assays (R2 = 0.99; Figure 7B), and almost complete inhibition was evident at 10 μM imatinib.
Discussion
Imatinib is a potent tyrosine kinase inhibitor with impressive single agent activity that is now increasingly used as an adjunct in the allogeneic transplant setting for leukemia. We report here that imatinib inhibits primary T-cell proliferation and activation in response to TCR engagement in vitro. Importantly, imatinib also reduced expansion of primary cytotoxic T cells in response to their cognate CMV or EBV antigen. These effects were dose-dependent with some inhibition of proliferation detectable at 1 μM, which corresponds to the mean steady-state level achieved after daily dosing with 400 mg imatinib. Proliferation was almost completely suppressed at 10 μM. No maximum tolerated dose has been established for imatinib, and doses up to 500 mg twice daily have been used in several studies.3,27 The mean peak plasma concentration after a single administration of 400 mg is 4 μMto 5 μM, but can reach 7.5 μM at steady state with 400 mg administered twice daily.27
Our results extend a recent study that analyzed effects of imatinib on T-cell proliferation induced by PHA, which reported an IC50 for proliferation of 3.9 μM. We confirmed that inhibition of proliferation at these concentrations is not due to the induction of apoptosis. In contrast to the study by Dietz et al28 however, we found that imatinib suppressed T-cell activation in addition to proliferation. The up-regulation of CD25 and CD69 was significantly inhibited by imatinib at 1 μM with IC50 concentrations of 5.4 μM and 7.3 μM, respectively, while no inhibition of these markers was detectable in the Dietz study. This discrepancy is likely due to the different signaling pathways studied. Dietz and coworkers stimulated the T cells using PHA that activates CD2, whereas we used an anti-CD3 monoclonal antibody to stimulate the TCR, a more physiologic stimulus. Consistent with an inhibitory effect of imatinib on T-cell activation, we also found that imatinib reduced IL-2 production by activated T cells.
Under conditions that reproduce physiologic stimulation of T cells, we found that imatinib inhibits proliferation of CMV-specific CD8+ T cells stimulated by the clinically relevant HLA A2–restricted CMV peptide pp65495-503. To identify the CMV-specific T-cell population, we used an HLA-peptide tetramer as an affinity reagent. HLA-peptide tetramers have been widely applied to the study of human and murine immune responses and showed that the CD8+ cytotoxic T-lymphocyte (CTL) response to CMV was among the strongest immune responses studied.29 The in vitro assays may not completely reproduce in vivo conditions, but the dose-dependent inhibition of CMV-specific proliferation argues for interference of imatinib with clinically important T-cell effector functions. Expansion of specific CD8+ CTLs is important in clearance and control of viral infections. Several studies found an increased risk of CMV reactivation in patients with incomplete T-cell recovery after allogeneic stem cell transplantation, and a reduced CD8+ CTL response to CMV correlates with recurrent CMV reactivation in the first 100 days after transplantation and predicts reduced disease-free survival.30-37
To test whether imatinib inhibits early TCR signaling directly, we analyzed tyrosine phosphorylation of the early key signal transduction components ZAP70 and LAT. In agreement with the inhibitory effects of imatinib on T-cell activation, we observed a dose-dependent reduction in ZAP70 and LAT activation. To investigate which kinases involved in immunoreceptor signaling might be sensitive to imatinib, we conducted a homology search with a consensus sequence derived from the ATP binding pocket of the known imatinib targets. One of the most homologous kinases that also share the critical threonine 315 (Thr315) residue is LCK. It is the first tyrosine kinase activated upon TCR engagement and initiates TCR signal transduction by activating ZAP70 and phosphorylating tyrosine residues on several of the TCR chains that recruit additional signal transduction molecules. The ATP binding pocket of LCK closely resembles that of ABL (Figure 7A and Schindler et al26 ). However, structural differences in the activation loop of the 2 kinases likely underlie the much higher affinity of imatinib for ABL.26 In an in vitro kinase assay, we confirmed that imatinib directly inhibits LCK activity with an IC50 of 0.6 μM to 0.8 μM. ZAP70 on the other hand is very unlikely to be a target of imatinib because its ATP binding pocket is less well conserved, and because it lacks the Thr315 residue present inABL that is required for interaction with imatinib. This Thr315, when mutated inABL or PDGF-R, confers clinical resistance to imatinib in CML and hypereosinophilia, respectively, and abrogates the inhibitory effect of imatinib on the respective TKs.38,39 Therefore, it appears that imatinib effects on TCR signaling are mediated through inhibition of LCK rather than ZAP70.
Imatinib affects TCR signal transduction and T-cell activation at concentrations achieved in human plasma. The IC50 for most of these effects is in the range of the peak plasma concentrations measured in vivo. Thus, the significance of these observations in vivo remains to be determined. However, there are several considerations that support an in vivo role of imatinib inhibition on immune function. First, the effect of TCR signaling on T-cell activation in vivo is in part determined by the strength of ITAM phosphorylation by LCK, bearing on the subsequent activation of further signaling molecules. Thus, a graded reduction of LCK activity may be sufficient to change cell fate after TCR activation.40 Second, imatinib may have synergistic effects with drugs affecting the same pathways, notably the calcineurin inhibitors CsA and FK506, which may be particularly important for TCR-mediated IL-2 production.41 Third, inhibition of the costimulatory capacity of APCs by imatinib8 and the effect of imatinib on c-KIT, which has been reported to induce profound changes in B- and T-cell development in mice,42 may contribute to an immunosuppressive effect of imatinib.
In keeping with these findings, there are recent reports of imatinib-induced immunosuppressive effects in nonhuman primates as well as in patients on imatinib treatment. In a safety study, nonhuman primates treated for 39 weeks with imatinib showed an increased incidence of opportunistic infections.43 Anecdotal case reports have described both viral infections44,45 and improvement in rheumatoid arthritis activity46,47 in patients on imatinib treatment, supporting an in vivo immunosuppressive effect of the agent.
Effects of imatinib on immune reconstitution and T-cell function may be especially relevant in the setting of allogeneic transplantation, where regulation of immune functions in the posttransplant period is especially critical and directly impacts morbidity and mortality. We have shown here that imatinib directly inhibits TCR signaling and this effect might potentiate the effects of immunosuppressive drugs. Thus, close monitoring of patients on imatinib for CMV reactivation or other opportunistic infections, especially in stem cell transplant recipients, appears warranted. Imatinib is being increasingly used as a means to prevent or treat relapse of Ph+ leukemias after allogeneic stem cell transplantation. Since graft-versus-host and graft-versus-leukemia effects are predominantly T-cell–mediated, and imatinib appears to have powerful suppressive effects on T-cell proliferation and function, it is a matter of importance to look for possible imatinib-induced failure of leukemia control after stem cell transplantation. Until the impact of imatinib on posttransplant T-cell function is better defined, imatinib should be used judiciously in these patients.
Prepublished online as Blood First Edition Paper, November 30, 2004; DOI 10.1182/blood-2004-07-2527.
R.S. is supported by the German Cancer Research Foundation. D.A.P. is a Medical Research Council (United Kingdom) Clinician Scientist.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Jason M. Brenchley and Michael R. Betts for helpful suggestions, and A. John Barrett for critical reading of the manuscript. OKT-3 was a kind gift of Barbara E. Bierer.


![Figure 5. Imatinib inhibits virus-specific T-cell proliferation in response to cognate antigen. (A) T cells from healthy HLAA2+ human blood donors with tetramer-defined CD8+ T-cell responses to CMV pp65495-503 were purified, CFSE-labeled, and kept in culture for 8 days in the presence of CMV peptide and antibodies against the costimulatory molecules CD28 and CD49d without imatinib (dashed line) or with imatinib at 5 μM (dotted line) or 10 μM (solid line). Data presented are overlaid histograms representing percentage fluorescence intensity of CFSE-labeled HLA A2–CMV pp65495-503 tetramer-positive CD8+ T cells (donors 1-3 [d1-3]) and CMV tetramer-positive CD3+ T cells (donor 4 [d]). The y-axis is scaled to 100% defined by the highest value on the y-axis for each condition. (B) Effects of imatinib on expansion of CMV-specific CD8+ T cells over a 4-day period in response to cognate peptide. (C) Effects of imatinib on expansion of EBV-specific CD8+ T cells over a 4-day period in response to cognate peptide.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/6/10.1182_blood-2004-07-2527/6/m_zh80060575760005.jpeg?Expires=1769089460&Signature=vrmQB2lcHt8vphnh7VQbW0yk-S-lvtVvkcGht3CEyhunQ8TEJUBTY-rafmSW4eqHVUkSu~lIn4CGrGrcz5NylxcPCiYFqdmKFyEebzz6iUZB8OhzaRWVxXd8m2IzsAWlNh-ofBW7GfEAiF9h58uRqvmJu1SKFrh0d4-1tFGJDc1kXuZQ1J6F32L1PfpFMNe-81GZbRApKicJNSmiFFL1YNRijvT-uMHzvyaEH8GUOBC~Z-B0wmZLaiO4G8ndf6PZ0uNakA9pBhtisNCFTnEmBh4uqP-PQnwlARFqSY6mGnkvjz4PyC3GhjqfTI38sW5ozWBvCIHaf68dg-iVZQuNFw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal