Abstract
Although dendritic cells (DCs) strongly stimulate the immune response, they can also induce unresponsiveness. Recently, a human monocyte-derived DC subpopulation was described that constitutively expresses indoleamine 2,3-dioxygenase (IDO). These DCs were defined as nonadherent CD123+/CC chemokine receptor 6+ (CCR6+) cells that suppress the allogeneic T-cell response. In the present study, we generated nonadherent, mature DCs from human blood monocytes. As expected, in addition to the classic markers, these cells expressed CD123 and CCR6. Reverse transcription–polymerase chain reaction (RT-PCR), however, did not show IDO gene transcription, nor did we detect enzymatic IDO activity. Treating the cells with interferon-γ (IFN-γ) resulted in significant IDO production. Subsequently, we studied the regulatory properties of IDO-producing DCs on autologous and allogeneic T-cell responses. Neither OKT3-stimulated T cells of healthy donors nor myelin basic protein (MBP)–specific T cells of patients with multiple sclerosis (MS) were suppressed by autologous IDO DCs. However, whereas IDOneg DCs supported further stimulation of preactivated MBP-specific T cells of an MS patient, IDOpos DCs had lost this capacity. The allogeneic T-cell response was only marginally suppressed by IDO DCs. Our findings show that nonadherent CD123+/CCR6+ human DCs do not constitutively express IDO, and, even if they express the enzyme after IFN-γ treatment, they possess only limited T-cell regulatory function.
Introduction
Since 1998, when a series of brilliant mouse experiments1 showed that a placental enzyme called indoleamine 2,3-dioxygenase (IDO) was able to prevent rejection of the fetus during pregnancy, the scientific community has been intrigued by a novel, basic immunoregulatory mechanism whose main player is IDO. Munn et al1 presented a convincing experiment: they implanted time-release capsules containing the IDO inhibitor 1-methyl-tryptophan (1-MT) into pregnant mice bred to genetically different fathers. This treatment induced fetal rejection. The experimental design relied on the observation that, under certain circumstances, macrophages inhibit the T-cell response, apparently because they produce IDO,2,3 an enzyme that is also manufactured in the placenta by the fetus-derived syncytiotrophoblast.4 Based on their experimental findings, Munn et al forwarded the hypothesis that once the embryo implants and begins establishing connections with the mother's blood supply, fetal-derived cells located in the placenta begin making IDO. By destroying tryptophan—so went the speculation—IDO suppresses maternal T cells that otherwise would make their way through the placenta and attack the fetus.
Subsequent studies addressed the mechanism by which tryptophan degradation affects the T-cell response and came to the conclusion that certain metabolites have a strong T-cell inhibitory action.5,6 Among the tryptophan metabolites, 3-OH-kynurenine and 3-OH-anthranilic acid were shown to be strongly inhibitory, whereas kynurenine had a significant but weaker effect.5
The mechanism that is able to efficiently regulate the immune reaction during pregnancy, a phenomenon of outstanding importance for the perpetuation of species, can be expected to be “used” by nature for controlling other unwanted immune reactions. A series of interesting studies emerged, shedding light on the mechanism of IDO up-regulation and its hypothetical role in immunoregulation. Most notable is the study of Grohmann et al7 showing that cytotoxic T-lymphocyte antigen-4–immunoglobulin (CTLA4-Ig) up-regulates IDO in murine dendritic cells (DCs) by ligation to B7 molecules via induction of interferon-γ (IFN-γ) synthesis. DCs from CTLA4-Ig–treated mice showed an increased rate of IDO production, suggesting that this mechanism also works in vivo. If murine IDO-producing DCs inhibit T-cell responses, as shown by some studies,8,9 administration of the IDO inhibitor 1-MT would be expected to reverse CTLA4-Ig–induced immunosuppression. Indeed, in the same series of experiments, Grohmann et al showed that long-term survival of pancreatic islet allografts induced by CTLA4-Ig can be reverted by treatment with 1-MT.7 Another interesting study pointed to a role of IDO in the mediation of tumoral resistance against immune attack.10 The authors showed that many human tumors constitutively express IDO. In mice, expression of IDO by immunogenic tumor cells prevented rejection in preimmunized recipients, an effect that could be partly reverted by systemic treatment with an IDO inhibitor.
Because IDO is synthesized by certain cells of the hematopoietic system, its role in hematologic diseases has been the focus of several studies. Up-regulation of IDO in monocytic leukemia or in other malignant cells was held to exert an inhibitory effect on tumor growth.11,12 It has also been speculated that IDO is involved in tolerance induction in patients with allogeneic stem cell transplantation.13 Human bone marrow stromal cells regulate allogeneic T-cell responses14,15 and, in addition to supporting hematopoiesis, are capable of differentiating along multiple mesenchymal lineages.16,17 Because of these properties, marrow stromal cells are a promising tool for clinical applications, such as allogeneic hematopoietic stem cell transplantation, tissue engineering, or gene therapy.18-21 Recently, Meisel et al22 showed that marrow stromal cells express IDO and that this activity affects the T-cell response.
From the findings that IDO suppresses the immune-mediated rejection of the fetus during pregnancy in mice1 and is detectable and functionally active in murine8,9 as well as human DCs,23 it was only a small step to search for a specialized subpopulation of IDO-producing DCs that regulate the T-cell response. No doubt, such an inhibitory cell population would be of pivotal importance for the control of autoimmunity and for other immunoregulatory mechanisms. It was therefore exciting when Munn et al24 reported on the existence of a subset of human DCs that constitutively express IDO and have T-cell–suppressive properties. These cells were generated in vitro by differentiation of blood monocytes and defined as a nonadherent cell population coexpressing the surface markers CD123 and chemokine receptor 6 (CCR6), in addition to the classic DC phenotype.
In the current series of experiments, we analyzed the nonadherent CD123+/CCR6+ human DC subpopulation with regard to IDO-mediated regulation of allo- and selfreactive T cells in health and disease.
Materials and methods
Generation of human monocyte-derived dendritic cells from peripheral blood mononuclear cells (PBMCs) of healthy donors and multiple sclerosis (MS) patients
Dendritic cells were generated according to a standard protocol.25 PBMCs were isolated from heparinized blood from either healthy donors or MS patients by density gradient centrifugation on lymphocyte cell separation medium (Lymphodex; Inno-Train Diagnostik, Kronberg, Germany). Approval was obtained from the University of Heidelberg institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki. After washing, the cells were incubated in Petri dishes (Nunc, Wiesbaden, Germany) at 37°C and 5% CO2 for 90 minutes and gently washed, and nonadherent cells were removed. The adherent monocytes were then cultured in the presence of 1000 U/mL recombinant human (rh) interleukin-4 (IL-4; Promocell, Heidelberg, Germany) and 666 U/mL rh granulocyte-macrophage colony-stimulating factor (GM-CSF; Sigma-Aldrich Chemie, Taufkirchen, Germany) in RPMI 1640 supplemented with 10% fetal calf serum (FCS), 2 mM l-glutamine (Promocell), and penicillin (100 U/mL)/streptomycin (100 μg/mL) (Gibco BRL Life Technologies, Eggenstein, Germany). Cytokines (IL-4, GM-CSF) were replenished every 2 to 3 days by removing half the volume of medium and adding back the same volume of fresh medium containing cytokines. On day 6 of culture, nonadherent cells (immature DCs) were collected by moderate aspiration and seeded into 24- or 96-well plates (Nunc). For maturation, the cells were treated for 36 hours with a combination of 500 ng/mL CD40 ligand (CD40L; Alexis Biochemicals, Gruenberg, Germany) and 5 μg/mL lipopolysaccharide (LPS; Sigma-Aldrich) or a cytokine cocktail comprising 1 μg/mL prostaglandin E2 (PGE2; Sigma-Aldrich), 1000 U/mL rh IL-6 (R&D System, Wiesbaden-Nordenstadt, Germany), 10 ng/mL rh tumor necrosis factor-α (TNF-α; Sigma-Aldrich), and 10 ng/mL rh IL-1β (Roche Diagnostics, Mannheim, Germany). IDO synthesis was induced by incubating immature DCs with IFN-γ (Roche Diagnostics) along with maturation factors. In order to obtain maximum IDO production, in preliminary testings, DCs were treated with increasing amounts of IFN-γ (100-2000 U/mL), and IDO synthesis was assessed by reverse transcription–polymerase chain reaction (RT-PCR) and by measuring tryptophan and kynurenine concentrations in the cell-culture medium. Maximum IDO production was noted at 1000 U/mL IFN-γ and was comparable with that obtained by IDO transgene expression via transfection with recombinant IDO adenoviruses. For myelin basic protein (MBP)–specific T-cell studies, immature DCs from HLA-DRB1*0301+ healthy blood donors or MS patients were incubated with 30 μg/mL MBP (Sigma-Aldrich) before adding the maturation factors.
Flow cytometric analysis
DCs kept in phosphate-buffered saline (PBS) + 0.1% bovine serum albumin (BSA) were incubated at 4°C for 30 minutes with the following fluorochrome-conjugated mouse antihuman monoclonal antibodies at the concentrations indicated by the manufacturer: anti-CD1a, -CD80, -CD86, -CD83, -CD11a, -CD11c, -CD123, -CCR6, and -CD14 and anti–HLA-DR (BD Biosciences, Heidelberg, Germany). Isotope controls were IgG1, IgG2a, and IgG2b (BD Biosciences). The cells were then washed and resuspended in 200 μL/sample. DCs were gated to exclude dead cells and contaminating lymphocytes. Events (10 000) were collected for each sample on a FACScan flow cytometer and analyzed using the CellQuest Pro software (BD Biosciences).
Reverse transcription–polymerase chain reaction (RT-PCR) analysis of IDO mRNA
After incubation of immature DCs with maturation factors ± IFN-γ, total RNA was extracted from DCs using a RNA-isolation kit (Qiagen, Hilden, Germany). Total RNA (1 μg) was reverse transcribed into cDNA using a transcription kit (TaqMan Reverse Transcription Reagents; Applied Biosystems, Darmstadt, Germany). The resulting cDNA was amplified by PCR using IDO sense (595-614: 5′-GCG CTG TTG GAA ATA GCT TC-3′) and antisense (770-752: 5′-TTT GGG TCT TCC CAG AAC C-3′) primers. A fragment of 175 base pair (bp) was obtained. As positive control served 293 cells transfected with recombinant IDO adenovirus, and as negative controls, water instead of RNA or PCR template.
High-pressure liquid chromatography (HPLC) determination of tryptophan and kynurenine
HPLC was carried out as previously described26 with minor modifications. DCs were seeded into 96-well plates (5 × 104 cells/well) in 200 μL RPMI 1640 + 10% FCS culture medium containing maturation factors as described with or without 1000 U/mL IFN-γ ± 1-methyl-DL-tryptophan (1-MT; final concentration: 1 mM; Sigma-Aldrich). Culture supernatants of 2 to 3 wells were collected and deproteinized by mixing with 2.4 M perchloric acid (10:1, vol/vol). After centrifugation (3500g, 15 minutes, 4°C), supernatants were transferred into new tubes, the pH value adjusted to 7, and 100 μL filtered supernatant was injected into a C-18 column (Supelco, Aschaffenburg, Germany). Samples were eluted with PBS buffer over 30 minutes, and the absorbance of column effluent was monitored at 230 nm for tryptophan and kynurenine. The peaks of kynurenine and tryptophan were identified by comparison with the retention time of previously determined standard compounds, and quantification was based on the ratios of the peak areas of the compound to the internal standard.
Lymphocyte cultures
T-cell proliferation assays were set up in 96-well plates with triplicate wells for each experimental condition in the same culture medium as that described for DCs. In one experiment, DCs were generated as described above and coincubated in the presence of anti-CD3 monoclonal antibody (mAb, final dilution 1:6400; BD Biosciences) with autologous peripheral lymphocytes, or in the absence of anti-CD3 mAb with allogeneic lymphocytes obtained from blood of healthy donors by density gradient centrifugation. In another experiment, MBP-loaded DCs were coincubated with autologous peripheral lymphocytes prepared from blood of MS patients. In a third experiment, DRB1*0301-expressing DCs obtained from healthy donors were loaded with MBP and coincubated with MBP-specific T cells (clone ES-BP8T) in the presence or absence of 1-MT. This clone comprised CD4+ T helper 1 (Th1) lymphocytes derived from an MS patient27 reactive against a DRB1*0301-restricted MBP epitope and transformed to continuous cell growth by Herpesvirus saimiri infection.28,29 If not otherwise mentioned, cocultures were performed at a DC/T-cell ratio of 1:10 in a final volume of 200 μL culture medium for 3 to 5 days. [3H]thymidine (1 μCi/well [0.037 MBq]; Amersham Biosciences, Freiburg, Germany) was added 18 hours before termination of the culture, and the number of counts per minute (cpm) was measured in a β-counter (Berchtold-Inotech, Rockville, MD).
Statistics
Results are shown as mean ± SD. Single values of T-cell proliferation represent the mean [3H]thymidine incorporation (cpm) of triplicate cultures and are given in percentage of positive control (= 100% proliferation). The 2-tailed Student t test was used for statistical analysis.
Results
Nonadherent, CCR6+/CD123+ dendritic cells do not produce IDO but can be induced to do so by IFN-γ treatment
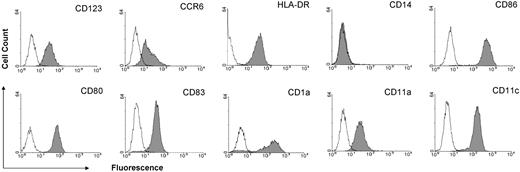
Mature DCs were generated from blood monocytes, and nonadherent cells were collected and analyzed in fluorescence-activated cell sorter (FACS). As shown in Figure 1, they expressed the classic maturation antigens as well as CD123 and CCR6, the characteristic markers for the suppressive, IDO-producing DC subset.24
Phenotypic characteristics of monocyte-derived mature DCs. mDCs were generated from blood monocytes as described. Nonadherent cells were stained with fluorochrome-conjugated mouse antihuman monoclonal antibodies. FACScan data show the cell surface expression of the indicated marker (gray area) or isotope controls (bold lines) with a life gate from one representative experiment of DCs matured with CD40L + LPS.
Phenotypic characteristics of monocyte-derived mature DCs. mDCs were generated from blood monocytes as described. Nonadherent cells were stained with fluorochrome-conjugated mouse antihuman monoclonal antibodies. FACScan data show the cell surface expression of the indicated marker (gray area) or isotope controls (bold lines) with a life gate from one representative experiment of DCs matured with CD40L + LPS.
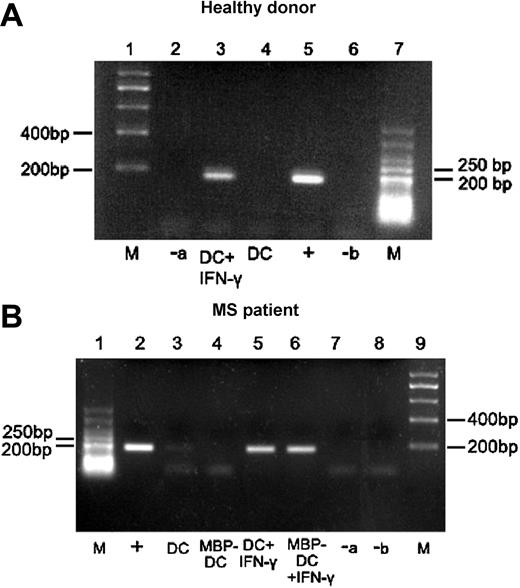
To test whether these DCs, matured with CD40L + LPS or cytokine cocktail, express IDO, gene transcription was studied by RT-PCR using IDO-specific primers. Figure 2 shows that nonadherent, CD123+/CCR6+ DCs derived from healthy individuals or MS patients did not transcribe IDO (healthy: lane 4; MS: lanes 3-4). Because in further cell-culture studies of MS patients we loaded the DCs with myelin basic protein (MBP) and coincubated them with T cells, we analyzed both, DCs with and without MBP. The results were identical. In some cases, weak expression was observed particularly in DCs matured with the cytokine cocktail. However, a strong RT-PCR signal was detected following treatment of DCs with IFN-γ (Figure 2, healthy: lane 3; MS: lanes 5-6).
Expression of IDO-specific transcripts in nonadherent CD123+/CCR6+ DCs from healthy donors and MS patients before and after treatment with IFN-γ. Immature DCs (iDCs) obtained from healthy donors (A) or MS patients with or without MBP loading (B) were cultured overnight with medium containing maturation factors, supplemented or not with IFN-γ. Total RNA was isolated and reverse transcribed into cDNA. PCR was performed using IDO-specific primers, and the products were analyzed by agarose gel electrophoresis. The positive control (+) consisted of material extracted from IDO transgene expressing 293 cells, whereas negative control “a” (-a) was water instead of RNA for reverse transcription and negative control “b” (-b) was water instead of cDNA template for PCR; M indicates DNA molecular marker. One representative example for healthy donors and MS patients is shown. A faint IDO band occurred in 2 of 9 tested DCs.
Expression of IDO-specific transcripts in nonadherent CD123+/CCR6+ DCs from healthy donors and MS patients before and after treatment with IFN-γ. Immature DCs (iDCs) obtained from healthy donors (A) or MS patients with or without MBP loading (B) were cultured overnight with medium containing maturation factors, supplemented or not with IFN-γ. Total RNA was isolated and reverse transcribed into cDNA. PCR was performed using IDO-specific primers, and the products were analyzed by agarose gel electrophoresis. The positive control (+) consisted of material extracted from IDO transgene expressing 293 cells, whereas negative control “a” (-a) was water instead of RNA for reverse transcription and negative control “b” (-b) was water instead of cDNA template for PCR; M indicates DNA molecular marker. One representative example for healthy donors and MS patients is shown. A faint IDO band occurred in 2 of 9 tested DCs.
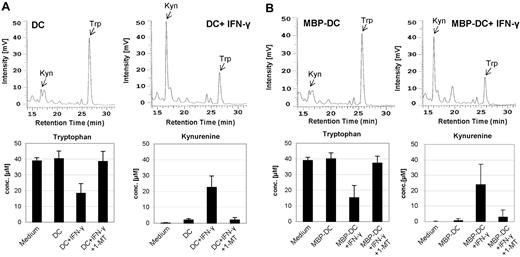
Because the IDO enzyme is known to degrade tryptophan, which results in the metabolite kynurenine, a functional consequence of IDO activity is a low tryptophan and high kynurenine concentration. HPLC analysis did not show decreased tryptophan and increased kynurenine concentrations in cultures of nonadherent, CD123+/CCR6+ DCs derived from healthy donors (Figure 3A) or MS patients (Figure 3B). As expected from the gene-transcription analysis (Figure 2) when treated with IFN-γ, mature DCs (mDCs) generated with CD40L + LPS or cytokine cocktail expressed functionally active enzyme fully capable of degrading tryptophan and generating kynurenine, both in experiments using cells derived from healthy persons and MS patients. The upper panels of Figure 3A-B show representative HPLC curves of DCs before and after IFN-γ treatment, whereas the lower panels present the cumulative tryptophan and kynurenine concentrations of DCs derived from healthy individuals or MS patients. Treatment of DCs with the IDO-inhibitor 1-MT abrogated the IFN-γ–induced IDO activity (last columns). Interestingly, in 4 of 13 DCs derived from healthy persons and in 1 of 11 DCs derived from MS patients, induction of IDO activity by IFN-γ was not possible. Only IDO-producing DCs were used in this study.
Kynurenine and tryptophan concentrations measured in the culture medium of DCs. iDCs were matured with CD40L + LPS without or with IFN-γ (± 1-methyl-tryptophan). iDCs of MS patients received MBP in addition to maturation factors. After 36 hours, the cell culture supernatants were harvested and the concentrations of kynurenine and tryptophan were measured by HPLC. The chromatograms (top rows of A and B) show the total free kynurenine (Kyn) and tryptophan (Trp) in cell-culture supernatants of DCs—without (left) and with (right) IFN-γ treatment—from one representative healthy donor (A) and MS patient (B). The mean ± SD concentrations of tryptophan and kynurenine of DCs from 7 healthy donors and 10 MS patients are shown in the bottom rows of panels A and B.
Kynurenine and tryptophan concentrations measured in the culture medium of DCs. iDCs were matured with CD40L + LPS without or with IFN-γ (± 1-methyl-tryptophan). iDCs of MS patients received MBP in addition to maturation factors. After 36 hours, the cell culture supernatants were harvested and the concentrations of kynurenine and tryptophan were measured by HPLC. The chromatograms (top rows of A and B) show the total free kynurenine (Kyn) and tryptophan (Trp) in cell-culture supernatants of DCs—without (left) and with (right) IFN-γ treatment—from one representative healthy donor (A) and MS patient (B). The mean ± SD concentrations of tryptophan and kynurenine of DCs from 7 healthy donors and 10 MS patients are shown in the bottom rows of panels A and B.
Nonadherent CD123+/CCR6+ dendritic cells treated with interferon-γ produce IDO but do not suppress autologous T cells of healthy persons, whereas they marginally suppress the allogeneic T-cell response
It has been held that nonadherent CD123+/CCR6+ DCs suppress T-cell activity.24 Because of lacking IDO expression, we did not expect them to exert a suppressive action. To analyze their T-cell regulatory function, we coincubated the DCs with autologous T cells stimulated with anti-CD3 antibody. The DCs were not able to suppress the T-cell response (Figure 4A). In the next experiment, we induced IDO by treatment with IFN-γ. To our surprise, even after IDO up-regulation no suppression of autologous T cells could be detected (Figure 4A-B). The same result was obtained when DCs were cultured in a medium containing, in addition to IFN-γ, an IDO enhancer (TNF-α) (supplemental Figure 1, located on the Blood website; see the Supplemental Figure link at the top of the online article). IFN-γ–treated DCs marginally suppressed the allogeneic T-cell response (Figure 4C). This effect could not be significantly augmented by increasing the DC/T-cell ratio (Figure 4D).
Effect of IFN-γ–treated DCs on anti-CD3 antibody-activated autologous and allogeneic peripheral lymphocytes of healthy donors. Untreated or IFN-γ–treated mDCs generated with CD40L + LPS were coincubated for 3 days with anti-CD3 antibody-stimulated autologous blood lymphocytes at a ratio of 1:10 (n = 7) (A) or at increasing ratios (n = 6) (B). Alternatively, DCs were cocultured for 5 days with allogeneic lymphocytes at a ratio of 1:10 (n = 8) (C) or at increasing ratios (n = 5) (D). Proliferation was assessed by adding [3H]thymidine (1 μCi [0.037 MBq]/well) during the last 18 hours of culture. The positive control consisted of DCs plus anti-CD3 antibody-activated autologous or allogeneic lymphocytes, and the negative control consisted of lymphocytes and DCs alone. Single values represent mean ± SD and are expressed as a percentage of the positive control values (= 100%) (mean stimulation: autologous, 23 000 ± 6000 cpm; allogeneic, 27 800 ± 8000 cpm). The difference between the T-cell stimulatory capacity of native DCs and IDO DCs was statistically not significant in experiment A and borderline significant (P = .026) in experiment C.
Effect of IFN-γ–treated DCs on anti-CD3 antibody-activated autologous and allogeneic peripheral lymphocytes of healthy donors. Untreated or IFN-γ–treated mDCs generated with CD40L + LPS were coincubated for 3 days with anti-CD3 antibody-stimulated autologous blood lymphocytes at a ratio of 1:10 (n = 7) (A) or at increasing ratios (n = 6) (B). Alternatively, DCs were cocultured for 5 days with allogeneic lymphocytes at a ratio of 1:10 (n = 8) (C) or at increasing ratios (n = 5) (D). Proliferation was assessed by adding [3H]thymidine (1 μCi [0.037 MBq]/well) during the last 18 hours of culture. The positive control consisted of DCs plus anti-CD3 antibody-activated autologous or allogeneic lymphocytes, and the negative control consisted of lymphocytes and DCs alone. Single values represent mean ± SD and are expressed as a percentage of the positive control values (= 100%) (mean stimulation: autologous, 23 000 ± 6000 cpm; allogeneic, 27 800 ± 8000 cpm). The difference between the T-cell stimulatory capacity of native DCs and IDO DCs was statistically not significant in experiment A and borderline significant (P = .026) in experiment C.
Interferon-γ–treated dendritic cells do not suppress myelin basic protein–specific T cells of patients with multiple sclerosis
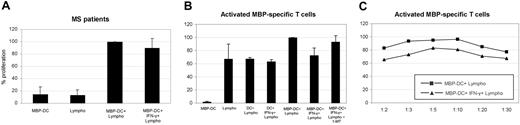
In the previous experiment, we analyzed the action of the nonadherent CD123+/CCR6+ DC subpopulation of healthy blood donors on T cells. Next, we addressed the question of its regulatory properties in an autoimmune disease in which T cells participate in the process of tissue damage. We took MS as a model for this study. A frequently discussed target antigen of the immune attack in MS is myelin basic protein.30,31 In order to study the regulatory effect of DCs on MBP-specific T cells, we generated nonadherent CD123+/CCR6+ DCs from peripheral blood of MS patients. The cells were loaded with MBP and coincubated with autologous T cells. As shown in Figure 5A, the DCs were capable of inducing specific T-cell proliferation. In the next step, the DCs were manipulated by treatment with IFN-γ to produce IDO. Even so, they did not suppress the specific T-cell response.
Effect of IFN-γ–treated DCs on MBP-specific T-cell proliferation of MS patients. mDCs were generated with CD40L + LPS, treated with IFN-γ, loaded with MBP, and cocultured with autologous peripheral lymphocytes obtained from MS patients (n = 10) (A). Alternatively, IFN-γ–treated MBP-DCs (± 1-MT) of HLA-DR compatible healthy donors were coincubated with H saimiri–transformed MBP-specific T cells (clone ES-BP8T) at a ratio of 1:10 (n = 8) (B) or at increasing ratios (n = 5) (C). For experiment A, controls consisted of MBP-loaded DCs plus autologous peripheral lymphocytes, MBP-DCs only, or lymphocytes only. For experiment B, controls consisted of MBP-loaded (without IFN-γ) or -unloaded DCs (± IFN-γ) plus ES-BP8T cells, ES-BP8T cells only, or MBP-loaded DCs only. Cell proliferation was measured after 4 days. Data represent mean ± SD and are expressed as a percentage of the positive control values (= 100%) (mean stimulation for A: 11 000 ± 5600 cpm and for B-C: 19 200 ± 5500 cpm). No statistically significant difference between the T-cell stimulatory capacity of native DCs and IDO DCs was noted in experiment A. IDO DCs significantly suppressed the T-cell response in experiment B (P = 10-4).
Effect of IFN-γ–treated DCs on MBP-specific T-cell proliferation of MS patients. mDCs were generated with CD40L + LPS, treated with IFN-γ, loaded with MBP, and cocultured with autologous peripheral lymphocytes obtained from MS patients (n = 10) (A). Alternatively, IFN-γ–treated MBP-DCs (± 1-MT) of HLA-DR compatible healthy donors were coincubated with H saimiri–transformed MBP-specific T cells (clone ES-BP8T) at a ratio of 1:10 (n = 8) (B) or at increasing ratios (n = 5) (C). For experiment A, controls consisted of MBP-loaded DCs plus autologous peripheral lymphocytes, MBP-DCs only, or lymphocytes only. For experiment B, controls consisted of MBP-loaded (without IFN-γ) or -unloaded DCs (± IFN-γ) plus ES-BP8T cells, ES-BP8T cells only, or MBP-loaded DCs only. Cell proliferation was measured after 4 days. Data represent mean ± SD and are expressed as a percentage of the positive control values (= 100%) (mean stimulation for A: 11 000 ± 5600 cpm and for B-C: 19 200 ± 5500 cpm). No statistically significant difference between the T-cell stimulatory capacity of native DCs and IDO DCs was noted in experiment A. IDO DCs significantly suppressed the T-cell response in experiment B (P = 10-4).
Dendritic cells loaded with myelin basic protein stimulate preactivated specific T cells derived from an MS patient but lose their stimulatory capacity after IFN-γ–induced IDO up-regulation
Our findings showed that nonadherent CD123+/CCR6+ DCs do not prevent the activation of resting T cells, even after IDO induction by IFN-γ. An important regulatory function of DCs might consist in down-regulation of already activated T cells. Therefore, we analyzed the impact of the relevant DC subset on preactivated T cells derived from an MS patient whose receptors specifically recognize a HLA-DRB1*0301–restricted MBP epitope.27,28 Continuous activation was achieved by transformation with Herpesvirus saimiri as previously described.29 When these T cells were coincubated with DR-matched nonadherent CD123+/CCR6+ DCs loaded with MBP, they were additionally stimulated instead of being suppressed. If MBP-loaded DCs were manipulated by IFN-γ to produce IDO, they significantly reduced T-cell proliferation but only to the level of the basic T-cell response (proliferation without MBP-DC stimulation; Figure 5B), a process that could be partially reverted by the IDO-inhibitor 1-methyl tryptophan. The suppressive effect could not be augmented by increasing the DC/T-cell ratio (Figure 5C). In contrast, MBP-loaded DCs pretreated with a partial protein synthesis inhibitor completely inhibited T-cell proliferation, showing that abrogation of the T-cell response by suppressive DCs is possible (supplemental Figure 2). These findings suggest that IDO DCs have lost their T-cell stimulatory capacity.
Discussion
Dendritic cells are well known for their capacity to induce strong innate and adaptive immunity.32,33 There has been increasing evidence, however, indicating that DCs can also induce unresponsiveness and tolerance.34,35 Cells possessing such properties can be expected to play a central role in the regulation of immune responses as well as in tolerance induction toward self-antigens. It is important to exactly define the tolerogenic DC subpopulation. Recently, a subset of monocyte-derived DCs has been described that constitutively expresses IDO and suppresses the allogeneic T-cell response.24 These cells were characterized by nonadherence and coexpression of CD123 and CCR6 in addition to the classic DC phenotype (CD83+, CD80+, CD86hi, HLA-DRhi, CD14neg). The main purpose of this study was to analyze the immunoregulatory IDO-mediated action of such nonadherent CD123+/CCR6+ DCs on allo- and autoreactive T cells in health and disease. Allogeneic- or anti-CD3–stimulated T cells served as a model for studying the regulatory influence of DCs derived from healthy donors. Because myelin-specific T cells often have been discussed as playing a role in the pathogenesis of MS,30,31 we chose such cells as a model for analyzing the impact of IDO DCs on autoreactive T cells in an autoimmune disease.
If nonadherence and expression of CD123 and CCR6 are the key characteristics of the human monocyte-derived IDO-producing DC subpopulation, as previously held by Munn et al,24 DCs having the same origin and phenotype should express IDO. Starting from peripheral blood monocytes we generated such mDCs, but to our surprise the cells did not produce IDO. In our studies, the DCs were matured either by CD40 stimulation or the described cytokine cocktail. At variance with Munn et al,24 we added LPS to the CD40-induced maturation protocol, while the cytokine cocktail was the same. Munn et al24 reported that when their IDO-producing mature DC subset was treated with IFN-γ, the IDO protein completely disappeared. In our experiments, IDO, which initially was absent, could be strongly up-regulated by treatment with IFN-γ. This was not surprising since many earlier studies showed that IFN-γ is a strong IDO inducer,36-42 a process that is initiated by increasing the transcriptional activity of the IDO promoter via effects on the signal transducer and activator of transcription-1.43,44
We surmised that even if the nonadherent CD123+/CCR6+ DCs do not produce IDO, they might suppress T cells by other mechanisms. Therefore, we analyzed their effect on T cells but failed to obtain suppressive activity. On the contrary, they stimulated allogeneic T cells and, when loaded with MBP, induced a specific autoimmune response. The latter was shown with resting peripheral T cells of healthy persons as well as with resting and preactivated T cells derived from MS patients. Interestingly, even if the DCs were manipulated with IFN-γ to produce functional IDO, they did not inhibit the primary autologous T-cell response and only marginally suppressed allogeneic T cells. However, in contrast to IDO-negative DCs, IDO-producing DCs were not able to stimulate preactivated MBP-specific T cells—an effect that was partially reverted by the IDO inhibitor 1-MT. This latter effect can be explained by our previous observation that activated T cells are more susceptible to inhibition by tryptophan metabolites.5
There are 2 mechanisms that have been proposed as mediators of the T-cell–suppressive action of IDO: (1) degradation and consequently reduction of tryptophan, an essential amino acid required for T-cell proliferation, and (2) generation of inhibitory tryptophan metabolites. Regardless of whether tryptophan deprivation, kynurenine-mediated inhibition, or a combination of both is responsible for IDO-mediated immunosuppression, it is clear that sufficient amounts of IDO must be generated in order to induce an inhibitory effect. We were therefore concerned that insufficient IDO activity may have been generated in our cell cultures. A possible reason for that could have been that the DCs were not sufficiently stimulated by IFN-γ and did not synthesize the maximal amount of IDO protein. However, we find that highly unlikely. IFN-γ is known to be a strong IDO inducer.36-42 In preliminary studies, we defined the IFN-γ concentration that induced maximum IDO synthesis. As a consequence, the obtained IDO activity per DC was as high as that noted following IDO transgene expression and corresponded to the activities described by others in similar experiments.3,24 A second reason for insufficient IDO activity might have been that although the DCs produced a maximum amount of IDO, their number in the cell culture was too low. However, we found that increasing the DC number did not induce more suppression. If tryptophan metabolites are the main mediators of suppression, as shown in our previous study,5 and if a sufficient amount of IDO was produced, another reason for the lack of T-cell suppression might have been that the culture medium contained too little tryptophan for generating a sufficient amount of suppressive metabolites. However, the tryptophan concentration in our cell cultures was comparable with that in human plasma45,46 and corresponded to that used by others.24 Moreover, as indicated by HPLC analysis, in most cases tryptophan was not completely degraded and thus could not have been a limiting factor for enzymatic IDO activity. We therefore conclude that neither suboptimal IDO production per DC, nor an insufficient number of DCs, nor a limiting tryptophan concentration can serve as plausible explanations for the lack of T-cell suppression in our experiments. Other factors might have influenced the regulatory function of IDO DCs.
It has been shown that certain cell types, when expressing IDO, inhibit cell proliferation, particularly that of T lymphocytes.42,47 Most impressive are the data of Uyttenhove et al10 showing that IDO-producing tumor cells have immunosuppressive effects. The finding that IDO-induced suppression is mediated by tryptophan degradation—an unspecific mechanism—easily leads to the conclusion that once the suppressive mediators are generated, they always inhibit the immune response, regardless of the original cell that initiates the process. There are many examples, however, that show that identical biomolecules produced by different cell types can have different functional effects. Perhaps the best example is that of major histocompatibility complex (MHC) molecules that either can stimulate T cells (if they are expressed on cells with costimulatory signals) or anergize T cells (if they are expressed on cells lacking such stimuli).48 The same might apply to IDO, a biomolecule that may or may not suppress T cells depending on the concomitant secretion of inhibitory or stimulatory agents. Activated DCs produce biomolecules that strongly stimulate lymphocytes and thus have the potential to override the suppressive action of IDO. It has been shown that the immunostimulatory capacity of DCs can be further augmented by IFN-γ, the cytokine that has been used by us and others to induce IDO synthesis.3,23 The presence of IFN-γ during CD40L/LPS activation of human dendritic cells, for instance, up-regulates IL-12p70,49 which in turn enhances the immune response and induces inflammatory processes.50,51 These effects may counteract the T-cell inhibitory action of IDO.
Apart from competing immunostimulatory molecules, it is known that the synthesis and function of IDO as well as that of tryptophan metabolites are regulated by the redox potential of the microenvironment.52 We have previously shown that among the tryptophan metabolites generated by IDO, 3-OH-kynurenine and 3-OH-anthranilic acid are the most important mediators of immunosuppression.5 Both compounds are good electron donors that reduce cytochrome c and are readily oxidized under aerobic conditions.53 Their oxidation leads to the generation of quinoneimines, which oxidatively modify various amino acid side chains of proteins.54 Thus, 3-OH-kynurenine and 3-OH-anthranilic acid, 2 reducing molecules, are the immediate precursors of potentially oxidizing agents in vivo, contributing to oxidation stress. Not surprisingly, the in vivo pro- and antioxidant properties and hence the biologic activities of these species depend on other redox agents present in the microenvironment.53 DCs have been shown to generate such redox-active substances, an example being the production of cysteine and thioredoxin.55,56 The IDO activator IFN-γ also influences the redox potential of DCs and therefore might interfere with IDO effector functions.57-59 In addition to the presence of immunostimulatory molecules, the redox activity is another factor by which DCs can influence the T-cell regulatory effect of IDO.
Whether a suppressive subset of IDO-producing DCs exists is of pivotal importance for the regulation of the immune response, particularly for the control of autoimmune reactions. Our findings show that under the experimental conditions used, nonadherent CD123+/CCR6+ mDCs do not produce IDO, nor do they suppress the T-cell response. Even if these cells are triggered to synthesize IDO by treatment with IFN-γ, they are lacking inhibitory action on primary autologous T-cell responses and only weakly suppress allogeneic T cells. However, in contrast to DCs that do not produce IDO, IDO-positive DCs lose their capacity to sustain stimulation of preactivated T cells.
Prepublished online as Blood First Edition Paper, November 30, 2004; DOI 10.1182/blood-2004-06-2103.
Supported by the Roche Organ Transplantation Research Foundation.
P.T. and J.-J.C. contributed equally to this work.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The expert technical assistance of Helmut Simon, Christiane Christ, and Stephanie Grimm is gratefully acknowledged. The T-cell clone ES-BP8T was kindly provided by Dr Edgar Meinl, Department of Neuroimmunology, Max Planck Institute of Neurobiology, Martinsried, Germany.

![Figure 4. Effect of IFN-γ–treated DCs on anti-CD3 antibody-activated autologous and allogeneic peripheral lymphocytes of healthy donors. Untreated or IFN-γ–treated mDCs generated with CD40L + LPS were coincubated for 3 days with anti-CD3 antibody-stimulated autologous blood lymphocytes at a ratio of 1:10 (n = 7) (A) or at increasing ratios (n = 6) (B). Alternatively, DCs were cocultured for 5 days with allogeneic lymphocytes at a ratio of 1:10 (n = 8) (C) or at increasing ratios (n = 5) (D). Proliferation was assessed by adding [3H]thymidine (1 μCi [0.037 MBq]/well) during the last 18 hours of culture. The positive control consisted of DCs plus anti-CD3 antibody-activated autologous or allogeneic lymphocytes, and the negative control consisted of lymphocytes and DCs alone. Single values represent mean ± SD and are expressed as a percentage of the positive control values (= 100%) (mean stimulation: autologous, 23 000 ± 6000 cpm; allogeneic, 27 800 ± 8000 cpm). The difference between the T-cell stimulatory capacity of native DCs and IDO DCs was statistically not significant in experiment A and borderline significant (P = .026) in experiment C.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/6/10.1182_blood-2004-06-2103/6/m_zh80060575620004.jpeg?Expires=1769085295&Signature=QGQht52geSMrz0n~oY6cJaUxwOWDQV-1NV7v4wPtBCXtAb83x2IvuGWvvA~OzuAYBEmk4BO3q9BlnCoUixxnWK4IB5IFJyjYePE32FB7Sv~0iZMv8PlhQVeHnV02hzH87xQUmhfwAU4FJc4xX5y3qSAh6oE64yR8Dl9v-ArbaiZVa6pCmxXgF~1n7L0Eiha~fOTBkqWUjPxsF2~tNu85urWih9JC-L~WE9Jx90e~deIKBnzmO02AVxNRXrQSU~PmMwEtE4O3jVDlQmAJP~bodoNNs6rPE~QjMDLeeTaHnPqXalbxeBJFyYZBp7SGg5AR5zkFAm4mkh-1atQfoS464Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal