Abstract
Hematopoietic cells (HCs) promote blood vessel formation by producing various proangiogenic cytokines and chemokines and matrix metalloproteinases. We injected mouse colon26 colon cancer cells or human PC3 prostate adenocarcinoma cells into mice and studied the localization of HCs during tumor development. HCs were distributed in the inner tumor mass in all of the tumor tissues examined; however, the localization of HCs in the tumor tissue differed depending on the tumor cell type. In the case of colon26 tumors, as the tumor grew, many mature HCs migrated into the tumor mass before fine capillary formation was observed. On the other hand, although very few HCs migrated into PC3 tumor tissue, c-Kit+ hematopoietic stem/progenitor cells accumulated around the edge of the tumor. Bone marrow suppression induced by injection of anti–c-Kit neutralizing antibody suppressed tumor angiogenesis by different mechanisms according to the tumor cell type: bone marrow suppression inhibited the initiation of sprouting angiogenesis in colon26 tumors, while it suppressed an increase in the caliber of newly developed blood vessels at the tumor edge in PC3 tumors. Our findings suggest that HCs are involved in tumor angiogenesis and regulate the angiogenic switch during tumorigenesis.
Introduction
Most tumors require new blood vessel formation in the tumor mass for their growth and for invasion of tumor cells.1,2 Therefore, antitumor angiogenesis is a promising approach for managing cancer.3 It is valuable to study how blood vessels are formed in vivo, and the mechanism of blood vessel formation has been studied using gene-targeting strategies and transgenic models. Although the molecular mechanism of blood vessel formation during embryogenesis is well understood, 4,5 the mechanism of tumor angiogenesis does not always correspond to physiological blood vessel formation in the embryo.6 However, there may be common aspects between angiogenesis in the embryo and tumor angiogenesis, and elucidating such general rules of angiogenesis is important for developing methods of suppressing tumor angiogenesis.
Sprouting of new vessels from a pre-existing blood vessel, a process termed angiogenesis, occurs in the embryo as well as in a number of physiological and pathological situations including tumor development. On the other hand, de novo blood vessel formation, termed vasculogenesis, in which endothelial cells (ECs) form from progenitor cells, is rarely observed in tumors.7 It was previously reported that hematopoietic cells (HCs) and ECs develop from common progenitor cells called hemangioblasts, 8 and ECs can support hematopoiesis as a component of stromal cells9 ; therefore, HCs and ECs are closely related developmentally and functionally. We have found that HCs play several critical roles in angiogenesis. Hematopoietic stem cells (HSCs) migrate into an avascular area before ECs migrate into the area, and they promote chemotaxis of ECs by producing angiopoietin-1 (Ang-1), which is a ligand for Tie2 expressed on ECs.10 Although HSCs promote angiogenesis in somewhat restricted regions including the head, pericardium, and peritoneal wall during embryogenesis, ectopic transplantation of HSCs promotes angiogenesis in adult tissues, and this concept has been utilized for therapeutic angiogenesis in ischemic patients. Moreover, we recently reported the critical role of neuropilin-1 (NP-1) expression on HCs in angiogenesis.11,12 NP-1 is also expressed on ECs as well as on neuronal cells. Vascular endothelial growth factor (VEGF) binds to NP-1 as well as to the receptors VEGFR1 and VEGFR2. NP-1 does not contain a kinase domain or a binding site for adaptor proteins; therefore, NP-1 modifies the signal transduction of VEGFR2. When NP-1 was coexpressed with VEGFR2 in ECs, NP-1 enhanced the binding of VEGF to VEGFR2 and strongly promoted the proliferation and chemotaxis of ECs.13 We found that NP-1, which is expressed on a variety of HCs, bound VEGF and enhanced the signal transduction of VEGFR2 exogenously.12 Strong NP-1 expression is observed in B lymphocytes, the erythroid lineage, and the myeloid lineage during embryogenesis and in T lymphocytes, B lymphocytes, the myeloid lineage, and HSCs in adult bone marrow (Y.Y., unpublished data, October 2002). Therefore, most HCs such as lymphocytes and myeloid cells as well as HSCs may promote tumor angiogenesis.
Regarding the role of HCs in tumor angiogenesis, it has been reported that macrophages and mast cells produce various proangiogenic cytokines and extracellular matrix metalloproteinases and may promote tumor angiogenesis.14 Indeed, in a study on human melanoma samples, the degree of macrophage infiltration was associated with the depth of invasion of primary melanoma, and this was due in part to macrophage-regulated tumor-associated angiogenesis.15 It was recently reported that upon transplantation of genetically modified bone marrow (BM) cells tagged with green fluorescent protein, the BM cells actually migrated into the tumor mass.16 Moreover, the authors described that elimination of those BM cells that had migrated into the tumor by delivering a “suicide” gene inhibited tumor angiogenesis. These results strongly suggest that HCs regulate tumor angiogenesis, but when and how HCs migrate into the tumor and whether the localization of HCs in tumor tissue is similar in different tumor types are not known. Moreover, in tumor models inoculating tumor cells into mice subcutaneously, there are 2 systems: transplantation of mouse or human tumor cells in syngenetic mice (syngenetic model) or immunodeficient nude mice (xenograft model), respectively. Therefore, in this study, we observed the migration of HCs into the tumor mass during the development of tumors in experimental mice and precisely studied which HCs migrated into the tumor mass using 2 tumor cell lines, such as mouse colon cancer cell line (colon26) and human prostate cancer cell line (PC3). We provide evidence that the tissue distribution of migrated HCs differs depending on the type of tumor cells; however, when the migration of HCs to the tumor site was suppressed, blood vessel formation was clearly inhibited.
Materials and methods
Animals
C57BL/6 mice, KSN nude mice, and BALB/c mice were purchased from Japan SLC (Shizuoka, Japan). Animal care in our laboratory was in accordance with the guidelines of Kanazawa University for animal and recombinant DNA experiments.
Mouse study using tumor cell line
Mouse colon cancer (colon26) cells (a gift from Dr N. Mukaida, Kanazawa University, Japan) and human prostate adenocarcinoma (PC3) cells (a gift from Dr T. Naruse, Chemo-Sero-Therapeutic Institute Foundation, Kumamoto, Japan) were maintained in RPMI 1640 medium (GIBCO, Grand Island, NY) with 10% fetal calf serum (FCS) (HyClone, Logan, UT) and penicillin/streptomycin (GIBCO). Colon26 cells (2 × 107 cells) or PC3 cells (2 × 107 cells) were inoculated subcutaneously into 6- to 8-week-old BALB/c female mice or nude female mice, respectively. Tumor was measured with a dial caliper, and the tumor volume was calculated using the following formula: width2 × length × 0.52. Ten to 20 μL of peripheral blood was drawn from the tail vein of the anesthetized mouse using a heparin-treated microcapillary tube (Terumo, Tokyo, Japan) and stained with trypan blue dye (GIBCO). The number of mononuclear cells was counted under a microscope. Anti–c-Kit (ACK2) monoclonal antibody (mAb) (a gift from S.-I. Nishikawa, CDB, Kobe, Japan) or anti-B220 (6B2) mAb was injected through the tail vein, and cyclophosphamide (170 mg/kg; Sigma, St Louis, MO) was injected subcutaneously.
CFU-c analysis and calculation of vascular area in an in vitro study using P-Sp cultures
Para-aortic splanchnopleural mesoderm (P-Sp) culture conditions were as described previously.10 To this culture, we added ACK2 monoclonal antibody or anti-B220 mAb. After 10 days of culturing, all cells were harvested and subjected to the colony formation unit in culture (CFU-c) assay as described previously.17 In other cultures, after 10 days of culturing, the cultured cells on plates were fixed and stained with anti–platelet endothelial cell adhesion molecule-1 (anti–PECAM-1) mAb (MEC13.3; Pharmingen, San Diego, CA) as previously described.18 The length of the vascular network areas (straight-line length of endothelial cells from the edge of the vascular bed to the top of the migrating endothelial cells in the network area) was measured under each culture condition. The length was calculated as described previously.12
Immunohistochemistry
Tissue fixation, preparation of tissue sections, and staining of sections with antibodies were performed as described previously.19 An anti–PECAM-1 mAb and/or biotin-conjugated anti-CD45 (Pharmingen) mAb, and anti–c-Kit (ACK2) mAb were used in double staining of tumor tissue sections. Anti–PECAM-1 antibody was developed with horseradish peroxidase (HRP)–conjugated antirat immunoglobulin G (IgG) antibody (Biosource, Camarillo, CA). In the final step of staining, samples were soaked in phosphate-buffered saline (PBS) containing 250 μg/mL diaminobenzidine (Dojin Chemicals, Kumamoto, Japan) in the presence of 0.05% NiCl2 for 10 minutes, and 0.01% hydrogen peroxidase was added for the enzymatic reaction. Biotinylated anti-CD45 antibody was developed with alkaline phosphatase (ALP) conjugated with streptavidin (DAKO, Glostrup, Denmark). The enzymatic reaction with fuchsin (New Fuchsin substrate system; DAKO) was allowed to proceed until the desired color intensity was reached. For lineage analysis of HCs in tumor tissue sections, anti-CD11b/Mac-1 (M1/70), anti-B220 (RA3-6B2), anti-CD4 (L3T4), anti-CD8 (53-6.72) anti–Ly-76 mAbs (TER-119), all of which were purchased from Pharmingen, and c-Kit mAb (ACK2) were used. The sections were mounted on glass slides under No. 1 coverslips (Matsunami Glass, Osaka, Japan) using an Aquatex (Merck, Darmstadt, Germany). The sections were observed using an Olympus IX-70 microscope equipped with UPlanFI 4×/0.13 and LCPlanFI 20×/0.04 dry objective lenses (Olympus, Tokyo, Japan). Images were acquired with a CoolSnap digital camera (Roper Scientific, Tokyo, Japan). For detection of apoptotic cells in tumor tissues, we performed the transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) assay using the In situ Cell Death Detection Kit (Roche, Mannhein, Germany) according to the manufacturer's instructions.
Immunohistochemistry of whole mount tissue was performed as described elsewhere.19 In brief, fixed tissues were stained with anti–PECAM-1 and anti-CD45 mAb. Second antibody and substrates for color reaction were the same as performed in staining of tissue sections. Stained tumor tissues were observed under a Leica MZ12.5 stereomicroscope equipped with a PlanApo 1× objective lens (Leica, Heerbrugg, Switzerland) and photographed with a DC120 digital camera (Pixera, Los Gatos, CA). In all assays, we used an isotype-matched control Ig as a negative control and confirmed that the positive signals were not derived from nonspecific background. Images were processed using Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA).
Cell preparation and flow cytometry
Tumors were staged by their size, and tumors with a volume of approximately 1.0 × 103 mm3 were used for flow cytometric analysis. Tumor tissues were minced with a scissor in PBS. Minced tumor tissues were incubated with 0.1% collagenase (Sigma) in 10% FCS at 37°C for 30 minutes, and then the dispersed tumor tissue cells were drawn through a 23-gauge needle. The cell staining procedure for flow cytometry was as described previously.17 The mAbs used in immunofluorescence staining were anti–c-Kit (2B8), –Sca-1, –Mac-1 (M1/70), –Gr-1 (RB6-8C5), -B220 (RA3-6B2), -CD4 (L3T4), -CD8 (53-6.72), and –Ly-76 (TER-119), all of which were purchased from Pharmingen. A mixture of mAbs against Mac-1, Gr-1, B220, CD4, CD8, and Ly-76 was used as a lineage marker (Lin). The stained cells were analyzed by FACSCalibur (Becton Dickinson, San Jose, CA) and sorted by EPICS ALTRA (Beckman Coulter, Fullerton, CA).
Cell lysate from tumor cells in culture or tumor tissue
Cells harvested from culture dishes using 5 mM EDTA (ethylenediaminetetraacetic acid) in PBS or cells from minced tumor tissue were homogenized with 18-gauge and 22-gauge needles. The cells were suspended in 0.25 mM Tris (tris(hydroxymethyl)aminomethane)–HCl and frozen at –80°C and thawed at 37°C repeatedly for a total of 7 cycles. Finally, the cell lysate was sonicated and filtered through Ultrafree-MC (Millipore, Bedford, MA).
Migration assay of BM cells
BM mononuclear cells were obtained from adult mice using standard methods.17 The BM cells were stained with Lin marker, anti–c-Kit (2B8) mAb, and anti-Tie2 (TEK4) mAb as described in “Cell preparation and flow cytometry.” The migration assay of BM cells was performed using 24-well Transwell chambers with 5-μm pore size bare filter (Costar, Corning, NY). The migration assay was performed as previously described.10 In brief, 600 μL of the lysate from tumor cells that was diluted to a protein concentration of 200 μg/mL in serum-free medium (RPMI 1640) was placed in the lower chamber; 5 × 105 stained BM cells suspended in 100 μL RPMI 1640 medium containing 0.5% bovine serum albumin (BSA) were seeded in the upper compartment. The chamber was then incubated at 37°C in humidified 5% CO2 air for 4 hours to allow cell migration. The chamber was then removed, and the phenotype of the cells in the lower well was analyzed by FACSCalibur.
CFU-c analysis and LTC-IC assay using Lin–c-Kit+Sca-1+ cells from BM or tumor tissue
A population of 2 × 102 Lin–c-Kit+Sca-1+ HSCs/hematopoietic progenitor cells (HPCs) that had been sorted from BM or PC3 tumor tissue were cultured under the semisolid culture condition as previously reported.17 Hematopoietic colonies containing more than 30 cells were counted as CFU-c's. The assay of long-term culture-initiating cells (LTC-ICs) was performed as previously reported.20 Briefly, 2 × 102 Lin–c-Kit+Sca-1+ cells were suspended in complete medium composed of α-minimum essential medium (α-MEM) (GIBCO) supplemented with 12.5% fetal bovine serum (FBS), 12.5% horse serum (GIBCO), 100 U/mL penicillin (Sigma), 2 mM l-glutamine (GIBCO), 10–4 M 2-mercaptoethanol (GIBCO), and freshly dissolved 10–6 M hydrocortisone (Sigma). Test cells were seeded into cultures containing a feeder layer of murine OP9 cells that had been treated with 10 mg/mL mitomycin C for 4 hours. Half of the culture medium was changed weekly. After 5 weeks of culture, nonadherent and adherent cells were harvested by trypsinization, pooled, washed, and cultured under the semisolid condition using MethoCult (GFM3434; Stem Cell Technologies, Vancouver, BC, Canada). The total number of hematopoietic colonies (ie, mixed colonies [CFU-Mix] plus erythroid burst-forming unit [BFU-E] plus granulocyte-macrophage colony-forming unit [CFU-GM]) was counted after 7 days of culturing.
Results
Localization of HCs during the development of colon26 colon cancer tumor
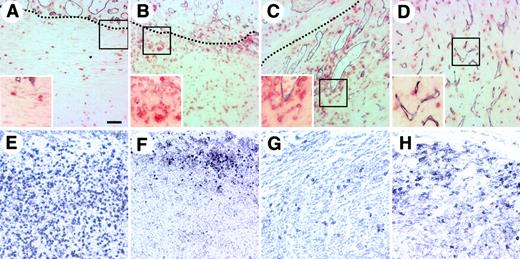
To examine when HCs migrate from a pre-existing vessel near the tumor mass and how angiogenesis occurs in association with migration of HCs, the localization of HCs was observed during the development and growth of colon26 tumors. After subcutaneous injection of colon26 tumor cells, the mice were killed at various time points and the tumor mass was excised. A few HCs were observed in the tumor on day 1 after injection, and numerous HCs were distributed in the tumor mass on day 3 (Figure 1A-B). However, PECAM-1–positive ECs did not migrate into the tumor within the first 3 days after subcutaneous injection of colon26 tumor cells. Five to 7 days after tumor cell injection, as the tumor grew, PECAM-1–positive ECs rapidly migrated into the tumor mass, which already contained many HCs (Figure 1C). Finally, as the tumor grew larger, a very fine capillary networklike structure formed at the center of the tumor mass where HCs were already located (Figure 1D).
Localization of HCs in colon26 tumor tissues. (A-D) Sections of colon26 tumor tissues from tumors with a volume of (A) 30 to 40 mm3, (B) 60 to 70 mm3, (C) 100 and 120 mm3, and (D) more than 500 mm3 were stained with anti–PECAM-1 mAb (dark blue) and anti-CD45 mAb (red). The dotted line (A-C) shows the border between the fibrous cap surrounding the tumor mass and the tumor mass. The area beneath the dotted line is the tumor mass. (E-H) Sections from colon26 tumors of 60 to 70 mm3 that were stained with anti-CD11b (Mac-1) (E), anti-B220 (F), a mixture of anti-CD4 and anti-CD8 (G), or anti–Ly-76 (Ter119) (H) mAb. Insets in panels A-D show high-power views of the area indicated by box. Bar indicates 50 μm.
Localization of HCs in colon26 tumor tissues. (A-D) Sections of colon26 tumor tissues from tumors with a volume of (A) 30 to 40 mm3, (B) 60 to 70 mm3, (C) 100 and 120 mm3, and (D) more than 500 mm3 were stained with anti–PECAM-1 mAb (dark blue) and anti-CD45 mAb (red). The dotted line (A-C) shows the border between the fibrous cap surrounding the tumor mass and the tumor mass. The area beneath the dotted line is the tumor mass. (E-H) Sections from colon26 tumors of 60 to 70 mm3 that were stained with anti-CD11b (Mac-1) (E), anti-B220 (F), a mixture of anti-CD4 and anti-CD8 (G), or anti–Ly-76 (Ter119) (H) mAb. Insets in panels A-D show high-power views of the area indicated by box. Bar indicates 50 μm.
We studied the characteristics of the HCs that migrated into the tumor mass by immunohistochemical staining for several hematopoietic markers. In the early stage of tumorigenesis, in tumors of 60 to 70 mm3 (Figure 1B), most CD45+ HCs were CD11b/Mac-1+ monocytes or macrophages (Figure 1E). Other cells were Ly-76/Ter119+ erythroid cells that were located at the edge of the tumor mass (Figure 1F), a very small number of CD4+ or CD8+ T lymphocytes, and a relatively large number of B220+ B lymphocytes compared with the number of T lymphocytes (Figure 1G-H). Similar staining profiles were observed in tumor sections from C57BL/6 mice that had been injected with Lewis lung carcinoma cells or B16 mouse melanoma cells (data not shown).
Suppression of hematopoiesis inhibits angiogenesis in vitro
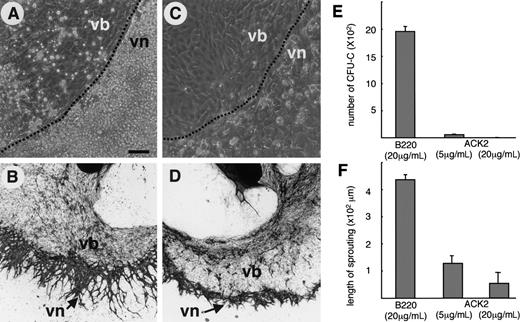
Based on the results of the histologic analysis, we hypothesized that if the migration of HCs into the tumor was inhibited, tumor angiogenesis might be inhibited. To address this issue, we tried to use an anti–c-Kit neutralizing antibody (ACK2) that can inhibit the binding of stem cell factor (SCF) to c-Kit—which is an important receptor for the survival of HSCs and for the proliferation of HPCs—to suppress hematopoiesis. Before using ACK2 in vivo, we investigated whether suppression of hematopoiesis by ACK2 inhibits angiogenesis in vitro. Regarding in vitro analysis of angiogenesis, we previously reported that HC-dependent angiogenesis was observed in the para-aortic splanchnopleural mesoderm (P-Sp) culture system.10 In the present study, in P-Sp explants that had been treated with the control antibody, ECs migrated from the P-Sp explants to form a sheetlike structure (Figure 2B, vascular bed [vb]); subsequently, ECs sprouted from the sheetlike structure and formed a networklike structure (Figure 2B, vascular network [vn]). HSCs appeared on the sheetlike structure and migrated into the networklike area; the HSCs then proliferated and underwent differentiation. Finally, the HCs formed a very large colony on this networklike area of ECs (Figure 2A). Using P-Sp explants from AML1 null mice in which definitive hematopoiesis is completely suppressed, we previously showed that the lack of HCs in this culture completely disrupted the formation of a networklike structure of ECs.10 The addition of ACK2 severely suppressed the proliferation of HCs on the vascular network (Figure 2C), and the number of CFU-c's decreased in a dose-dependent manner according to the concentration of ACK2 (Figure 2E). As the number of HCs decreased, vn formation decreased (Figure 2D,F). Therefore, we confirmed that suppression of hematopoiesis inhibits the formation of an endothelial networklike structure in vitro.
Suppression of hematopoiesis inhibits vascular network formation of ECs in P-Sp culture system. (A-D) P-Sp explants were cultured on OP9 stromal cells in the presence of anti-B220 mAb (20 μg/mL) as a control (A-B) or anti–c-Kit neutralizing antibody, ACK2 (20 μg/mL) (C-D), and the development of HCs and ECs was observed. (A,C) Phase contrast view of P-Sp cultures. The dotted line shows the presumptive border between the vascular bed (vb) and the vascular network area (vn). (B,D) PECAM-1 staining of culture plates after 10 days of culture. Bar in panel A indicates 50 μm (A,C). Original magnification, ×4 (B,D). (E) Total number of CFU-c's observed in the P-Sp culture system in the presence of anti-B220 mAb (20 μg/mL) or ACK2 (5 or 20 μg/mL). Results are expressed as the mean number of CFU-c's in 3 experiments (± SEM). (F) Length of elongated ECs (sprouting) from the vb. Results are expressed as mean length at 10 random points. Standard deviations are indicated.
Suppression of hematopoiesis inhibits vascular network formation of ECs in P-Sp culture system. (A-D) P-Sp explants were cultured on OP9 stromal cells in the presence of anti-B220 mAb (20 μg/mL) as a control (A-B) or anti–c-Kit neutralizing antibody, ACK2 (20 μg/mL) (C-D), and the development of HCs and ECs was observed. (A,C) Phase contrast view of P-Sp cultures. The dotted line shows the presumptive border between the vascular bed (vb) and the vascular network area (vn). (B,D) PECAM-1 staining of culture plates after 10 days of culture. Bar in panel A indicates 50 μm (A,C). Original magnification, ×4 (B,D). (E) Total number of CFU-c's observed in the P-Sp culture system in the presence of anti-B220 mAb (20 μg/mL) or ACK2 (5 or 20 μg/mL). Results are expressed as the mean number of CFU-c's in 3 experiments (± SEM). (F) Length of elongated ECs (sprouting) from the vb. Results are expressed as mean length at 10 random points. Standard deviations are indicated.
Tumor angiogenesis is inhibited by leukocytopenia
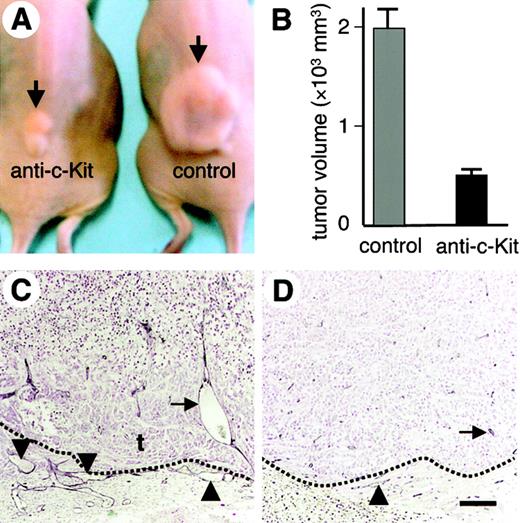
As previously reported, injection of ACK2 into mice results in a reduction in the number of c-Kit+ HSCs/HPCs.21 We injected ACK2 into mice and counted the number of mononuclear cells in the peripheral blood. As shown in Figure 3I, upon injection of 1 mg per mouse per day of ACK2 for 4 days on day –4today –1, the number of mononuclear cells on day 0 was nearly 10 times lower than that in mice that had been injected with control antibody. Injection of larger amounts of ACK2 promoted death of the mice due to paralytic ileus, because c-Kit is expressed on Cajal cells that work as a pacemaker to maintain the movement of the gut. Next, after injection of ACK2 at 1 mg per mouse per day on day –4 to day –1, we subcutaneously inoculated colon26 tumor cells into the mice on day 0 (denoted day 0 in Figure 3I) and observed the initiation of tumor angiogenesis without additional injection of ACK2. In the control tumors of mice that had been injected with control antibody, 2 to 3 days after tumor cell inoculation, sprouting angiogenesis was initiated (Figure 3A-B) with migration of massive numbers of HCs into the tumor mass (Figure 3C). On the other hand, in mice that had been injected with ACK2 and then inoculated with colon26 tumor cells, fewer HCs migrated into the tumor (Figure 3F), and sprouting angiogenesis from a pre-existing vessel was completely suppressed (Figure 3D-E). A delay in angiogenesis around the tumor mass was still observed on day 5 in ACK2-injected mice (Figure 3H) compared with that in control mice (Figure 3G).Although tumor growth was still inhibited on day 7 in ACK2-injected mice (Figure 3J), the tumor thereafter rapidly grew as the number of HCs in the peripheral blood increased without additional ACK2 injection (Figure 3I-J). ACK2 did not alter the in vitro proliferative ability of colon26 cells and primary ECs that had been isolated from skin specimens from the mice (data not shown). Therefore, we concluded that HCs that migrated into the tumor mass promoted the initiation of sprouting angiogenesis.
Suppression of tumor angiogenesis in colon26 tumor mass by leukocytopenia. (A-F) Whole mount staining of colon26 tumor mass with anti–PECAM-1 mAb (dark blue) (A,B,D,E) or anti–PECAM-1 (dark blue) and anti-CD45 (red) mAbs (C,F). The tumor mass along with skin was dissected 3 days after inoculation of colon26 tumor cells from mice after treatment with the anti–c-Kit mAb, ACK2 (D-F), or anti-B220 mAb as a control (A-C). The dotted line shows the mass of inoculated tumor cells (A,D). (B,E) High-power view of the location indicated by the arrow in panels A,D, respectively. (G,H) Gross appearance of tumor mass. The photographs of the dissected tumors were taken 5 days after inoculation of colon26 tumor cells from ACK2-treated mice (H) or anti-B220–treated mice (G). (I) Number of white blood cells (WBCs) in the peripheral blood after treatment with ACK2 (blue line) or anti-B220 mAb (red line; control [CTL]) for 4 days from day –4 to day –1. Results are expressed as the mean number of WBCs in 3 experiments (± SEM). (J) Tumor volume in mice that had been treated with ACK2 (blue line) or anti-B220 mAb (red line; CTL). Day 0 in this graph corresponds to the day 0 indicated in panel I. The tumor volumes measured on day 5, day 7, and day 14 were as follows: ACK2 (c-Kit): 52 ± 13 mm3 at day 5, 65 ± 15 mm3 at day 7, and 1020 ± 358 mm3 at day 14; B220 (CTL): 86 ± 15 mm3 at day 5, 890 ± 211 mm3 at day 7, and 1856 ± 325 mm3 at day 14. Results are expressed as the mean volume of 6 experiments (± SEM).
Suppression of tumor angiogenesis in colon26 tumor mass by leukocytopenia. (A-F) Whole mount staining of colon26 tumor mass with anti–PECAM-1 mAb (dark blue) (A,B,D,E) or anti–PECAM-1 (dark blue) and anti-CD45 (red) mAbs (C,F). The tumor mass along with skin was dissected 3 days after inoculation of colon26 tumor cells from mice after treatment with the anti–c-Kit mAb, ACK2 (D-F), or anti-B220 mAb as a control (A-C). The dotted line shows the mass of inoculated tumor cells (A,D). (B,E) High-power view of the location indicated by the arrow in panels A,D, respectively. (G,H) Gross appearance of tumor mass. The photographs of the dissected tumors were taken 5 days after inoculation of colon26 tumor cells from ACK2-treated mice (H) or anti-B220–treated mice (G). (I) Number of white blood cells (WBCs) in the peripheral blood after treatment with ACK2 (blue line) or anti-B220 mAb (red line; control [CTL]) for 4 days from day –4 to day –1. Results are expressed as the mean number of WBCs in 3 experiments (± SEM). (J) Tumor volume in mice that had been treated with ACK2 (blue line) or anti-B220 mAb (red line; CTL). Day 0 in this graph corresponds to the day 0 indicated in panel I. The tumor volumes measured on day 5, day 7, and day 14 were as follows: ACK2 (c-Kit): 52 ± 13 mm3 at day 5, 65 ± 15 mm3 at day 7, and 1020 ± 358 mm3 at day 14; B220 (CTL): 86 ± 15 mm3 at day 5, 890 ± 211 mm3 at day 7, and 1856 ± 325 mm3 at day 14. Results are expressed as the mean volume of 6 experiments (± SEM).
The c-Kit+ HCs are involved in blood vessel formation in the fibrous cap of the tumor mass
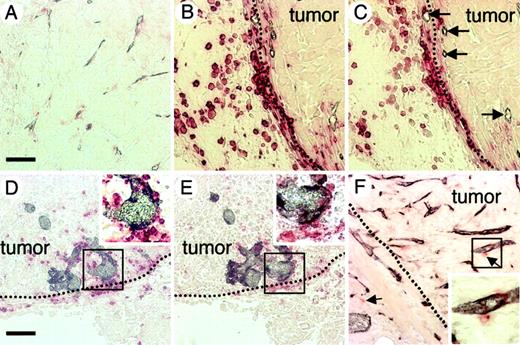
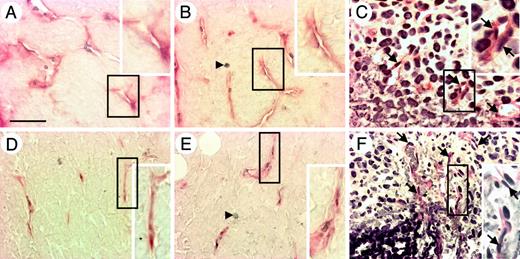
Although CD45+ HCs such as the macrophage/monocyte lineage, erythroid cells, and T and B lymphocytes were abundant in tumors of colon26, Lewis lung carcinoma, and B16 melanoma (data not shown in later 2 tumors), interestingly, a different staining profile of hematopoietic markers was observed in the tumors of nude mice that had been inoculated with PC3 human prostate cancer cells. There were fewer CD45+ HCs and capillaries in the tumor tissue of PC3 tumors (Figure 4A) than in colon26 tumor tissue (Figure 1D). Particularly, 5 to 7 days after inoculation of PC3 cells, many CD45+ HCs were located at the edge of the tumor tissue (Figure 4B) and most of the CD45+ HCs expressed c-Kit (Figure 4C). At this stage, very small capillaries were observed near the c-Kit+ HCs (arrows in Figure 4C), but as the tumor grew, c-Kit+ HCs accumulated around the newly formed blood vessel and the caliber of the capillaries increased (Figure 4D-E). On the other hand, in colon26 tumor tissues, a very small number of c-Kit+ HCs were observed (Figure 4F).
Localization of HCs in PC3 tumor tissues. (A-E) Sections of PC3 tumor tissues from PC3 tumors with a volume of (A) more than 500 mm3, (B-C) 60 to 70 mm3, and (D-E) 100 to 120 mm3 were double stained with anti–PECAM-1 mAb (dark blue) and anti-CD45 mAb (red) (A,B,D) or with anti–PECAM-1 mAb (dark blue) and anti–c-Kit mAb (red) (C,E). Arrows in panel C indicate a small capillary at the tumor edge. Panels C,E are serial sections of panels B,D, respectively. (F) Colon26 tumor tissue section from a tumor with a volume of 60 to 70 mm3 was stained with anti–PECAM-1 mAb (dark blue) and anti–c-Kit mAb (red). Arrows indicate cells that are positive for c-Kit. The dotted line (B-F) shows the border between the fibrous cap surrounding the tumor mass and the tumor mass. Insets (D-F) show high-power views of the area indicated by box. The bar in panel A indicates 50 μm (A-C). The bar in panel D indicates 100 μm (D-F).
Localization of HCs in PC3 tumor tissues. (A-E) Sections of PC3 tumor tissues from PC3 tumors with a volume of (A) more than 500 mm3, (B-C) 60 to 70 mm3, and (D-E) 100 to 120 mm3 were double stained with anti–PECAM-1 mAb (dark blue) and anti-CD45 mAb (red) (A,B,D) or with anti–PECAM-1 mAb (dark blue) and anti–c-Kit mAb (red) (C,E). Arrows in panel C indicate a small capillary at the tumor edge. Panels C,E are serial sections of panels B,D, respectively. (F) Colon26 tumor tissue section from a tumor with a volume of 60 to 70 mm3 was stained with anti–PECAM-1 mAb (dark blue) and anti–c-Kit mAb (red). Arrows indicate cells that are positive for c-Kit. The dotted line (B-F) shows the border between the fibrous cap surrounding the tumor mass and the tumor mass. Insets (D-F) show high-power views of the area indicated by box. The bar in panel A indicates 50 μm (A-C). The bar in panel D indicates 100 μm (D-F).
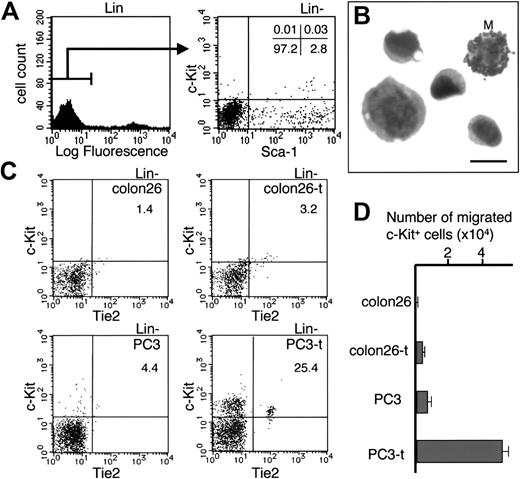
Based on the appearance of the capillaries, we hypothesized that the role of c-Kit+ HCs in tumor angiogenesis is to stabilize or support the maturation of a newly developed unstable capillary. First, we further characterized the c-Kit+ HCs in this tumor by flow cytometric analysis. These c-Kit+ HCs did not express CD4, CD8, B220, or Ter119 and weakly expressed CD11b on immunohistochemical staining of tumor sections (data not shown), and we observed the c-Kit+ cell fraction among lineage–/dull cells on flow cytometry (Figure 5A). Approximately 60% of the Lin–/dull c-Kit+ cells also expressed Sca-1, suggesting an HSC/HPC population. Moreover, we confirmed that approximately 80% of the sorted Lin–/dull c-Kit+ cells were phenotypically an HSC/HPC population, and the remaining cells were mast cells (Figure 5B). Additionally, we tested whether the phenotypic HSC/HPC population that was observed in PC3 tumor tissues has hematopoietic potential or not using the CFU-c and LTC-IC assays. Compared with the Lin–c-Kit+Sca-1+ HSC/HPC population in BM, the frequencies of CFU-c's and LTC-ICs among the Lin–c-Kit+Sca-1+ population from PC3 tumor tissue were low at 63% to 66% (Table 1); however, we confirmed that Lin–c-Kit+Sca-1+ cells in PC3 tumor tissue are an HSC/HPC population.
Phenotype of c-Kit+ HCs located in the tumor. (A) Fluorescence-activated cell sorter (FACS) analysis of cells in PC3 tumor tissue. The numbers in each quadrant represent the percentage of gated Lin– cells. (B) Phenotype of c-Kit+ HCs sorted from PC3 tumor tissue by FACS. Cells fixed on glass were analyzed by May-Grünwald-Giemsa staining. M indicates mast cell. Bar indicates 10 μm. (C) FACS analysis of migrated BM cells by tumor lysates. The number in the upper right quadrant indicates the total percentage of migrated c-Kit+ cells. (D) Chemotaxis of c-Kit+ HCs in the presence of the lysates of various tumor cells. Colon26 indicates cell lysate from in vitro culture of colon26 cells; colon26-t, lysate of cells from colon26 tumor; PC3, cell lysate from in vitro culture of PC3 cells; PC3-t, lysate of cells from PC3 tumor. Results are expressed as the mean number in 3 experiments (± SEM).
Phenotype of c-Kit+ HCs located in the tumor. (A) Fluorescence-activated cell sorter (FACS) analysis of cells in PC3 tumor tissue. The numbers in each quadrant represent the percentage of gated Lin– cells. (B) Phenotype of c-Kit+ HCs sorted from PC3 tumor tissue by FACS. Cells fixed on glass were analyzed by May-Grünwald-Giemsa staining. M indicates mast cell. Bar indicates 10 μm. (C) FACS analysis of migrated BM cells by tumor lysates. The number in the upper right quadrant indicates the total percentage of migrated c-Kit+ cells. (D) Chemotaxis of c-Kit+ HCs in the presence of the lysates of various tumor cells. Colon26 indicates cell lysate from in vitro culture of colon26 cells; colon26-t, lysate of cells from colon26 tumor; PC3, cell lysate from in vitro culture of PC3 cells; PC3-t, lysate of cells from PC3 tumor. Results are expressed as the mean number in 3 experiments (± SEM).
Number of CFU-c's and LTC-ICs from BM and tumor
Source of KSL cells, 2 × 102 . | CFU-c . | LTC-IC . |
|---|---|---|
| BM | 56 ± 7 | 35 ± 8 |
| PC3 tumor | 35 ± 4 | 23 ± 5 |
Source of KSL cells, 2 × 102 . | CFU-c . | LTC-IC . |
|---|---|---|
| BM | 56 ± 7 | 35 ± 8 |
| PC3 tumor | 35 ± 4 | 23 ± 5 |
The number of CFU-c's or LTC-ICs was evaluated after 1 week or 5 weeks of culturing, respectively, as described in “Materials and methods.” The results are expressed as the mean ± SD (n = 4).
KSL indicates Lin-c-Kit+Sca-1+.
To determine the function of these c-Kit+ HCs surrounding the blood vessel at the tumor edge, we injected ACK2 into mice according to the schedule described in the legend of Figure 3 and inhibited the accumulation of c-Kit+ HCs near the tumor (data not shown). In the tumors of mice that had been injected with control antibody, the caliber of the vessels in the fibrous cap and vessels in the inner tumor mass had increased (Figure 6C). On the other hand, in mice that had been injected with ACK2, the caliber of vessels barely increased and sprouting angiogenesis into the tumor mass was barely observed (Figure 6D). In accordance with the poor vasculature around the tumor mass in ACK2-injected mice, tumor growth was severely inhibited (Figure 6A-B). ACK2 did not alter the in vitro proliferative ability of PC3 cells and primary ECs that had been isolated from skin specimens from the mice (data not shown). Therefore, we concluded that BM suppression that had been induced by injection of ACK2 suppressed blood vessel formation at the tumor edge of PC3 tumors.
Suppression of tumor angiogenesis in PC3 tumor mass by anti–c-Kit mAb. The anti–c-Kit mAb, ACK2, was injected for 4 days (1 mg/d per mouse) into nude mice as described in the legend to Figure 3. (A) Photographs of mice taken 14 days after inoculation with PC3 cells. Arrows indicate the tumor mass in mice that had been treated with ACK2 (anti–c-Kit) or anti-B220 mAb (control). (B) Tumor volume on day 14 in mice that had been treated with ACK2 (anti–c-Kit) or anti-B220 mAb (control). The tumor volumes measured on day 14 were 486 ± 53 mm3 (ACK2: n = 8), and 1927 ± 258 mm3 (B220: n = 8). Results are expressed as the mean volume of 8 experiments (± SEM). (C-D) Tissue sections of the tumors from the mice observed in panel A, stained with anti–PECAM-1 mAb and counterstained with fast red. Tissue sections of the tumors from mice that had been injected with anti-B220 mAb (C) or ACK2 (D) are shown. The dotted line shows the border between the fibrous cap surrounding the tumor mass and the tumor mass. The area above the dotted line is the tumor mass. Arrow indicates a capillary that migrated into the tumor. The arrowheads show a vascular bed observed in the fibrous cap surrounding the tumor mass. Bar indicates 150 μm in panels C,D.
Suppression of tumor angiogenesis in PC3 tumor mass by anti–c-Kit mAb. The anti–c-Kit mAb, ACK2, was injected for 4 days (1 mg/d per mouse) into nude mice as described in the legend to Figure 3. (A) Photographs of mice taken 14 days after inoculation with PC3 cells. Arrows indicate the tumor mass in mice that had been treated with ACK2 (anti–c-Kit) or anti-B220 mAb (control). (B) Tumor volume on day 14 in mice that had been treated with ACK2 (anti–c-Kit) or anti-B220 mAb (control). The tumor volumes measured on day 14 were 486 ± 53 mm3 (ACK2: n = 8), and 1927 ± 258 mm3 (B220: n = 8). Results are expressed as the mean volume of 8 experiments (± SEM). (C-D) Tissue sections of the tumors from the mice observed in panel A, stained with anti–PECAM-1 mAb and counterstained with fast red. Tissue sections of the tumors from mice that had been injected with anti-B220 mAb (C) or ACK2 (D) are shown. The dotted line shows the border between the fibrous cap surrounding the tumor mass and the tumor mass. The area above the dotted line is the tumor mass. Arrow indicates a capillary that migrated into the tumor. The arrowheads show a vascular bed observed in the fibrous cap surrounding the tumor mass. Bar indicates 150 μm in panels C,D.
Tumor environment promotes chemotaxis of c-Kit+Tie2+ HSCs
The localization of HSCs/HPCs suggested that PC3 tumor cells may promote the migration of c-Kit+ HCs to the tumor edge. To test this, we studied the migration of BM cells using the lysates of cells obtained from in vitro cultures of colon26 cells or PC3 cells or the lysates of cells obtained from colon26 or PC3 tumor tissue in vivo. As observed in Figure 5C-D, c-Kit+ HCs did not migrate toward the cell lysate from colon26 cells (colon26: 876 ± 57 cells, n = 3) or toward the cell lysate from colon26 tumor (colon26-t: 2540 ± 351 cells, n = 3). Although PC3 cell lysate did not effectively promote the migration of c-Kit+ HCs (PC3: 3766 ± 520 cells, n = 3), the PC3 tumor tissue lysate significantly promoted the migration of c-Kit+ HCs (47 100 ± 5720 cells, n = 3). Very interestingly, PC3 tumor tissue lysate promoted the migration of very immature c-Kit+Tie2+ HSCs (Figure 5C).
The c-Kit blocking antibody does not act directly on endothelial cells
The c-Kit+ cells that had been sorted from colon26 or PC3 tumor tissues barely differentiated into ECs when cultured on OP9 stromal cells, which can support EC development from embryonic stem cells, or in fibronectin-coated culture plates in the presence of VEGF (data not shown). Moreover, ECs in the tumor environment did not seem to express c-Kit (Figure 4C,F). Therefore, it is suggested that c-Kit blocking antibody would not directly affect the survival, proliferation, or migration of ECs in tumors. However, to test the possibility that c-Kit blocking antibody acts directly on ECs in vivo, we injected ACK2 antibody into mice (1 mg per mouse per day) daily from day 2 to day 5 after subcutaneous inoculation of tumor cells on day 0 and observed whether angiogenesis was affected, focusing on the migration and apoptosis of ECs on day 7. As a positive control of apoptosis, we subcutaneously injected a low dose (170 mg/kg per single injection) of cyclophosphamide (CPM) into mice on day 0 and day 6 after inoculation of tumor cells (Figure 7C,F). It was previously reported that this schedule of CPM injection effectively promoted the apoptosis of ECs.22
Analysis of the survival and migration of ECs into tumor tissues after injection of anti–c-Kit mAb in tumor-bearing mice. Colon26 tumor cells (A-C) or PC3 tumor cells (D-F) were subcutaneously inoculated into mice. Then, anti-B220 control mAb (A,D), anti–c-Kit mAb (B,E), or CPM (C,F) was injected into the mice. Anti-B220 mAb or anti–c-Kit mAb (1 mg/d per mouse) was injected daily from day 2 to day 5 after inoculation of tumor cells, and CPM (170 mg/kg per single injection) was injected on day 0 and day 6. Tumors were dissected on day 7. Sections were stained with anti–PECAM-1 mAb (red) and were subsequently analyzed for the presence of apoptotic cells by the TUNEL assay (dark blue). Arrowheads in panels B,E indicate round, apoptotic cells, and arrows in panels C,F indicate ECs. The inset in each panel shows a high-power view of the area indicated by the box. Bar in panel A indicates 40 μm in panels A-C and 60 μm in panels D-F.
Analysis of the survival and migration of ECs into tumor tissues after injection of anti–c-Kit mAb in tumor-bearing mice. Colon26 tumor cells (A-C) or PC3 tumor cells (D-F) were subcutaneously inoculated into mice. Then, anti-B220 control mAb (A,D), anti–c-Kit mAb (B,E), or CPM (C,F) was injected into the mice. Anti-B220 mAb or anti–c-Kit mAb (1 mg/d per mouse) was injected daily from day 2 to day 5 after inoculation of tumor cells, and CPM (170 mg/kg per single injection) was injected on day 0 and day 6. Tumors were dissected on day 7. Sections were stained with anti–PECAM-1 mAb (red) and were subsequently analyzed for the presence of apoptotic cells by the TUNEL assay (dark blue). Arrowheads in panels B,E indicate round, apoptotic cells, and arrows in panels C,F indicate ECs. The inset in each panel shows a high-power view of the area indicated by the box. Bar in panel A indicates 40 μm in panels A-C and 60 μm in panels D-F.
As observed in Figure 7B,E, when c-Kit blocking antibody was injected into tumor-bearing mice, apoptosis of ECs was not induced and the number of blood vessels in the deep layer of the tumor did not significantly differ from that in tumors injected with control antibody (Figure 7A,D). These results suggest that c-Kit antibody does not directly influence the survival in tumor tissue or migration of ECs into tumor tissues. Moreover, this schedule of ACK2 injection did not clearly suppress angiogenesis or tumor growth (data not shown). Therefore, we concluded that HCs regulate tumor angiogenesis during tumor growth.
Discussion
In the present report, we showed that a large number of CD45+ HCs migrate into tumor tissue and play important roles in tumor angiogenesis. Recent data have suggested that inflammation is a critical component of tumor progression.14 Many cancers arise from sites of infection, chronic stimulation, or inflammation where many inflammatory cells such as neutrophils, dendritic cells, macrophages, eosinophils, and mast cells as well as lymphocytes localize. Such HCs produce many chemokines, cytokines, matrix metalloproteinases, and others and have been suggested to promote angiogenesis.14,23 In addition to such proangiogenic effect, HCs have the capacity to suppress tumor growth; for instance, tumor-associated macrophages may kill neoplastic cells directly14 and have been reported to produce angiogenesis inhibitors such as thrombospondin-1.24 Because HCs play dual roles in tumor growth, it has been unclear whether suppression of HC migration actually inhibits tumor growth. One study showed that impaired recruitment of bone marrow cells blocked tumor angiogenesis using Id1+/–Id3–/– mutant mice.25 However, it was not clear whether CD45+ HCs show the same responses in different tumors. In the present study, we induced BM suppression by administering an anti–c-Kit neutralizing antibody, and the resultant leukocytopenia inhibited sprouting angiogenesis from pre-existing vessels in colon26 tumors. In PC3 tumors, the resultant leukocytopenia inhibited the migration of c-Kit+ HSCs/HPCs into the fibrous cap surrounding the tumor, thereby suppressing maturation or dilation of the newly formed blood vessel.
In terms of maturation of blood vessel by HSCs/HPCs, we reported that HSCs promote angiogenesis and vascular remodeling during embryogenesis by producing Ang-1, a ligand for Tie2.10 Moreover, the HSC/HPC populations designated as Lin–c-Kit+Sca-1+ or Lin–c-Kit+Tie2+ in adult bone marrow also express Ang-1 (data not shown). As previously reported, in transgenic mice that overexpressed Ang-1 in keratinocytes, significantly enlarged vessels formed in the dermis.26 Ang-1 is usually expressed in the mural cells that surround ECs, and it promotes adhesion between ECs and mural cells, resulting in stabilization of the blood vessel.10,18,27,28 Therefore, these suggest that HSCs/HPCs accumulating at the tumor edge of PC3 tumors promote the stability or promote an increase in caliber of the newly developed blood vessels around the tumor.
HSCs and endothelial progenitor cells (EPCs) are difficult to distinguish by their surface phenotype, and EPCs may express c-Kit. Therefore, although c-Kit antibody did not affect the migration or survival of ECs in tumors, it is possible that c-Kit antibody inhibits the recruitment of c-Kit+ EPCs into tumor tissues. However, our analysis indicated that a very low frequency of c-Kit+ cells in tumor tissue may not contribute to tumor angiogenesis, even if such c-Kit+ cells could differentiate into ECs. This concept is supported by a recent paper showing the minor contribution of EPCs in tumor angiogenesis.29 Therefore, c-Kit+ HSCs/HPCs are suggested to contribute to the establishment of blood vessel maturation in the early stage of tumor development, especially in PC3 tumor.
How tumor dormancy and progression are regulated is not clearly understood. Among many stimuli, angiogenic switch is thought to be one trigger for tumor progression from the quiescent stage.2 In this paper, we showed that HCs migrated into the tumor mass prior to the initiation of angiogenesis in the tumor mass. Therefore, it is suggested that HCs regulate the angiogenic switch during tumorigenesis, and inhibition of the migration of HCs into tumors will be an effective strategy for suppression of tumor angiogenesis.
Prepublished online as Blood First Edition Paper, November 30, 2004; DOI 10.1182/blood-2004-08-3317.
Supported in part by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan (N. Takakura).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr S.-I. Nishikawa (CDB, Kobe, Japan) for providing c-Kit mAb and Miss M. Sato, K. Ishida, Y. Shimizu, and Mrs Y. Nakano for technical support.

![Figure 3. Suppression of tumor angiogenesis in colon26 tumor mass by leukocytopenia. (A-F) Whole mount staining of colon26 tumor mass with anti–PECAM-1 mAb (dark blue) (A,B,D,E) or anti–PECAM-1 (dark blue) and anti-CD45 (red) mAbs (C,F). The tumor mass along with skin was dissected 3 days after inoculation of colon26 tumor cells from mice after treatment with the anti–c-Kit mAb, ACK2 (D-F), or anti-B220 mAb as a control (A-C). The dotted line shows the mass of inoculated tumor cells (A,D). (B,E) High-power view of the location indicated by the arrow in panels A,D, respectively. (G,H) Gross appearance of tumor mass. The photographs of the dissected tumors were taken 5 days after inoculation of colon26 tumor cells from ACK2-treated mice (H) or anti-B220–treated mice (G). (I) Number of white blood cells (WBCs) in the peripheral blood after treatment with ACK2 (blue line) or anti-B220 mAb (red line; control [CTL]) for 4 days from day –4 to day –1. Results are expressed as the mean number of WBCs in 3 experiments (± SEM). (J) Tumor volume in mice that had been treated with ACK2 (blue line) or anti-B220 mAb (red line; CTL). Day 0 in this graph corresponds to the day 0 indicated in panel I. The tumor volumes measured on day 5, day 7, and day 14 were as follows: ACK2 (c-Kit): 52 ± 13 mm3 at day 5, 65 ± 15 mm3 at day 7, and 1020 ± 358 mm3 at day 14; B220 (CTL): 86 ± 15 mm3 at day 5, 890 ± 211 mm3 at day 7, and 1856 ± 325 mm3 at day 14. Results are expressed as the mean volume of 6 experiments (± SEM).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/7/10.1182_blood-2004-08-3317/6/m_zh80070576490003.jpeg?Expires=1769135012&Signature=HaSXMBPNCHfuBosdBz8-nAPUWp8lBUyIuLKiXqC7Zn0y4NQSUV0~GC~Fr6bizLFLm14E1IaliacE2RCbdS3K-MnxIbtv6IdoKiiy21e0aFdwRAFIdM8uv2v2KGME0d~eVSGUgsBG2uTAcnk~O4dbE7wnxolRABn~xwnwhQB8si1vWWtg2aOQ2xcIJ14RMoMLEcwmjz8PJeyOqwWTaMzE6ojzbg6V6~W-T3JsduC2TXNKVrYATNN4SnZu1XiIiMHXY6Kw6zUzo8EaJ4hv3ikTriLem5IotQHfYbkOb2bhyU5AcEo8v2r~KOHmZs-uUZjN3A51QwwnFhCCzch2iGCUkQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal