Abstract
Eph receptors, the largest subfamily of receptor tyrosine kinases, and their ephrin ligands are important mediators of cell-cell communication regulating cell attachment, shape, and mobility. Here we demonstrate that CD4+ T lymphocytes express the EphA1 and EphA4 receptors and that these cells bind the ligand ephrin-A1. Further we show ephrin-A1 expression in vivo on high endothelial venule (HEV) endothelial cells. Ephrin-A1 binding to CD4+ T cells stimulates both stromal cell-derived factor 1α (SDF-1α)- and macrophage inflammatory protein 3β (MIP3β)-mediated chemotaxis. In line with the increased chemotactic response, increased actin polymerization is observed in particular with the combination of ephrin-A1 and SDF-1α. Signaling through EphA receptors induces intracellular tyrosine phosphorylation. In particular, proline-rich tyrosine kinase 2 (PYK2) is phosphorylated on tyrosine residues 402 and 580. Ephrin-A1-induced chemotaxis and intracellular tyrosine phosphorylation, including EphA1 and Pyk2, was inhibited by Tyrphostin-A9. In conclusion, ligand engagement of EphA receptors on CD4+ T cells stimulates chemotaxis, induces intracellular tyrosine phosphorylation, and affects actin polymerization. This, together with our finding that ephrin-A1 is expressed by HEV endothelial cells, suggests a role for Eph receptors in transendothelial migration.
Introduction
Eph receptors are the largest subfamily of receptor tyrosine kinases (RTKs) interacting with a family of ligands, the ephrins. These receptors can be divided into 2 subgroups, EphA and EphB. The EphA class consists of 9 members that in general bind ephrin-A members that are linked to the plasma membrane through a glycosylphosphatidylinositol anchor. The EphB class consists of 6 members that in general bind ephrin-B members that transverse the cell membrane.1 Eph receptors are important mediators of cell-cell communication regulating cell attachment, shape, and mobility in neuronal and endothelial cells. Well-characterized examples of Eph-regulated processes include embryonic pattern formation, axon path finding, and vascular remodeling. One prominent function of the Eph receptors is to establish cell positioning and to maintain cellular organization.2-5 It is well documented that Eph receptor signaling is involved in cytoskeletal organization.6 The small guanosine 5′-triphosphatases (GTPases) of the Rho family, like RhoA, Rac1, and Cdc42, are key intermediates in cellular signaling originating from cell adhesion receptors and eliciting distinct effects on the actin cytoskeleton.7-10 They have been implicated in biologic effects of ephrins in neuronal cells, melanoma cells, and muscle cells.11-14 Eph receptors also influence other signaling molecules that regulate cell behavior. In particular, Eph receptor activation has been shown to either repress or promote integrin activity depending on the developmental or environmental context, leading to either increase in cell adhesion or cell detachment.15-18 A role for focal adhesion kinase (FAK) has been suggested in integrin activation or deactivation.15,19
Lymphocytes also express certain Eph receptors of both subfamilies. The significance of these receptors in lymphocyte biology is not firmly established, but several studies have suggested a role in the differentiation and modulation of T-cell responses.20-23 In view of the highly complex nature of lymphocyte traffic and the role of Eph receptors in other cell systems, it is likely that these receptors are also involved in cell migration or cell positioning in lymphatic tissues. Interestingly, a recent study showed that ephrin-A1 stimulation inhibited movement of CD4+ T cells and the Jurkat cell line toward a gradient of either of the chemoattractants stromal cell-derived factor 1α (SDF-1α) or macrophage inflammatory protein 3β (MIP3β). The phenomenon was associated with elevated Rho activation and decreased Cdc42 activity in response to chemotactic stimuli.24
In the present study we describe the expression of EphAmembers on CD4+ T cells and show that stimulation of CD4+ T cells with the EphA ligand ephrin-A1 leads to increased chemotaxis, affects actin polymerization in combination with SDF-1α, and induces tyrosine phosphorylation of the FAK-related kinase, proline-rich tyrosine kinase 2 (PYK2). In addition, we show that ephrin-A1 is expressed on high endothelial venules. Together, our results suggest that EphA expression is important for migration of T cells through high endothelial venules in the T-cell area of secondary lymphoid tissues.
Materials and methods
Antibodies and reagents
The following antibodies were used in this study: anti-EphA1 (R&D Systems, McKinley Place, MN), anti-PYK2 (Santa Cruz Biotechnology, Santa Cruz, CA), antiphospho-PYK2 sampler kit (Biosource, Camarillo, CA), antiphosphotyrosine PY99 (Santa Cruz Biotechnology), anti-ephrin-A1 (Santa Cruz Biotechnology), anti-ephrin-A1 (Zymed Laboratories, South San Francisco, CA), antiactin (Santa Cruz Biotechnology), fluorescein isothiocyanate (FITC)-labeled anti-CD45RA and FITC-labeled anti-CD62L (BD Biosciences, San Jose, CA), FITC-labeled anti-CD45RO and FITC-labeled anti-CD4 (DakoCytomation, Via Real Carpinteria, CA), goat anti-mouse immunoglobulin R-phycoerythrin (Ig-RPE; Southern Biotechnology Associates, Birmingham, AL), horseradish peroxidase (HRP)-labeled goat antirabbit and HRP-labeled rabbit antimouse (DakoCytomation), mouse gamma globulin (Jackson ImmunoResearch Laboratories, West Grove, PA). Rhodamine-labeled phalloidin was used for F-actin staining (Molecular Probes, Eugene, OR). The following inhibitors were used in this study: Tyrphostin-A9, SU6656, and PP2 (all from Calbiochem, Darmstadt, Germany). The following recombinant human proteins were used in this study: EphA1-Fc chimera (R&D Systems), recombinant human SDF-1α (CXCL12), and MIP3β (CCL19; R&D Systems).
Cell separation procedures
B cells and T cells from buffy coats were isolated with anti-CD19-coated or anti-CD4-coated beads, respectively (Dynal, Oslo, Norway). CD4+ T cells were also negatively isolated using Dynal CD4 Negative Isolation Kit (Dynal). For some experiments, CD4+ T cells were kept in serum-free medium X-Vivo 15 (Bio-Whittaker, Verviers, Belgium) overnight at 37°C. CD4+ T cells were stimulated with anti-CD3/CD28-coated magnetic beads (Dynal), with the ratio of 1 bead per 2 cells, in RPMI 1640 with 10% fetal bovine serum. Tonsils, obtained from children undergoing tonsillectomy on medical grounds, were minced, and mononuclear cells were purified by Lymphoprep (Axis-Shields, Oslo, Norway) density gradient centrifugation. CD4+, CD19+, and CD8+ cells were isolated using Dynabeads (Dynal). For isolation of endothelial cells from tonsils, tonsils were minced and washed before adding a collagenase solution (Collagenase/Dispase/DnaseI; Boehringer Mannheim, Mannheim, Germany) followed by incubation for 15 minutes at 37°C. The solution was discarded, and fresh solution was added to the minced tissue for an additional 2 hours at 37°C. The resulting cell suspension was further purified by Lymphoprep (Nycomed Pharma, Oslo, Norway) density gradient centrifugation. Endothelial cells were then positively isolated by anti-CD34-coated magnetic beads (Dynal).
Fusion protein generation
The generation of control Fc fusion protein (CD19-Fc) construct has previously been described.25 The construction of soluble ephrin-A1-Fc was performed in principle as previously described for CD19-Fc. Ephrin-A1, without the glycosyl-phosphatidylinositol (GPI) linkage, was amplified by polymerase chain reaction (PCR) from a previously described cDNA clone26 with specific primers including the restriction sites HindIII and BamH1. The PCR product was cloned in-frame with the Fc part of mouse IgG2b in pcDNA1-Fc.25 For protein production, HEK293T cells were transfected with control-Fc or ephrin-A1-Fc vectors using lipofectAMINE (InVitrogen, Carlsbad, CA). Soluble control-Fc and ephrin-A1-Fc were purified from culture supernatant by affinity chromatography on a protein-G column (Pharmacia, Uppsala, Sweden) as previously described.25
Expression analysis
CD4+ T cells and CD19+ B cells were stained with control-Fc (20 μg/mL) or ephrin-A1-Fc (20 μg/mL) followed by phycoerythrin (PE)-labeled antimouse second layer. Further immunophenotypic analysis of ephrin-A1-binding cells was performed by incubating with ephrin-A1-Fc, subsequently with a PE-labeled antimouse second layer, thereafter blocking with mouse gamma globulin (100 μg/mL), followed by staining with relevant FITC-labeled antibodies. The EphA1 antibody (5 μg/mL) was preincubated with 2 times the amount in micrograms of EphA1-Fc fusion protein before staining of cells to test for specificity of the anti-EphA1 antibody. Surface expression was analyzed by FACSCalibur flow cytometer (Becton Dickinson, Heidelberg, Germany).
Total RNA was extracted from cells or tissues by standard methods, and 10 μg was size fractionated on a 1% agarose formaldehyde denaturing gel and transferred to nitrocellulose membranes.25 Hybridization was performed with 32P-deoxycytosine triphosphate (32P-dCTP)-labeled EphA1-, EphA4-, or ephrin-A1-specific cDNA probes as previously described.25 Hybridization with a β-actin cDNA probe was used as control.
Immunohistochemistry
The primary antibodies used for immunohistochemistry on frozen tonsil or lymph node sections were anti-ephrin-A1 from Santa Cruz Biotechnology and Zymed Laboratories. For detection, a goat antirabbit antibody labeled with peroxidase-conjugated polymer (Dako En Vision System; Dakopatts, Glostrup, Denmark) was used. The color reaction was developed using diaminobenzidine and H2O2 as substrates.
Photographs of the histologic slides were obtained using an Olympus BX50 microscope equipped with 18 ×/0.3, 40 ×/0.75, and 60 ×/0.90 Uplan FI objective lenses. Images were acquired through an ORCA-285 IEEE 1394-Based Digital Camera (Hamamatsu Photonics, Hamamatsu City, Japan) by using Paint Shop Pro 7.0 acquisition software (Corel, Ottawa, Canada).
Subcellular fractionation
CD4+ T cells were stimulated with ephrin-A1-Fc (20 μg/mL) at 37°C with or without various inhibitors at different time intervals. The cells were then washed in ice-cold phosphate-buffered saline (PBS), resuspended in buffer A (5 mM Tris-HCl, pH 8.5; mM EDTA [ethylenediaminetetraacetic acid]; 75 mM sucrose; proteinase and phosphatase inhibitors), and sonicated 4 times for 15 seconds. Nuclei were pelleted by centrifugation at 400g for 5 minutes at 4°C in a microcentrifuge. The supernatants were centrifuged at 32 000g for 30 minutes at 4°C in a Beckman centrifuge (Beckman Coulter, Fullerton, CA). The subsequent supernatants were collected and used as the cytosol + plasma membrane fraction. The samples were boiled prior to separation on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Chemotaxis assay
The chemotaxis assay was performed in standard Transwell plates using 5-μm diameter pores (Costar, Corning, NY). Control-Fc (10 μg/mL) and ephrin-A1-Fc (10 μg/mL) were cross-linked with antimouse Ig for 20 minutes at 37°C before addition to cells. In some experiments cells were preincubated with Tyrphostin-A9, SU6656, and PP2 inhibitors. CD4+ T-cell chemotaxis assays were in most cases performed in RPMI in the absence of heat-inactivated fetal bovine serum (FBS). Cells (2.5 × 10-5) were added to the top of the chamber (transwell) in the presence of control-Fc or ephrin-A1-Fc. Assays were performed in duplicate at 37°C for 2 hours. A fluorescent bead internal control (Bangs Laboratories, Fishers, IN) was added to the cells that had passed through the membrane into the lower chamber. The cells with the fluorescent bead control were collected and counted by flow cytometry and normalized by the reference to the internal fluorescent bead control.
Actin polymerization
Prewarmed (37°C) CD4+ T cells in PBS were stimulated with ephrin-A1 (10 μg/mL), SDF-1α (10 ng/mL), or the combination of both reagents. The reactions were stopped by the addition of solution A from an intracellular staining kit (DakoCytomation). Permeabilization and staining with rhodamine-phalloidin (5 U/mL) was performed in solution B. The F-actin content shown by rhodamine-phalloidin staining was analyzed by flow cytometry.
Results
CD4+ lymphocytes express ephrin-A1 binding receptor(s)
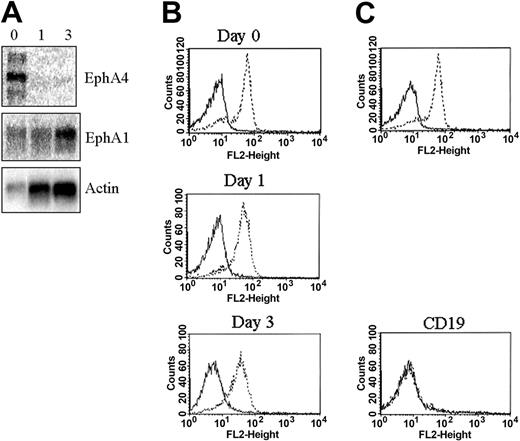
CD4+ T cells from blood and tonsils were compared for their capacity to bind ephrin-A1. The large majority (80%-90%) of peripheral blood CD4+ T cells bound ephrin-A1-Fc as shown by flow cytometry analysis (Figure 1A). The nonbinding cells corresponded to a subset of CD45RO+CD62L- cells (Figure 1B), indicative of a subset of memory cells.27 Tonsil CD4+ T cells, on the other hand, presented as 2 distinct populations of approximately equal numbers based on ephrin-A1-Fc binding (Figure 1C). Ephrin-A1-Fc binding was further examined after stimulation of peripheral blood CD4+ T cells with anti-CD3/CD28. Cross-linked CD3 and CD28 led to less binding of ephrin-A1-Fc already after 1 day of stimulation compared with nonstimulated cells, and 3 days of stimulation showed the same as for day 1 (Figure 1 D). These data demonstrate that EphA receptors exist both on nonstimulated and stimulated CD4+ cells and might indicate that they exert distinct functions at different activation/differentiation stages.
Ephrin-A1 binds to CD4+ lymphocytes. (A) Binding of control-Fc (—) and ephrin-A1-Fc (- - -) to peripheral blood isolated CD4+ T cells (CD4+) or CD19+ B cells (CD19+). No binding is observed to CD19+ B cells. (B) Costaining of peripheral blood CD4+ T cells with ephrin-A1-Fc and either CD45RA, CD45RO, or CD62L. The ephrin-A1 nonbinding cells are CD45RO+ and CD62L-. (C) Costaining of control-Fc or ephrin-A1-Fc with anti-CD4 on a total tonsil cell preparation. (D) Binding of control-Fc (—) or ephrin-A1-Fc (- - -) to peripheral blood CD4+ T cells stimulated with anti-CD3/CD28 (day 1, day 3). Day 0 indicates freshly isolated cells.
Ephrin-A1 binds to CD4+ lymphocytes. (A) Binding of control-Fc (—) and ephrin-A1-Fc (- - -) to peripheral blood isolated CD4+ T cells (CD4+) or CD19+ B cells (CD19+). No binding is observed to CD19+ B cells. (B) Costaining of peripheral blood CD4+ T cells with ephrin-A1-Fc and either CD45RA, CD45RO, or CD62L. The ephrin-A1 nonbinding cells are CD45RO+ and CD62L-. (C) Costaining of control-Fc or ephrin-A1-Fc with anti-CD4 on a total tonsil cell preparation. (D) Binding of control-Fc (—) or ephrin-A1-Fc (- - -) to peripheral blood CD4+ T cells stimulated with anti-CD3/CD28 (day 1, day 3). Day 0 indicates freshly isolated cells.
Ephrin-A1 binds to most Eph receptors of the EphA subfamily.28 By PCR analysis, mRNA expression of the ephrin-A1 binding receptors EphA1 and EphA4 in CD4+ lymphocytes has previously been described.29 We performed Northern blot analysis on CD4+ T cells isolated from blood with or without activation by anti-CD3/CD28. These results show that both EphA1 and EphA4 mRNA are expressed in freshly isolated CD4+ T cells (Figure 2A). No EphA4 mRNA expression could be observed after anti-CD3/CD28 stimulation for 1 or 3 days, whereas EphA1 expression could be observed at all time points (Figure 2A). Cell surface expression with an anti-EphA1 antibody was evaluated on freshly isolated and anti-CD3/CD28-stimulated CD4+ T cells. The data show similar EphA1 expression levels on both freshly isolated and anti-CD3/CD28-stimulated cells (Figure 2B). In addition, no binding of the EphA1 antibody could be observed to CD19+ B cells (Figure 2B).
EphA1 and EphA4 are expressed in CD4+ T cells. (A) Expression of EphA4 and EphA1 mRNA by Northern blot analysis of peripheral blood CD4+ T cells (0 indicates freshly isolated) and after CD3/CD28 stimulation (1 indicates day 1; and 3, day 3). Control gene hybridization with an actin probe. (B) EphA1 cell surface expression on peripheral blood CD4+ T cells (Day 0), after CD3/CD28 stimulation (Day 1, Day 3), or on peripheral blood CD19+ B cells (CD19+). Anti-EphA1 (- - -), isotype specific control antibody (—). No binding is observed to CD19+ B cells. (C) Specificity of the anti-EphA1 antibody was shown by the loss of cell surface binding after preincubating the antibody with the extracellular part of recombinant EphA1 (EphA1-Fc). Peripheral blood CD4+ T cells were stained. (- - -) EphA1 expression, (—) EphA1 expression after preincubation of the anti-EphA1 antibody with EphA1-Fc before cell surface staining.
EphA1 and EphA4 are expressed in CD4+ T cells. (A) Expression of EphA4 and EphA1 mRNA by Northern blot analysis of peripheral blood CD4+ T cells (0 indicates freshly isolated) and after CD3/CD28 stimulation (1 indicates day 1; and 3, day 3). Control gene hybridization with an actin probe. (B) EphA1 cell surface expression on peripheral blood CD4+ T cells (Day 0), after CD3/CD28 stimulation (Day 1, Day 3), or on peripheral blood CD19+ B cells (CD19+). Anti-EphA1 (- - -), isotype specific control antibody (—). No binding is observed to CD19+ B cells. (C) Specificity of the anti-EphA1 antibody was shown by the loss of cell surface binding after preincubating the antibody with the extracellular part of recombinant EphA1 (EphA1-Fc). Peripheral blood CD4+ T cells were stained. (- - -) EphA1 expression, (—) EphA1 expression after preincubation of the anti-EphA1 antibody with EphA1-Fc before cell surface staining.
Ephrin-A1 is expressed in secondary lymphoid organs
Expression of ephrin-A1 was first investigated in isolated cell populations from tonsils by Northern blot analysis. The results show that ephrin-A1 mRNA is expressed in CD34+ cells but not in CD19+ B cells, CD4+ T cells, or CD8+ T cells (Figure 3A). CD34 is a general marker for endothelial cells in tonsils.30 Immunohistochemical analysis of tonsils (Figure 3B) and lymph nodes (data not shown) with ephrin-A1-specific antibodies was then performed to analyze ephrin-A1-expressing cells in situ. We found that in particular, the endothelial cells of high endothelial venules (HEVs) highly express ephrin-A1, whereas endothelial cells of other vessels show weaker expression of ephrin-A1 (Figure 3B). Closer examination shows that the staining is concentrated toward the luminal side of the HEV endothelial cells (Figure 3C). No other cells were stained by anti-ephrin-A1.
Ephrin-A1 is expressed on endothelial cells in tonsils. (A) Northern blot analysis of ephrin-A1 mRNA expression in selected cell populations isolated from tonsils. Two CD34+ cell isolations from 2 different tonsils are shown. Actin hybridization serves as a loading control. (B) Immunohistochemical analysis of ephrin-A1 expression in tonsils. Vessels, in particular HEVs, are stained (top panel left and right, bottom panel right). Anti-ephrin-A1 antibody from Santa Cruz Biotechnology. Original magnification is × 10 or × 40. (C) Immunohistochemical analysis of ephrin-A1 expression in tonsils. Bright staining is observed to the luminal side of HEV endothelial cells. Anti-ephrin-A1 antibody from Zymed. Original magnification is × 63.
Ephrin-A1 is expressed on endothelial cells in tonsils. (A) Northern blot analysis of ephrin-A1 mRNA expression in selected cell populations isolated from tonsils. Two CD34+ cell isolations from 2 different tonsils are shown. Actin hybridization serves as a loading control. (B) Immunohistochemical analysis of ephrin-A1 expression in tonsils. Vessels, in particular HEVs, are stained (top panel left and right, bottom panel right). Anti-ephrin-A1 antibody from Santa Cruz Biotechnology. Original magnification is × 10 or × 40. (C) Immunohistochemical analysis of ephrin-A1 expression in tonsils. Bright staining is observed to the luminal side of HEV endothelial cells. Anti-ephrin-A1 antibody from Zymed. Original magnification is × 63.
Ephrin-A1 stimulation of CD4+ T lymphocytes facilitates chemotaxis
Previously, ephrin stimulation of T cells has been shown to modulate SDF-1α-induced chemotaxis.24 In an experiment presented by Sharfe et al,24 different ephrins were immobilized to transwells in standard chemotaxis Transwell assay plates and shown to inhibit SDF-1α-induced chemotaxis of both CD4+ T cells isolated from blood and the Jurkat T-cell line. We set out to investigate the effect of ephrin-A1 stimulation on chemokine-mediated chemotaxis using a cross-linked soluble dimeric ephrin-A1-Fc fusion protein instead of immobilized ephrin-A1. The effect of 2 different chemokines was tested on peripheral blood CD4+ T cells: SDF-1α that binds to the CXCR4 receptor and MIP3β that binds to the CCR7 receptor.31 In the Transwell assay plates, 100 ng/mL SDF-1α was added to the lower well, and in some experiments SDF-1α was added both in the transwell and in the lower well. CD4+ T cells (2.5 × 105) were incubated for 2 hours at 37°C, and the cells that passed through the membrane were counted by flow cytometry. Under serum-free conditions, low migration was observed in response to SDF-1α when control Fc-protein (Figure 4A) or no Fc-protein at all was added to the transwell (data not shown). When ephrin-A1-Fc was added to the transwell without SDF-1α in the lower chamber, increased migration through the transwell was observed (Figure 4A), indicating that ephrin-A1 by itself can induce random migration (chemokinesis). Interestingly, ephrin-A1-Fc also potentiated SDF-1α-induced chemotaxis (Figure 4A). Typically, between 15% and 20% of input cells migrated through the membrane after ephrin-A1 stimulation toward an SDF-1α chemotactic gradient. No migration was observed when either control-Fc or ephrin-A1-Fc was added only to the lower chamber, indicating that ephrin-A1 by itself did not induce any chemotactic response. Less migration was observed when SDF-1α was added both to the transwell and to the lower chamber, indeed indicating that ephrin-A1-induced migration in the presence of an SDF-1α gradient is a chemotactic response (Figure 4A). No significant difference in ephrin-A1-induced migration could be observed when cells were exposed to ephrin-A1-Fc both in the lower chamber and in the transwell compared with ephrin-A1-Fc added only in the transwell (data not shown). The ephrin-A1 effect on migration could be observed both in positively selected (Figure 4A) or negatively isolated CD4+ T cells (data not shown). The effect of serum on ephrin-A1-induced chemotactic response was also investigated. As shown in Figure 4B, the most pronounced relative effect of ephrin-A1-Fc-induced migration compared with control-Fc was under no-serum or low-serum (0.2% FBS) conditions. This effect was much less pronounced at higher serum concentrations (0.5% and 1%; Figure 4B). We have not observed inhibition of chemotaxis after ephrin-A1 stimulation under any of the conditions tested in contrast to what has been reported previously by others.24
Ephrin-A1 stimulates migration. (A) SDF-1α chemotaxis assay with peripheral blood CD4+ T cells. Fc indicates control-Fc; A1, ephrin-A1-Fc; FcU, Fc only in the lower chamber; A1U, A1 only in the lower chamber; Fc SDF-1α and A1 SDF-1α, 100 ng/mL SDF-1α added to the lower chamber and Fc and A1 added to the transwell; and Fc SDF-1 O/U and A1 SDF-1 O/U, 100 ng/mL SDF-1α added to both the transwell and the lower chamber and Fc and A1 added to the transwell. Standard error of the mean (SEM) is shown for 4 different experiments. (B) Effect of FBS on ephrin-A1-induced chemotaxis. Different FBS concentrations (in %) were included in the media added to the transwell. One hundred nanograms per milliliter SDF-1α was added to the lower chamber. Fc indicates control-Fc (▪); and A1, ephrin-A1-Fc (▦). SEM is shown for 4 different experiments. (C) MIP3β chemotaxis assay. Fc indicates control-Fc; A1, ephrin-A1-Fc; Fc MIP3β and A1 MIP3β, 500 ng/mL MIP3β added to the lower chamber and Fc and A1 added to the transwell; and Fc MIP3β O/U and A1 MIP3β O/U, 500 ng/mL MIP3β added to both the transwell and the lower chamber and Fc and A1 added to the transwell. SEM is shown for 4 different experiments.
Ephrin-A1 stimulates migration. (A) SDF-1α chemotaxis assay with peripheral blood CD4+ T cells. Fc indicates control-Fc; A1, ephrin-A1-Fc; FcU, Fc only in the lower chamber; A1U, A1 only in the lower chamber; Fc SDF-1α and A1 SDF-1α, 100 ng/mL SDF-1α added to the lower chamber and Fc and A1 added to the transwell; and Fc SDF-1 O/U and A1 SDF-1 O/U, 100 ng/mL SDF-1α added to both the transwell and the lower chamber and Fc and A1 added to the transwell. Standard error of the mean (SEM) is shown for 4 different experiments. (B) Effect of FBS on ephrin-A1-induced chemotaxis. Different FBS concentrations (in %) were included in the media added to the transwell. One hundred nanograms per milliliter SDF-1α was added to the lower chamber. Fc indicates control-Fc (▪); and A1, ephrin-A1-Fc (▦). SEM is shown for 4 different experiments. (C) MIP3β chemotaxis assay. Fc indicates control-Fc; A1, ephrin-A1-Fc; Fc MIP3β and A1 MIP3β, 500 ng/mL MIP3β added to the lower chamber and Fc and A1 added to the transwell; and Fc MIP3β O/U and A1 MIP3β O/U, 500 ng/mL MIP3β added to both the transwell and the lower chamber and Fc and A1 added to the transwell. SEM is shown for 4 different experiments.
Also MIP3β was also tested in the same experimental setup. The data show that ephrin-A1 also stimulated migration toward an MIP3β chemotactic gradient (Figure 4C). This was a chemotactic response since addition of MIP3β both in the upper and lower chamber showed less migrating cells.
Altogether, these data indicate that ephrin-A1 facilitates SDF-1α- and MIP3β-induced chemotaxis of blood isolated CD4+ cells.
Ephrin-A1 stimulation induces tyrosine phosphorylation in CD4+ T cells
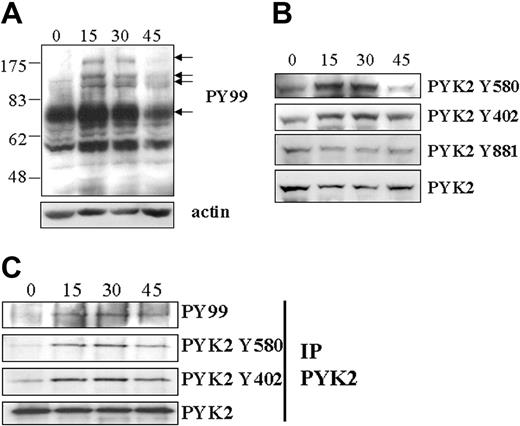
Ephrin stimulation of the EphA receptor subclass has been shown to induce autophosphorylation of EphA receptors, binding of adapter proteins, and further signaling involving intracellular tyrosine phosphorylation.6 Induction of tyrosine phosphorylation after ephrin-A1 stimulation of CD4+ T cells was therefore investigated. Soluble cross-linked ephrin-A1-Fc was incubated with T cells at 37°C. Tyrosine phosphorylation of intracellular proteins was then investigated in subcellular fractionated cell lysates (see “Subcellular fractionation”). Phosphorylation of several proteins could be observed after 15-minute incubation with ephrin-A1 in the cytosol + plasma membrane fraction (Figure 5A). Approximate molecular masses of these proteins were 170, 120, and 83 kDa. Notably, the antiphosphotyrosine-specific antibody PY99 detected an induced tyrosine phosphorylation pattern. This tyrosine phosphorylation induction could not be seen using the 4G10 antiphosphotyrosine-specific antibody from Upstate Biotechnology (Lake Placid, NY).
Ephrin-A1 induces intracellular tyrosine phosphorylation in CD4+ T cells. (A) CD4+ T cells were stimulated for the indicated times (in minutes) with ephrin-A1-Fc, and subcellular fractionation was performed. The Western blot was incubated with the antiphosphotyrosine antibody PY99. Molecular mass in kDa is shown to the left. Arrows indicate induced phosphorylation. Actin serves as loading control. (B) Incubation of antiphospho-PYK2 antibodies (PYK2 Y580, PYK2 Y403, and PYK2 Y881; Y indicates tyrosine) and total PYK2 antibody to Western blot of subcellular fractionated lysates after ephrin-A1 stimulation. Induced PYK2 phosphorylation is seen on tyrosine residues 580 and 402. (C) PYK2 was immunoprecipitated (IP PYK2) from CD4+ T cells after ephrin-A1 stimulation. Phosphorylated PYK2 was then detected using antiphosphotyrosine antibody PY99 or antiphospho-PYK2 antibodies. Total PYK2 expression serves as control.
Ephrin-A1 induces intracellular tyrosine phosphorylation in CD4+ T cells. (A) CD4+ T cells were stimulated for the indicated times (in minutes) with ephrin-A1-Fc, and subcellular fractionation was performed. The Western blot was incubated with the antiphosphotyrosine antibody PY99. Molecular mass in kDa is shown to the left. Arrows indicate induced phosphorylation. Actin serves as loading control. (B) Incubation of antiphospho-PYK2 antibodies (PYK2 Y580, PYK2 Y403, and PYK2 Y881; Y indicates tyrosine) and total PYK2 antibody to Western blot of subcellular fractionated lysates after ephrin-A1 stimulation. Induced PYK2 phosphorylation is seen on tyrosine residues 580 and 402. (C) PYK2 was immunoprecipitated (IP PYK2) from CD4+ T cells after ephrin-A1 stimulation. Phosphorylated PYK2 was then detected using antiphosphotyrosine antibody PY99 or antiphospho-PYK2 antibodies. Total PYK2 expression serves as control.
FAK, a protein with molecular mass of 120 kDa, has previously been shown to be phosphorylated and to be involved in morphologic changes of NIH3T3 cells after ephrin stimulation of the EphA2 receptor.19 Induction of tyrosine phosphorylation of FAK could be detected in CD4+ T cells after ephrin-A1 stimulation neither with a phospho-FAK-specific antibody nor after anti-FAK immunoprecipitation and detection using PY99 (data not shown). PYK2, an FAK-like kinase with a similar molecular mass, is involved in coupling signaling through several receptors, including integrin and chemokine receptors.32-34 The tyrosine phosphorylation status of this kinase was therefore investigated after ephrin-A1 stimulation of T cells. Subcellular fractionated cell lysates were first incubated with antiphospho-specific PYK2 (pY580). Phosphorylation of tyrosine 580 located in the catalytic domain of PYK2 is involved in the activation of PYK2.35 The results show that PYK2 phosphorylation is induced in T cells after 15-minute stimulation with ephrin-A1 (Figure 5B). In repeated experiments, the phosphorylation status of PYK2 was back to basal levels after 45 minutes. Furthermore, we investigated other PYK2 phosphorylation sites. No induced phosphorylation of tyrosine 579 (data not shown) or 881 was detected, whereas induced phosphorylation of tyrosine 402 was observed (Figure 5B). PYK2 was also immunoprecipitated and Western blots were incubated with either PY99 or antiphospho-specific PYK2 antibodies. PY99 recognizes phosphorylated immunoprecipitated PYK2. Also here, induced tyrosine phosphorylation of PYK2 Y580 and PYK2 Y402 was observed (Figure 5C). Taken together, our results indicate induced phosphorylation of PYK2 after ephrin-A1 stimulation of CD4+ T cells.
Tyrphostin-A9 inhibits ephrin-A1-induced migration
To further evaluate the role of induced phosphorylation after ephrin stimulation, the effect of a putative PYK2 inhibitor (Tyrphostin-A9) was investigated. Tyrphostin-A9 has recently been shown to inhibit tumor necrosis factor α (TNF-α)-induced tyrosine phosphorylation of PYK2 in neutrophils.36 First, the effect of Tyrphostin-A9 on ephrin-A1-induced chemotaxis toward SDF-1α was studied. CD4+ T cells were preincubated with different concentrations of Tyrphostin-A9 (10, 5, 1 μM) and then added to Transwell plates together with ephrin-A1-Fc or control Fc-protein. Nearly complete inhibition of chemotaxis was observed at 10 and 5 μM, and even at 1 μM significant inhibition of chemotaxis was observed (Figure 6A). We then evaluated the effect of ephrin-A1-induced tyrosine phosphorylation after Tyrphostin-A9 treatment. The phosphorylation level on several proteins was lowered after Tyrphostin-A9 at 10-μM treatment and ephrin-A1 stimulation (Figure 6B). Specifically, phosphorylation of both the EphA1 receptor and the PYK2 kinase was tested after Tyrphostin-A9 treatment. The results from these experiments show that Tyrphostin-A9 inhibits both EphA1- and PYK2-induced phosphorylation after ephrin-A1 stimulation. To conclude, Tyrphostin-A9 inhibits ephrin-A1-induced chemotaxis and inhibits phosphorylation of both EphA1 and PYK2. The inhibition of EphA1 receptor activity adds to the list of kinases inhibited by Tyrphostin-A9.
Effect of Tyrphostin-A9 and SU6656 on ephrin-A1-induced chemotaxis and intracellular signaling. (A) Effect of Tyrphostin-A9 on ephrin-A1-induced SDF-1α chemotaxis. Fc indicates control Fc; A1, ephrin-A1-Fc. Peripheral blood CD4+ cells were preincubated with the indicated concentrations of Tyrphostin-A9 (in μM) shown in parentheses. SDF-1α was added to the lower chamber and Fc and A1 to the transwell. SEM is shown for 4 different experiments. (B) Effect of Tyrphostin-A9 on ephrin-A1-induced intracellular tyrosine phosphorylation. CD4+ T cells were preincubated with or without 10 μM Tyrphostin-A9 (Tyr A9) and then stimulated for the indicated times (in minutes) with ephrin-A1-Fc. The Western blot of the cytosol + plasma membrane fraction was incubated with the antiphosphotyrosine antibody PY99. Molecular mass in kDa is shown to the left. Actin detection serves as loading control. (C) Effect of Tyrphostin-A9 on PYK2- and EphA1-induced tyrosine phosphorylation. CD4+ T cells were preincubated or not with 10 μM Tyrphostin-A9 (Tyr A9) before stimulation with ephrin-A1-Fc for the indicated time points (in minutes). Western blot was incubated with antiphospho-PYK2 Y580 antibody. Total PYK2 detection serves as loading control. EphA1 was immunoprecipitated (IP) from cell lysates before SDS-PAGE. Phosphorylation of EphA1 was detected with the PY99 antibody. The blot was then stripped and EphA1 levels were confirmed with an anti-EphA1 antibody. (D) Effect of src-family kinase inhibitor SU6656 on ephrin-A1-induced SDF-1α chemotaxis. Fc indicates control Fc; and A1, ephrin-A1-Fc. CD4+ cells were preincubated with the indicated concentrations of SU6656 (in μM) shown in parentheses. SDF-1α was added to the lower chamber and Fc and A1 to the transwell. SEM is shown for 4 different experiments. (E) Effect of SU6656 on ephrin-A1-induced intracellular tyrosine phosphorylation. CD4+ T cells, preincubated or not with 10 μM SU6656, were stimulated for the indicated times (in minutes) with ephrin-A1-Fc. Western blot of cytosol + plasma membrane fraction was incubated with the antiphosphotyrosine antibody PY99. Molecular mass in kDa is shown to the left. Actin detection serves as loading control. (F) Effect of SU6656 on ephrin-A1-induced tyrosine phosphorylation of PYK2. CD4+ T cells were preincubated or not with 10 μM SU6656 and lysates were subcellular fractionated. Western blot was incubated with antiphospho-PYK2 Y580 antibody. Total PYK2 detection serves as loading control.
Effect of Tyrphostin-A9 and SU6656 on ephrin-A1-induced chemotaxis and intracellular signaling. (A) Effect of Tyrphostin-A9 on ephrin-A1-induced SDF-1α chemotaxis. Fc indicates control Fc; A1, ephrin-A1-Fc. Peripheral blood CD4+ cells were preincubated with the indicated concentrations of Tyrphostin-A9 (in μM) shown in parentheses. SDF-1α was added to the lower chamber and Fc and A1 to the transwell. SEM is shown for 4 different experiments. (B) Effect of Tyrphostin-A9 on ephrin-A1-induced intracellular tyrosine phosphorylation. CD4+ T cells were preincubated with or without 10 μM Tyrphostin-A9 (Tyr A9) and then stimulated for the indicated times (in minutes) with ephrin-A1-Fc. The Western blot of the cytosol + plasma membrane fraction was incubated with the antiphosphotyrosine antibody PY99. Molecular mass in kDa is shown to the left. Actin detection serves as loading control. (C) Effect of Tyrphostin-A9 on PYK2- and EphA1-induced tyrosine phosphorylation. CD4+ T cells were preincubated or not with 10 μM Tyrphostin-A9 (Tyr A9) before stimulation with ephrin-A1-Fc for the indicated time points (in minutes). Western blot was incubated with antiphospho-PYK2 Y580 antibody. Total PYK2 detection serves as loading control. EphA1 was immunoprecipitated (IP) from cell lysates before SDS-PAGE. Phosphorylation of EphA1 was detected with the PY99 antibody. The blot was then stripped and EphA1 levels were confirmed with an anti-EphA1 antibody. (D) Effect of src-family kinase inhibitor SU6656 on ephrin-A1-induced SDF-1α chemotaxis. Fc indicates control Fc; and A1, ephrin-A1-Fc. CD4+ cells were preincubated with the indicated concentrations of SU6656 (in μM) shown in parentheses. SDF-1α was added to the lower chamber and Fc and A1 to the transwell. SEM is shown for 4 different experiments. (E) Effect of SU6656 on ephrin-A1-induced intracellular tyrosine phosphorylation. CD4+ T cells, preincubated or not with 10 μM SU6656, were stimulated for the indicated times (in minutes) with ephrin-A1-Fc. Western blot of cytosol + plasma membrane fraction was incubated with the antiphosphotyrosine antibody PY99. Molecular mass in kDa is shown to the left. Actin detection serves as loading control. (F) Effect of SU6656 on ephrin-A1-induced tyrosine phosphorylation of PYK2. CD4+ T cells were preincubated or not with 10 μM SU6656 and lysates were subcellular fractionated. Western blot was incubated with antiphospho-PYK2 Y580 antibody. Total PYK2 detection serves as loading control.
SU6656 stimulates ephrin-A1- and SDF-1α-induced migration
Phosphorylated PYK2 has been shown to associate with src-family kinases.37 We therefore investigated the effect of src inhibitors on the ephrin-A1-induced chemotactic response. First the src-family kinase inhibitor SU6656 was tested.38 To our surprise, the SDF-1α-induced migration was increased when cells were treated with SU6656. For cells treated with SU6656 and the control-Fc protein, an increased chemotaxis was observed to nearly the level of the ephrin-A1-induced chemotaxis. An approximately 2-fold increase of chemotaxis was observed for cells treated with SU6656 and ephrin-A1 when compared with ephrin-A1 alone (Figure 6D). The overall phosphorylation status and that of PYK2 was then evaluated after SU6656 treatment. Figure 6E-F shows that SU6656 treatment slightly affects the general ephrin-A1-induced phosphorylation pattern and that of PYK2 Y580.
Other src-family kinase inhibitors, like PP1 and PP2, have been shown to inhibit chemotaxis in different cell systems.39,40 We therefore tested the effect of PP2 in ephrin-A1-induced chemotaxis. No difference in ephrin-A1-Fc-induced migration was shown with this inhibitor at concentrations ranging from 10 to 1 μM (data not shown).
Taken together, SU6656 stimulates both control-Fc- and ephrin-A1-Fc-induced chemotaxis. Whether this effect is through src-kinases or other protein (s) that have not yet described to be inhibited by SU6656 (eg, phosphatases) awaits further study.
Actin polymerization
The increased migration after ephrin-A1 stimulation prompted us to investigate the effect on actin polymerization. Filamentous F-actin was detected by intracellular staining of CD4+ T cells with rhodamine-labeled phalloidin. Different time points were applied (0, 1, 2, 3, and 5 minutes) in the experiments. The results show that SDF-1α alone induces a rapid increase in actin polymerization with a maximum after 1 minute of stimulation as shown by increased phalloidin labeling (Figure 7). F-actin content declined thereafter. Low or no increase of actin polymerization after ephrin-A1 stimulation alone could be observed at the time points tested (Figure 7). Interestingly, when cells were stimulated with the combination of ephrin-A1 and SDF-1α, a faster increase and higher level of actin polymerization was observed than for SDF-1α alone (Figure 7). Thus the ephrin-A1-induced stimulation of migration in the presence of SDF-1α can be related to an increase in actin polymerization.
Ephrin-A1 induces actin polymerization. CD4+ T cells were incubated with ephrin-A1 (•), SDF-1α (○), or the combination of ephrin-A1 and SDF-1α (▾) for the indicated times. The cells were then fixed, permeabilized, and stained with rhodamine-phalloidin for analysis by flow cytometry. SEM is shown for 4 different experiments.
Ephrin-A1 induces actin polymerization. CD4+ T cells were incubated with ephrin-A1 (•), SDF-1α (○), or the combination of ephrin-A1 and SDF-1α (▾) for the indicated times. The cells were then fixed, permeabilized, and stained with rhodamine-phalloidin for analysis by flow cytometry. SEM is shown for 4 different experiments.
Discussion
In this study we present data supporting a role for EphA receptors in entrance of CD4+ T cells into secondary lymphoid organs. Although the mRNA expression of EphA1 and EphA4 in CD4+ T cells has previously been described by PCR analysis,29 we provide here conclusive evidence that these 2 receptors indeed are expressed by peripheral blood CD4+ T cells by Northern blot and flow cytometry analysis. In addition, we present expression data of these receptors on activated CD4+ cells. EphA4 mRNA was down-regulated after CD3/CD28 stimulation whereas EphA1 was not. Data on EphA4 protein expression are not presented here. Only nonconclusive data were obtained from Western blot analysis by applying anti-EphA4 antibodies from different vendors. The level of EphA1 cell surface expression did not change after anti-CD3/CD28 stimulation. A ligand for EphA subclass receptors, ephrin-A1, bound to 80% to 90% of peripheral blood CD4+ T cells and to approximately 50% of tonsil CD4+ T cells. In addition, most ephrin-A1-binding cells expressed L-selectin, a prerequisite for entrance through high endothelial venules.41 We observed lowered ephrin-A1 binding intensity after anti-CD3/CD28 stimulation of CD4+ T cells. One might speculate that the combination of EphA4 and EphA1, as hetero-oligomeric complexes, generates a strong binding affinity for ephrin-A1 and that the lower ephrin-A1 binding intensity observed after anti-CD3/CD28 stimulation is due to binding to only the EphA1 receptor. In contrast to the promiscuous binding properties of other EphA members, EphA1 preferentially binds ephrin-A1 with reasonable affinity.28,42 Assembly of Eph receptor hetero-oligomeric complexes has previously been described for the combination of EphB1 and EphB6.43 Alternatively, the strong binding of ephrin-A1 to unstimulated cells is the result of binding to EphA1 and EphA4 existing as homo-oligomeric complexes. Our data therefore indicate distinct functions of ligand binding to EphA receptors on nonstimulated versus T-cell receptor (TCR)-stimulated CD4+ T cells. The role of Eph-ephrin interaction in TCR-stimulated cells is under study.
Although there have been several reports regarding the expression of Eph receptors on T cells,20-23 it is still not yet clear which cells express the corresponding ligands or in which context Eph receptors on T cells interact with ligand-bearing cells. We have previously shown ephrin-A4 expression on activated T cells25,44 and others have described ephrin-B2 expression on T cells and monocytes/macrophages.21 Here we demonstrate that ephrin-A1 is expressed on endothelial cells in secondary lymphoid organs and in particular on HEVs. In lymphoid organs, lymphocyte adherence and transendothelial migration occurs through HEVs; in addition HEV-like structures are observed in chronically inflamed nonlymphoid tissues.45 Ephrin-A1 was initially characterized as an immediate early response gene of endothelium, and TNF-α has been shown to induce transient ephrin A1 expression in human umbilical vein endothelial cell.46,47 A global gene expression profiling of diverse endothelial cells has shown ephrin-A1 expression in microvascular endothelial cells.48 The expression of EphA receptors on CD4+ T cells and the corresponding expression of ephrin-A1 on HEV endothelial cells indicate a role in adhesion/transmigration of these cells into secondary lymphoid organs or to inflamed tissues. The functional data presented here support this. Ephrin-A1 stimulated both SDF-1α- and MIP3β-induced chemotaxis of CD4+ T cells under serum-free conditions. In our experiments, cells were stimulated with soluble cross-linked ephrin-A1-Fc and exposed to gradients of chemokine. The addition of serum led to less relative effect, but we have not noted any inhibition of ephrin-A1-directed chemotaxis under any of the conditions tested. A previous study has shown inhibition of chemotaxis after ephrin-A1 binding to CD4+ T cells and the Jurkat cell line. However, in this study ephrin-A1 was immobilized to the transwells before addition of cells. The rationale for this experimental setup was to mimic the membrane-bound nature of ephrins in vivo.24 However, ephrin-A members are rapidly cleaved of the cell surface after receptor interaction, a process shown to involve metalloproteinases.26,49 Therefore, immobilizing ephrin-A1 to the transwell might not mimic a physiologic situation. The cells might adhere and spread on ephrin-A1-coated surfaces and the interaction between ligand and cells might be prolonged.19 Therefore the effect of ephrin-A1 on chemotaxis might be difficult to evaluate in such an experimental setup.
Ephrin-A1 stimulation of CD4+ T cells induced tyrosine phosphorylation on several proteins. In particular, we observed induced phosphorylation of PYK2, an FAK-like kinase, on tyrosine residues 580 and 402 but not on tyrosine 579 or 581. The result suggests that PYK2 is involved in EphA signaling in T cells, which has not been reported before. PYK2 has been shown to be involved in cytoskeletal remodeling, proliferation, and motility in several different cell systems.37 In T cells, PYK2 has been shown to be phosphorylated after T-cell receptor signaling and may act to link signals from the cell surface to the cytoskeleton.50-52 A potential role of PYK2 in T-cell costimulation mediated by β1 integrins has also been suggested.53 Thus the observed increase in mobility after ephrin-A1 stimulation might be related to PYK2 phosphorylation and might potentiate chemokine receptor signaling. Although we did not show a direct link from ephrin-A1-induced PYK2 activation to ephrin-A1-induced migration, the role of PYK2 in other cell systems might suggest this. However, we do present data showing inhibition of ephrin-A1-induced migration and intracellular tyrosine phosphorylation using a kinase inhibitor, Tyrphostin-A9. This inhibitor has previously been shown to inhibit the activity of the platelet-derived growth factor (PDGF) receptor and PYK2.36 We show here that Tyrphostin-A9 also inhibits autophosphorylation of the EphA1 receptor. Thus the inhibition of migration observed after Tyrphostin-A9 treatment is most likely due to the inhibition of EphA receptor activity further pointing to the involvement of these receptors in the ephrin-A1-induced stimulation of migration. We did also observe inhibition of chemotaxis using Tyrphostin-A9 in cells not stimulated by ephrin-A1. This might indicate the involvement of PYK2 in chemokine signaling as shown previously.54 Although src-kinase family inhibitors have been shown to inhibit chemotaxis,39,40 we did not observe this in our experiments. The src-kinase family inhibitor SU6656 surprisingly stimulated chemotaxis both in ephrin-A1-stimulated and nonstimulated cells, whereas PP2, another src-kinase inhibitor, did not affect migration of cells. Induction of PYK2 phosphorylation was also observed after SU6656 treatment. The stimulatory effect of SU6656 warrants further study.
The study presented by Sharfe et al24 showed inhibition of SDF-1α-induced actin polymerization in the Jurkat cell line after ephrin-A1 binding. In our studies, we demonstrate induced actin polymerization in peripheral blood CD4+ T cells after SDF-1α incubation, which is significantly increased after coincubation with ephrin-A1. Thus ephrin-A1 influences SDF-1α-induced actin polymerization. We have not explored the precise mechanism for this, although we speculate that it relates to the increased migration observed after ephrin-A1 stimulation toward an SDF-1α gradient. The involvement of Rho, Rac1, or Cdc42 after ephrin stimulation has not been investigated here.
In conclusion, interaction between EphA receptors on CD4+ T cells and corresponding ephrin-A1 expression on endothelial cells located in HEVs suggests a role in transendothelial migration of CD4+ T cells. This involves intracellular tyrosine phosphorylation, including PYK2 phosphorylation, and actin polymerization leading to an increased chemotactic response.
Prepublished online as Blood First Edition Paper, December 7, 2004; DOI 10.1182/blood-2004-08-2981.
Supported by the Norwegian Cancer Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Steinar Funderud and Fridtjof Lund-Johansen for their critical comments on the manuscript.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal