Abstract
This work addresses the question whether CD38, a negative prognostic marker in B-cell chronic lymphocytic leukemia (B-CLL), plays a role in neoplastic B-cell growth and survival. We show that CD38+ B-CLL cells bind to murine fibroblasts transfected with the CD31 ligand. The interaction triggers an extensive remodeling of the B-CLL membrane, with relocalization of BCR/CD19 to the CD38/CD31 contact areas, and it also increases cell survival and proliferation. A second event is the up-modulation of the survival receptor CD100, restricted to proliferating cells, and a concomitant decrease of CD72 (low-affinity CD100 ligand and negative regulator of immune responses). The most efficient signals are delivered through sequential interactions between CD38/CD31 and CD100/plexin-B1 (high-affinity CD100 ligand), as inferred by coculture experiments using specific transfectants and blocking monoclonal antibodies (mAbs). The finding that nurselike cells from B-CLL patients express CD31 and plexin-B1, which deliver growth and survival signals to CD38+/CD100+ B-CLL cells, further confirms the model proposed. These findings show that a set of normal receptors and ligands ruling physiologic signaling pathways in B lymphocytes becomes detrimental when expressed in the context of B-CLL cells, ultimately leading to the generation of a tumor reservoir.
Introduction
Human CD38 is an ectoenzyme that synthesizes cyclic adenosine diphosphate (ADP) ribose and nicotinic acid adenine dinucleotide phosphate (NAADP), key compounds in the regulation of cytoplasmic Ca2+ levels.1-3 Engagement of CD38 by its ligand CD314,5 or by monoclonal antibodies (mAbs) induces activation and differentiation signals in T,6 B,7 and natural killer (NK)8 cells.
CD38 expression is high in B-cell precursors and in terminally differentiated plasma cells, but low to absent in mature B cells, where it can be induced by activatory signals.9,10 Functional data suggest that CD38 mediates adhesion of B lymphocytes to stromal cells in the bone marrow (BM)11,12 and in peripheral lymphoid organs.13,14 Several malignancies deriving from B lymphocytes are reportedly CD38+,15 including B-cell chronic lymphocytic leukemia (B-CLL) cells, which vary from negative to highly CD38+.16 Patients with a CD38+ clone are characterized by an unfavorable clinical course with a more advanced stage of disease, poor responsiveness to chemotherapy, earlier call for initial treatment, and shorter survival.17-20
This work stems directly from our previous observation that CD38 performs as a receptor in B-CLL cells upon translocation into the lipid rafts and physical association with the BCR/CD19 complex. Environmental conditions were found to be crucial in modulating CD38 surface expression and functions: interleukin-2 (IL-2) was identified as a surrogate indicator of the relevance of soluble factor(s), working in synergy to determine the final biologic effects. The signals delivered by CD38 and IL-2 induce proliferation and prolong survival of a subpopulation of B-CLL cells.21 These observations obtained using agonistic mAbs are now extended to a model closely resembling the physiologic environment where B-CLL cells grow. We show that CD38+ B-CLL cells bind to murine fibroblasts transfected with the CD31 ligand with resulting increased growth and survival. Further, this work shows that CD38/CD31 crosstalk is part of an intricate network of communication between neoplastic cells and bystander nonneoplastic cells. Indeed, CD38/CD31 interactions lead to increased B-CLL proliferation and survival by means of a direct cooperation with CD100, a cell surface receptor member of the semaphorin family (also known as sema4D), which interacts with CD72 (low-affinity ligand, coexpressed by B-CLL cells) and with plexin-B1 (high-affinity ligand, expressed by stromal and endothelial cells).22,23 CD38-mediated signals are followed by increased expression of cell surface CD100 and simultaneous down-modulation of CD72, which is the prototype of negative regulation of B cells.24 The validity of these results was confirmed by the finding that nurselike cells, professional supporters of B-CLL cells,25 express high levels of CD31 and plexin-B1. Further, CD38+/CD100+ B-CLL cells interact with nurselike cells with the final result of a significant improvement of their growth potential.
The resulting complex pattern of relations provides the first evidence of stepwise interactions between CD38 and CD100 on the B-CLL side and CD31 and plexin-B1 on the stromal side. The consequence for the B-CLL cell is the acquisition of an increased survival potential and proliferative activity, providing clues that explain the poorer prognosis of CD38+ patients.
Patients, materials, and methods
Patients and cells
The sample included 12 patients with B-CLL at diagnosis or who had received no treatment in the prior 6 months (Table 1). B-CLL was diagnosed according to standard clinical and laboratory criteria.26 B cells were purified from the peripheral blood by Ficoll-Paque (Pharmacia, Uppsala, Sweden) density gradient centrifugation followed by negative selection using anti-CD3, anti-CD16, and anti-CD14 mAbs (produced locally) and immunomagnetic bead separation (Dynal, Oslo, Norway).21 Flow cytometric analysis showed that these cells were more than 95% CD19+ and CD5+.
Characteristics of the patient sample
Patient no. . | Age, y . | Sex . | Ig . | % CD38+ . | Year of diagnosis . | Treatment* . |
|---|---|---|---|---|---|---|
| 1 | 65 | F | UM | 54.7 | 1998 | Yes |
| 2 | 68 | F | UM | 88.6 | 2001 | Yes |
| 3 | 77 | M | ND | 81.5 | 2002 | No |
| 4 | 64 | F | Mut | 98.5 | 1999 | Yes |
| 5 | 63 | M | Mut | 75.0 | 1987 | Yes |
| 6 | 64 | M | UM | 56.8 | 2000 | No |
| 7 | 58 | F | Mut | 93.4 | 1997 | Yes |
| 8 | 50 | M | Mut | 2.0 | 2003 | No |
| 9 | 64 | F | Mut | 54.2 | 1987 | Yes |
| 10 | 67 | F | UM | 34.0 | 1988 | Yes |
| 11 | 55 | F | UM | 91.8 | 2001 | Yes |
| 12 | 56 | F | Mut | 10.3 | 2003 | No |
Patient no. . | Age, y . | Sex . | Ig . | % CD38+ . | Year of diagnosis . | Treatment* . |
|---|---|---|---|---|---|---|
| 1 | 65 | F | UM | 54.7 | 1998 | Yes |
| 2 | 68 | F | UM | 88.6 | 2001 | Yes |
| 3 | 77 | M | ND | 81.5 | 2002 | No |
| 4 | 64 | F | Mut | 98.5 | 1999 | Yes |
| 5 | 63 | M | Mut | 75.0 | 1987 | Yes |
| 6 | 64 | M | UM | 56.8 | 2000 | No |
| 7 | 58 | F | Mut | 93.4 | 1997 | Yes |
| 8 | 50 | M | Mut | 2.0 | 2003 | No |
| 9 | 64 | F | Mut | 54.2 | 1987 | Yes |
| 10 | 67 | F | UM | 34.0 | 1988 | Yes |
| 11 | 55 | F | UM | 91.8 | 2001 | Yes |
| 12 | 56 | F | Mut | 10.3 | 2003 | No |
M indicates male; F, female; Mut, mutated; UM, unmutated; and ND, not determined.
Treatment was administered at least 6 months prior to analysis.
CD31 and plexin-B1 transfectants were obtained by transfecting specific cDNAs in murine L fibroblasts (L-CD31+) and NIH-3T3 cells (NIH/Plex-B1).5,27 Mock-transfected L cells (L-mock) and wild-type NIH-3T3 cells were used as controls.
Nurselike cells were obtained from peripheral blood mononuclear cells of B-CLL patients, as described.25
The experiments reported were performed after having obtained the approval of the Ethical Committee and Review Board of the University of Torino Medical School. Informed consent was provided according to the Declaration of Helsinki.
IgV gene sequencing
Immunoglobulin heavy chain variable region (IgVH) sequencing was determined by previously described methods.28
Antibodies and reagents
The high-performance liquid chromatography (HPLC)–purified endotoxin-free IB4 mAb was used to ligate CD38. Other reagents were O1.65 (anti–human leukocyte antigen [HLA] class I), CB19 (anti-CD19), CB01 (anti-CD5), Moon-1 (anti-CD31), BD16 and BB18 (anti-CD100), B6-9 (anti–plexin-B1, also indicated as EC6.9),27 Ab2.9 (anti-HLA class II), CB14 (anti-CD14) and the control JAS (anti–HIV-1 glycoprotein [gp] 120). Fluoroscein isothiocyanate (FITC)–labeled reagents included anti-CD72, (Caltag Laboratories, Burlingame, CA), anti-CD100 (BD Biosciences, Milano, Italy), anti–immunoglobulin M (IgM; Southern Biotechnologies Associates, Birmingham, AL) and O1.65 (locally conjugated). FITC- and Texas Red–conjugated F(ab′)2 goat anti–mouse Ig (GαMIg) were from Caltag. Syto 59 was from Molecular Probes (Eugene, OR).
Cell cultures
Purified B-CLL cells were cultured (1 × 106/mL) in RPMI-1640 medium (Sigma, Milano, Italy) with 5% heat-inactivated fetal calf serum (FCS; Seromed, Berlin, Germany), 50 μg/mL gentamicin, 100 U/mL penicillin, 100 μg/mL streptomicin (all from Sigma), referred to as complete medium. When indicated, the IB4 mAb, or the control JAS mAb (both at 10 μg/mL), alone or in combination with recombinant IL-2 (100 IU/mL), were added. For coculture experiments, L-CD31+ and L-mock cells were irradiated (30 Gy) and plated (50 000 cells) in 24-well plates (Costar, Corning, NY). A ratio of 1 fibroblast to 10 B-CLL cells was used. NIH/Plex-B1 and control cells were plated at the same concentration without irradiation. For coculture experiments with nurselike cells, autologous purified B-CLL cells were thawed and plated on nurselike cell layers for the indicated time points. Blocking of specific receptors was obtained by preincubation (30 minutes) of transfectants/nurselike cells with Moon-1 (anti-CD31), B6-9 (anti–plexin-B1) or the irrelevant anti–HIV-1 gp120 JAS mAb (all at 10 μg/mL). The blocking mAbs were replaced every 48 hours.
Cell survival in long-term cultures was assessed daily by microscope examination and viability determined by staining with Annexin V–FITC (BioSource, Camarillo, CA). Cell proliferation was determined upon assessment of DNA content after staining with propidium iodide (PI; Sigma). Cell morphology was studied by Giemsa staining of cytospin preparations. Samples were analyzed by light microscopy and images acquired using a C-VIEW-12-BUND camera fitted to an Olympus 1 × 70 microscope (Milano, Italy).
Cytofluorographic analyses and cell sorting
Stained cells were analyzed with a FACSort flow cytometer (BD Biosciences). The reading parameters were constant in each acquisition (forward scatter [FSC] 1.49, side scatter [SSC] 381); in these conditions, B-CLL cells were clearly distinct from murine fibroblasts and nurse-like cells in coculture experiments. Data were processed using CellQuest and WinMDI 2.8 software and expressed as histograms of the fluorescence intensity versus cell number or as mean fluorescence intensity (MFI). Purified B-CLL cells were gated into R1 (FSC > 530), R2 (330 = FSC = 530) and R3 (FSC < 330) regions.
B-CLL cells (50 × 106) cultured for 5 days in the presence of anti-CD38 mAb and IL-2 were resuspended (10 × 106/mL) in phosphate-buffered saline (PBS; Ca/Mg2+-free) + 5 mM ethylenediaminetetraacetic acid (EDTA), 25 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; pH 7.0 (HEPES), and 1% FCS and sorted on the basis of size, using the MoFlo cell sorter (Dako Cytomation, Glostrup, Denmark).
Determination of soluble CD100
Soluble CD100 (sCD100) levels were quantified by means of a sandwich enzyme immunoassay.29
Evaluation of CD38/CD31 interactions
Purified B-CLL cells (5 × 105 cells/well) were mixed with L-CD31+ or L-mock fibroblasts (2.5 × 105 cells/well) in a final volume of 600 μL and plated onto polylysine-coated slides in flat-bottomed 24-well plates. Conjugates were either centrifuged on the plates and immediately fixed (5 minutes in 4% paraformaldehyde) or incubated (37°C, 5% CO2) for 10, 30, and 60 minutes and then fixed and stained with the appropriate primary and secondary antibodies. The cells were observed using confocal microscopy. Conjugates were identified by directly observing cell morphology under differential interference contrast. The proportion of conjugates with CD38 redistributed to the areas of cell-cell contacts was calculated by randomly choosing at least 100 conjugates in 5 different experiments and scoring those with an accumulation of the fluorescent signal at the contact region.
In situ immunofluorescence staining
Irradiated fibroblasts were plated on glass coverslips and cultured in the presence of B-CLL cells for specified times. Cover slips were then fixed (4% paraformaldehyde), incubated (1 hour at 37°C) with anti-CD100 mAb (BB18), followed by a Texas Red–conjugated secondary antibody and a directly FITC-conjugated anti–HLA class I mAb. Nurselike cells were also grown on coverslips, washed, and incubated with the indicated primary antibody (1 hour at 4°C). After adding a Texas Red–conjugated secondary antibody, cells were fixed as described, permeabilized (0.1% saponin for 30 minutes), and counterstained with Syto 59.
The coverslips were mounted on slides and analyzed with an Olympus 1 × 71 confocal microscope, using the FluoView software from Olympus, Milano, Italy. Images were processed using Adobe Photoshop CS software (San Jose, CA).
Results
CD38/CD31 crosstalk leads to remodeling of the B-CLL membrane
We previously showed that CD38 performs as a receptor in B-CLL cells and that the signals implemented upon CD38 ligation in the presence of IL-2 induce proliferation and immunoblast-like transformation of the neoplastic clone.21 The aim of this work is to verify whether the events observed following CD38 ligation with an agonistic mAb in the presence of a soluble factor (IL-2) reflect what happens when a CD38+ B-CLL cell interacts with a CD31+ cell. The complexity of the interaction was reduced by using murine fibroblasts expressing CD31 as the only human molecule. The reorganization of the B-CLL membrane was selected as a rapid and sensitive indication of the crosstalk underway between CD38 and its ligand CD31.
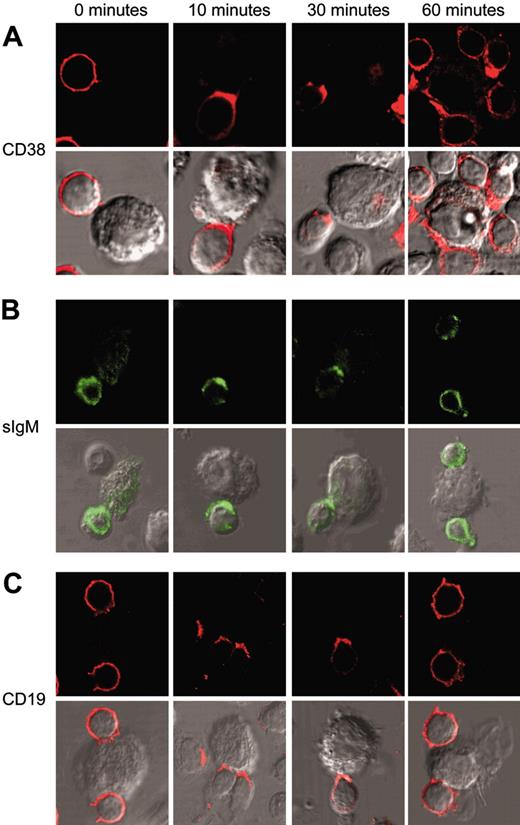
Exposure to L-CD31+ fibroblasts of B-CLL cells purified from 5 patients (nos. 2, 4, 6, 7, and 9; Table 1) induces an immediate redistribution of the CD38 molecules. CD38 molecules are evenly distributed on the surface of B-CLL cells: just 10 minutes of interaction with L-CD31+ cells is sufficient to induce the molecule to relocalize to the membrane contact areas, and the effect remains stable for more than 30 minutes. The CD38 molecules reacquire the original homogeneous distribution 1 hour after the interaction (Figure 1A). The same experiments performed with control L-mock cells yielded no recordable modifications of membrane CD38 distribution (not shown). Table 2 shows the percentage of relocalization of CD38 at the B-CLL/L-fibroblast contact areas.
CD38/CD31 crosstalk induces membrane remodeling of the CD38 receptor and of the BCR/CD19 complex. B-CLL cells purified from 5 patients (nos. 2, 4, 6, 7, and 9) were exposed to L-CD31+ transfectants for the indicated time intervals. Cells were then fixed and stained for CD38 (A) and CD19 (C) using a Texas Red–conjugated secondary antibody. sIgM molecules (B) were highlighted by means of a FITC-conjugated antibody. The cells were observed with an Olympus 1 × 71 confocal microscope (oil immersion × 60 objective), and the images were acquired using the FluoView software. Conjugates were identified by direct observation of cell morphology under differential interference contrast.
CD38/CD31 crosstalk induces membrane remodeling of the CD38 receptor and of the BCR/CD19 complex. B-CLL cells purified from 5 patients (nos. 2, 4, 6, 7, and 9) were exposed to L-CD31+ transfectants for the indicated time intervals. Cells were then fixed and stained for CD38 (A) and CD19 (C) using a Texas Red–conjugated secondary antibody. sIgM molecules (B) were highlighted by means of a FITC-conjugated antibody. The cells were observed with an Olympus 1 × 71 confocal microscope (oil immersion × 60 objective), and the images were acquired using the FluoView software. Conjugates were identified by direct observation of cell morphology under differential interference contrast.
Frequency of relocalization at the B-CLL/L-fibroblast contact areas
. | 0 min, % . | . | 10 min, % . | . | 30 min, % . | . | 60 min, % . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecule . | L-CD31+ . | L-mock . | L-CD31+ . | L-mock . | L-CD31+ . | L-mock . | L-CD31+ . | L-mock . | ||||
| CD38 | 5.3 ± 3.2 | 7.4 ± 3.8 | 58.2 ± 9.1 | 8.3 ± 4.8 | 65.5 ± 11.4 | 12.7 ± 7.5 | 22.2 ± 9.7 | 9.1 ± 5.9 | ||||
| CD19 | 8.6 ± 4.1 | 5.9 ± 2.4 | 44.3 ± 8.7 | 9.5 ± 6.3 | 57.9 ± 8.9 | 7.5 ± 5.4 | 15.6 ± 8.4 | 5.7 ± 4.4 | ||||
| slgM | 6.5 ± 4.3 | 6.8 ± 2.7 | 37.9 ± 11.4 | 7.8 ± 1.4 | 44.6 ± 7.9 | 3.2 ± 2.8 | 18.8 ± 6.9 | 7.4 ± 3.9 | ||||
| HLA class I | 4.7 ± 2.8 | 7.1 ± 3.3 | 7.5 ± 5.9 | 8.4 ± 4.2 | 4.2 ± 3.5 | 8.4 ± 5.2 | 9.3 ± 3.5 | 6.6 ± 5.1 | ||||
. | 0 min, % . | . | 10 min, % . | . | 30 min, % . | . | 60 min, % . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecule . | L-CD31+ . | L-mock . | L-CD31+ . | L-mock . | L-CD31+ . | L-mock . | L-CD31+ . | L-mock . | ||||
| CD38 | 5.3 ± 3.2 | 7.4 ± 3.8 | 58.2 ± 9.1 | 8.3 ± 4.8 | 65.5 ± 11.4 | 12.7 ± 7.5 | 22.2 ± 9.7 | 9.1 ± 5.9 | ||||
| CD19 | 8.6 ± 4.1 | 5.9 ± 2.4 | 44.3 ± 8.7 | 9.5 ± 6.3 | 57.9 ± 8.9 | 7.5 ± 5.4 | 15.6 ± 8.4 | 5.7 ± 4.4 | ||||
| slgM | 6.5 ± 4.3 | 6.8 ± 2.7 | 37.9 ± 11.4 | 7.8 ± 1.4 | 44.6 ± 7.9 | 3.2 ± 2.8 | 18.8 ± 6.9 | 7.4 ± 3.9 | ||||
| HLA class I | 4.7 ± 2.8 | 7.1 ± 3.3 | 7.5 ± 5.9 | 8.4 ± 4.2 | 4.2 ± 3.5 | 8.4 ± 5.2 | 9.3 ± 3.5 | 6.6 ± 5.1 | ||||
Quantification (percentage) of cell conjugates in which the indicated molecule was relocalized at the B-CLL/L-fibroblast contact areas. At least 100 conjugates from 5 independent experiments (patient nos. 2, 4, 6, 7, and 9) were analyzed. Results correspond to the arithmetic mean ± SD.
The effects of CD38/CD31 crosstalk were further tested on the localization of BCR and CD19, which were shown to be physically associated to CD38 in B-CLL cells.21 Surface IgM molecules cap to the contact areas in all the samples analyzed, with kinetics similar to those observed for CD38 (Figure 1B). Similar results are also obtained when analyzing the distribution of CD19 molecules (Figure 1C). Frequency of relocalization of BCR and CD19 at the B-CLL/L-fibroblast contact areas is reported in Table 2.
These results indicate that the effects observed upon mAb ligation are reproducible using CD31 as the trigger. The contribution of other molecules to the observed effects was ruled out, at least in this artificial model, by the absence of signals when incubating B-CLL cells with L-mock transfectants (not shown). Moreover, the absence of homotypic adhesion phenomena was confirmed by the lack of redistribution of the CD31 molecules.
CD38/CD31 crosstalk sustains viability and proliferation of B-CLL cells
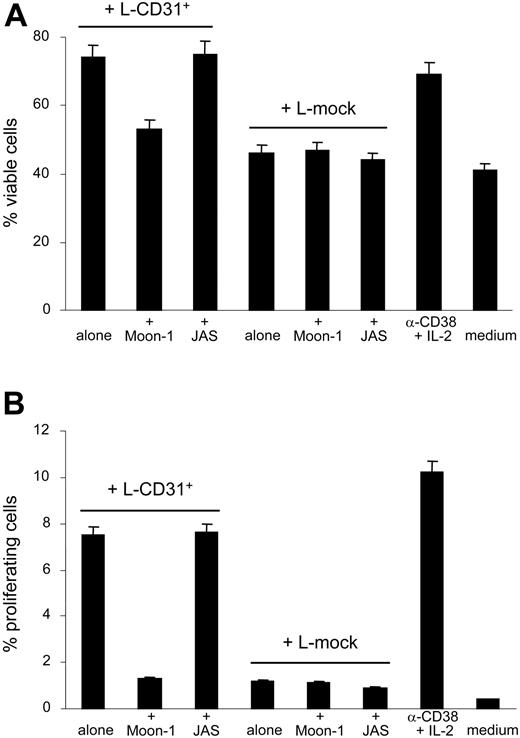
Long-term effects such as viability and proliferation following CD38/CD31 interactions were next examined. B-CLL cells purified from 4 patients (nos. 2, 4, 6, and 7) displayed a better viability and significantly increased their proliferative activity (ie, cells in the S, G2, and M phases of the cell cycle as shown by PI staining) when exposed to L-CD31+ cells compared with L-mock fibroblasts. The effect is apparent after 2 days of coculture and peaks at day 4. Results reported in Figure 2 indicate that proliferation and survival induced in B-CLL cells upon CD38/CD31 interaction are significantly inhibited by using an anti-CD31 mAb (Moon-1, added at a final concentration of 10 μg/mL and replaced every 48 hours, known as the best experimental blocking conditions5 ). An irrelevant isotype-matched mAb failed to alter the profile obtained. The specificity of the interaction was also witnessed by the failure of CD38– B-CLL cells (nos. 8 and 12) to increase viability or to proliferate when interacted with L-CD31+ fibroblasts (not shown). Of relevance, the effects are apparent even without the addition of IL-2, in contrast to what is observed using the agonistic mAb, likely reflecting a more efficient interaction in the presence of the ligand.
CD38/CD31 crosstalk promotes survival and proliferation of CD38+ B-CLL cells. Purified B-CLL cells from 4 patients (nos. 2, 4, 6, and 7) were cocultured with L-CD31+ or L-mock transfectants. After 3 days, B-CLL cell viability was determined by annexin-V staining (A), while proliferation rates were derived after assaying DNA content by PI staining (B). The anti-CD31 Moon-1 mAb was added to the coculture (10 μg/mL, replaced every 48 hours) to verify the specificity of the interaction. The anti–HIV-1 gp120 JAS mAb was used with similar modalities as the irrelevant control. Data are the mean values obtained using B-CLL cells from 4 patients in 2 separate experiments; error bars represent the SD.
CD38/CD31 crosstalk promotes survival and proliferation of CD38+ B-CLL cells. Purified B-CLL cells from 4 patients (nos. 2, 4, 6, and 7) were cocultured with L-CD31+ or L-mock transfectants. After 3 days, B-CLL cell viability was determined by annexin-V staining (A), while proliferation rates were derived after assaying DNA content by PI staining (B). The anti-CD31 Moon-1 mAb was added to the coculture (10 μg/mL, replaced every 48 hours) to verify the specificity of the interaction. The anti–HIV-1 gp120 JAS mAb was used with similar modalities as the irrelevant control. Data are the mean values obtained using B-CLL cells from 4 patients in 2 separate experiments; error bars represent the SD.
CD38/CD31 crosstalk up-modulates CD100 expression in a subset of B-CLL cells
The next issue was whether CD38 is directly responsible for increased proliferation and survival or whether these effects are exerted through a functional cooperation with other players. The attention was focused on the semaphorin family member CD100 because of its established involvement in the activation, survival, and differentiation of B-CLL cells.22,30,31
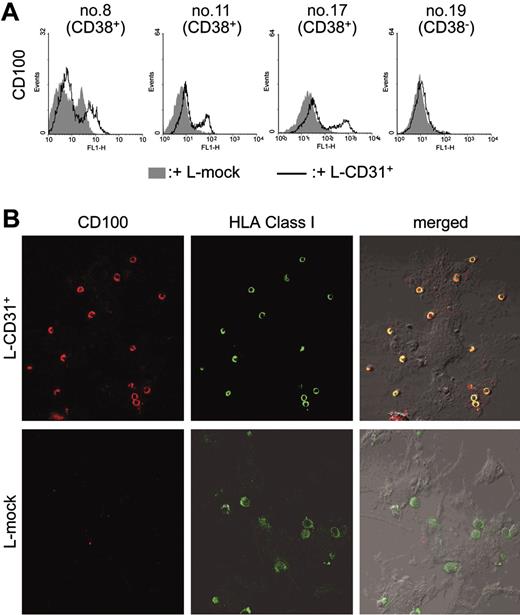
Five-day cocultures of purified B-CLL cells (nos. 2, 9, and 11) with irradiated L-CD31+ transfectants significantly increases CD100 levels in a subset of cells ranging from 16% to 23%. Comparative analysis was performed using the basal expression levels of the receptor and the effects induced by coculturing with L-mock transfectants as the control (Figure 3A). Increased CD100 expression becomes apparent 3 days after beginning of coculture and plateaus thereafter.
CD38/CD31 crosstalk up-modulates CD100 expression in a subset of B-CLL cells. B-CLL cells were purified from 3 CD38+ and 1 CD38– patients (nos. 8, 11, and 17, and no. 19, respectively) and cocultured with murine irradiated L-CD31+ or L-mock fibroblasts previously plated on glass coverslips. After 5 days, non–tightly adhering cells were collected from culture and stained for CD100 expression (A). The remaining conjugates were fixed and stained with an anti-CD100 mAb (BB18) followed by a Texas Red–conjugated secondary antibody. Counterstaining was performed with a FITC-conjugated anti–HLA class I mAb. The coverslips were mounted on slides and the cells observed using an Olympus 1 × 71 confocal microscope (oil immersion × 60 objective), and the images acquired using the FluoView software (B).
CD38/CD31 crosstalk up-modulates CD100 expression in a subset of B-CLL cells. B-CLL cells were purified from 3 CD38+ and 1 CD38– patients (nos. 8, 11, and 17, and no. 19, respectively) and cocultured with murine irradiated L-CD31+ or L-mock fibroblasts previously plated on glass coverslips. After 5 days, non–tightly adhering cells were collected from culture and stained for CD100 expression (A). The remaining conjugates were fixed and stained with an anti-CD100 mAb (BB18) followed by a Texas Red–conjugated secondary antibody. Counterstaining was performed with a FITC-conjugated anti–HLA class I mAb. The coverslips were mounted on slides and the cells observed using an Olympus 1 × 71 confocal microscope (oil immersion × 60 objective), and the images acquired using the FluoView software (B).
The effects are more evident when comparing cells tightly adhering to the fibroblast layer. This was revealed by removing non–tightly adhering cells by gentle washing and by staining the remaining B-CLL population with BB18, an anti-CD100 mAb (Figure 3B) and counterstaining with directly FITC-labeled anti–HLA class I mAb. Figure 3 shows that B-CLL cells adhering to the L-CD31+ fibroblasts express CD100 at high levels; on the contrary, B-CLL cells exposed to L-mock transfectants lack CD100. CD38– B-CLL cells (nos. 8 and 12) cocultured with L-CD31+ fibroblasts show no apparent modification in CD100 levels (Table 3).
Percentage of CD100+ cells after coculturing with L-CD31+ and CD38- B-CLL
. | . | L-CD31+ . | . | L-mock . | . | ||
|---|---|---|---|---|---|---|---|
| Patient no. . | % CD38+ . | Total cells, no. . | % CD100+ . | Total cells, no. . | % CD100+ . | ||
| 7 | 54.7 | 85 | 79 | 73 | 17 | ||
| 8 | 88.6 | 92 | 84 | 89 | 13 | ||
| 11 | 93.4 | 78 | 92 | 91 | 11 | ||
| 17 | 34.0 | 86 | 88 | 74 | 18 | ||
| 15 | 2.0 | 77 | 16 | 86 | 9 | ||
| 19 | 10.3 | 84 | 18 | 92 | 16 | ||
. | . | L-CD31+ . | . | L-mock . | . | ||
|---|---|---|---|---|---|---|---|
| Patient no. . | % CD38+ . | Total cells, no. . | % CD100+ . | Total cells, no. . | % CD100+ . | ||
| 7 | 54.7 | 85 | 79 | 73 | 17 | ||
| 8 | 88.6 | 92 | 84 | 89 | 13 | ||
| 11 | 93.4 | 78 | 92 | 91 | 11 | ||
| 17 | 34.0 | 86 | 88 | 74 | 18 | ||
| 15 | 2.0 | 77 | 16 | 86 | 9 | ||
| 19 | 10.3 | 84 | 18 | 92 | 16 | ||
CD100 expression is up-regulated exclusively in the proliferating cells
These results indicate that CD38-induced proliferation and differentiation events may be mediated, at least in part, by CD100. The obvious question at this point concerns the nature of the distinct subset of cells which up-regulate CD100. To address this issue, we used an agonistic anti-CD38 mAb and IL-2: this condition makes CD38-mediated signals more performing and does not require the presence of L-CD31+ fibroblasts, thus ruling out possible pitfalls due to the presence of a heterogeneous cell population.
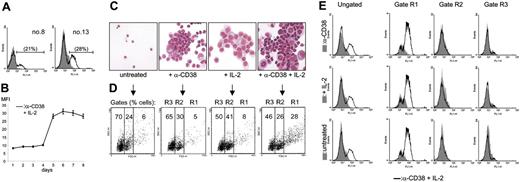
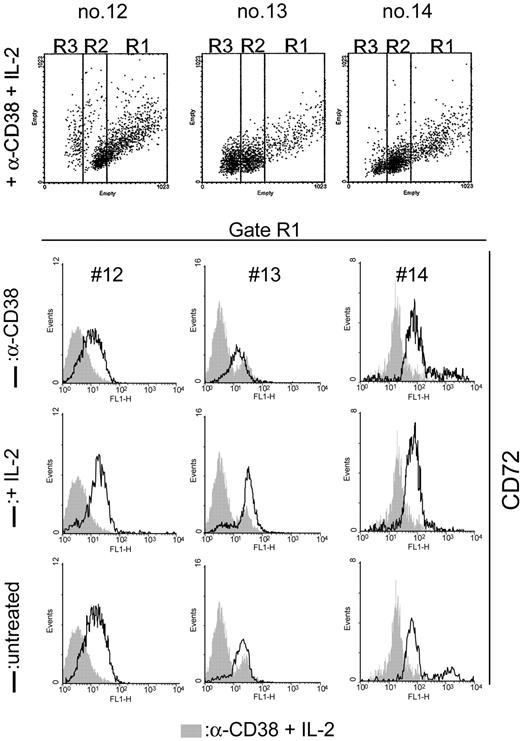
Ligation of CD38 by an agonistic mAb in IL-2–treated B-CLL cells from 7 different patients (nos. 1, 8, 9, 10, 12, 13, and 14) induces the appearance of a subset of CD100+ cells. Representative cytofluorimetric profiles (Figure 4A) confirm that the increased CD100 expression is limited to a subset (from 7% to 28%) of the whole B-CLL population, in line with what was observed using L-CD31+ fibroblasts. (1) The up-modulation of CD100 pertains exclusively to B-CLL cells, as determined after excluding contamination by monocytes, T or NK cells; (2) it is related to CD38 surface expression, given that CD38– B-CLL cells are unresponsive; (3) the phenomenon is independent from the basal levels of the CD100 receptor; (4) the increase in CD100 takes place at the cell surface 4 days after culture with anti-CD38 and IL-2, with expression levels peaking at day 6 and plateauing thereafter (Figure 4B); and (5) CD100 up-regulation is the result of the synergistic action of anti-CD38 mAb and IL-2, as indicated by the comparison of the MFI values of CD100 expression in the 4 culture conditions (not shown), in line with the notion that the agonistic mAb is per se inefficient in activating CD38 signals.21
CD100 up-regulation is restricted to the proliferating cells. (A) Purified B-CLL cells from patients nos. 8 and 13 were cultured for 5 days in the presence of agonistic anti-CD38 mAb + IL-2 and evaluated by flow cytometry using a directly labeled anti-CD100 mAb. The gray profiles represent the expression levels of the receptor in untreated cells, while the open profiles indicate the fluorescence intensity in the anti-CD38 + IL-2 samples. The percentages in parentheses refer to the CD100+ population. (B) The kinetics of CD100 up-regulation: the y-axis represents the MFI values of CD100 expression obtained from 7 different samples. Error bars represent the SD. Purified B-CLL cells from patient no. 6 were exposed to agonistic anti-CD38 mAb, IL-2, or a combination of the 2 for 5 days; the morphology of the resulting population was evaluated by Giemsa staining (C) and by cytofluorographic analysis (D). Gate R1 contains the proliferating cells, while R2 identifies resting and R3 the apoptotic population (as described in “Results”). The results in panel E show that the cells within the R1 gate display increased CD100 expression, while the other 2 subsets are unmodified. The open profiles reflect CD100 expression in the anti-CD38 + IL-2 condition compared with anti-CD38 alone (top row, gray profile), IL-2 alone (middle row, gray profile), and the untreated condition (bottom row, gray profile). Representative data from 7 patients.
CD100 up-regulation is restricted to the proliferating cells. (A) Purified B-CLL cells from patients nos. 8 and 13 were cultured for 5 days in the presence of agonistic anti-CD38 mAb + IL-2 and evaluated by flow cytometry using a directly labeled anti-CD100 mAb. The gray profiles represent the expression levels of the receptor in untreated cells, while the open profiles indicate the fluorescence intensity in the anti-CD38 + IL-2 samples. The percentages in parentheses refer to the CD100+ population. (B) The kinetics of CD100 up-regulation: the y-axis represents the MFI values of CD100 expression obtained from 7 different samples. Error bars represent the SD. Purified B-CLL cells from patient no. 6 were exposed to agonistic anti-CD38 mAb, IL-2, or a combination of the 2 for 5 days; the morphology of the resulting population was evaluated by Giemsa staining (C) and by cytofluorographic analysis (D). Gate R1 contains the proliferating cells, while R2 identifies resting and R3 the apoptotic population (as described in “Results”). The results in panel E show that the cells within the R1 gate display increased CD100 expression, while the other 2 subsets are unmodified. The open profiles reflect CD100 expression in the anti-CD38 + IL-2 condition compared with anti-CD38 alone (top row, gray profile), IL-2 alone (middle row, gray profile), and the untreated condition (bottom row, gray profile). Representative data from 7 patients.
Giemsa staining of the purified B-CLL cells cultured for 5 days in the presence of anti-CD38 and IL-2 induced the appearance of a cell population comprising large profilerating cells along with resting and apoptotic lymphocytes (Figure 4C). Using a dot-blot analysis of FSC (x-axis) and SSC (y-axis) (“Patients, materials, and methods”), this heterogeneous population was separated into 3 regions, namely, R1 (FSC > 530), R2 (330 = FSC = 530), and R3 (FSC < 330) (Figure 4D). Sorting followed by Giemsa staining of the cells belonging to the 3 regions also shows that R1 encompasses the majority of blast cells, R2 contains the resting cells, and R3 contains the apoptotic ones. CD38 ligation in IL-2–treated B-CLL cells increases the number of cells in the R1 gate (Figure 4D). Analysis of the FL1-H parameter after staining for CD100 and performed separately on the 3 gates clearly indicates that the increased expression of CD100 occurs exclusively in the R1 population, as no modifications are recorded among the resting and apoptotic cells (Figure 4E). The increase results from combined CD38 and IL-2 signals; either agent alone is ineffective or only weakly effective.
CD72 is down-modulated as a downstream event elicited upon CD38-mediated signals
The finding of CD100 up-modulation implemented by CD38 signaling in B-CLL cells led us to investigate the expression levels of CD72 (the CD100 low-affinity ligand), known to be coexpressed by B-CLL cells.32 This analysis was done exploiting the same morphologic approach adopted to distinguish among the discrete cell populations. CD38 ligation in IL-2–treated B-CLL cells is followed by a marked decrease in CD72 expression by the blasts obtained from the 7 patients studied (nos. 1, 2, 3, 4, 5, 6, and 7, with representative profiles shown in Figure 5). By contrast, CD72 remains unmodified in the populations of resting and apoptotic cells. This event becomes apparent 4 days after the beginning of the experiment and peaks at day 6, reproducing the same kinetics observed with the CD100 up-modulation. CD38– B-CLL cells (nos. 8 and 12) show no modification in surface CD72 expression. Plexin-B1 was never expressed on the surface of B-CLL cells.
CD72 is down-modulated as a downstream event elicited upon CD38-mediated signals. Purified B-CLL cells from CD38+ patients were exposed to agonistic anti-CD38 mAb, IL-2, or a combination of the 2 for 5 days; the surface levels of CD72 were then evaluated by exploiting the same morphologic approach described in Figure 4 to distinguish the discrete cell populations. The results indicate that the synergistic presence of anti-CD38 mAb and IL-2 (gray profiles) selectively down-regulates CD72 in the cells of the R1 region. Open profiles refer to anti-CD38 (top row), IL-2 (middle row), and the untreated conditions (bottom row).
CD72 is down-modulated as a downstream event elicited upon CD38-mediated signals. Purified B-CLL cells from CD38+ patients were exposed to agonistic anti-CD38 mAb, IL-2, or a combination of the 2 for 5 days; the surface levels of CD72 were then evaluated by exploiting the same morphologic approach described in Figure 4 to distinguish the discrete cell populations. The results indicate that the synergistic presence of anti-CD38 mAb and IL-2 (gray profiles) selectively down-regulates CD72 in the cells of the R1 region. Open profiles refer to anti-CD38 (top row), IL-2 (middle row), and the untreated conditions (bottom row).
These results indicate that the blasts obtained in IL-2-treated B-CLL cells after CD38-mediated signals express high levels of CD100, while CD72 (low-affinity CD100 ligand) is greatly reduced.
CD38/CD31 crosstalk cooperates with CD100/plexin-B1 in inducing survival of B-CLL cells
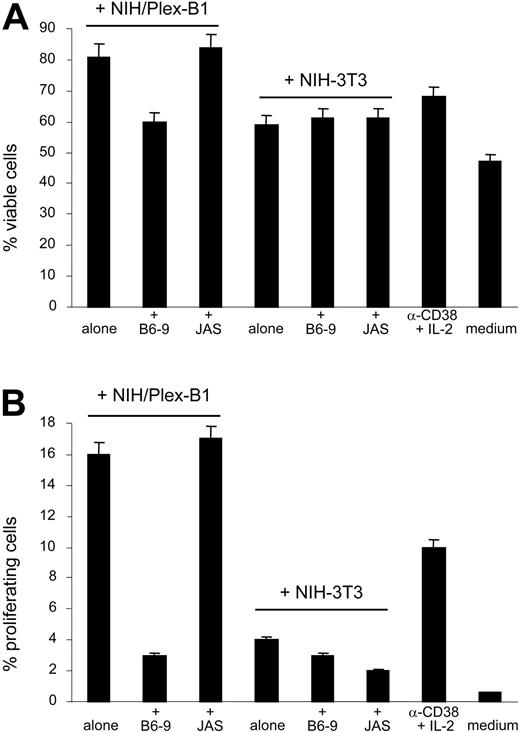
The next question was whether CD38 and CD100 might work in synergy in determining increased proliferation and survival of B-CLL cells. To address this issue, CD38+ B-CLL cells (from patients nos. 1, 4, and 6) were exposed to CD38-and IL-2–mediated signals for 5 days to induce the appearance of a subset of proliferating cells (highly CD100+). After removing anti-CD38 mAb and IL-2, cells were plated: (1) on a layer of murine NIH-3T3 fibroblasts stably transfected with plexin-B1 (the high-affinity stromal cell ligand for CD100); (2) on control wild-type cells; (3) in complete medium; or (4) maintained in the presence of anti-CD38 mAb and IL-2. CD38– B-CLL cells (patient no. 12) were used as the control.
B-CLL cells showed significantly better viability and a higher proliferation rate, as witnessed by Annexin-V (Figure 6A) and PI stainings (Figure 6B) following a 72-hour coculture with NIH/Plex-B1, compared with all 3 other conditions. These results indicate that the best effects in terms of survival and proliferation of B-CLL cells are obtained by combining the signals mediated by CD38 and CD100, pointing to a functional interplay between the 2 receptors. Moreover, when the availability of the plexin-B1 ligand was blocked by means of a specific mAb (B6-9, added at a final concentration of 10 μg/mL and replaced every 48 hours), the increased survival was significantly diminished, while an isotype-matched irrelevant mAb proved ineffective (Figure 6). CD38– B-CLL cells show no modification in the proliferation or survival rate in the presence of NIH/Plex-B1 (not shown).
CD38/CD31 crosstalk cooperates with CD100/plexin-B1 in inducing survival of B-CLL cells. CD38+ B-CLL cells (patients nos. 1, 4, 6, and 7) were exposed to CD38- and IL-2–mediated signals for 5 days to induce the appearance of a highly CD100+ subset. The cells were then collected, washed to remove anti-CD38 mAb and IL-2, and plated (1) on a layer of NIH/Plex-B1; (2) on control wild-type NIH-3T3 cells; (3) or in complete medium; or were maintained in the presence of anti-CD38 mAb and IL-2. Viability was tested after 72 hours by annexin-V staining (A), while proliferation was measured by PI staining (B). The percentages of annexin-V+ cells were derived from cells in the R1 and R2 gates (see Figure 5). The specificity of the system was confirmed by blocking the availability of the plexin-B1 ligand using the specific B6-9 mAb. The irrelevant JAS mAb was used as the control. Data are the mean values obtained using B-CLL cells from 4 patients in 2 separate experiments; error bars represent the SD.
CD38/CD31 crosstalk cooperates with CD100/plexin-B1 in inducing survival of B-CLL cells. CD38+ B-CLL cells (patients nos. 1, 4, 6, and 7) were exposed to CD38- and IL-2–mediated signals for 5 days to induce the appearance of a highly CD100+ subset. The cells were then collected, washed to remove anti-CD38 mAb and IL-2, and plated (1) on a layer of NIH/Plex-B1; (2) on control wild-type NIH-3T3 cells; (3) or in complete medium; or were maintained in the presence of anti-CD38 mAb and IL-2. Viability was tested after 72 hours by annexin-V staining (A), while proliferation was measured by PI staining (B). The percentages of annexin-V+ cells were derived from cells in the R1 and R2 gates (see Figure 5). The specificity of the system was confirmed by blocking the availability of the plexin-B1 ligand using the specific B6-9 mAb. The irrelevant JAS mAb was used as the control. Data are the mean values obtained using B-CLL cells from 4 patients in 2 separate experiments; error bars represent the SD.
These effects are directly mediated by cell-cell contacts, with no relevant participation of soluble CD100.22 sCD100 levels in supernatants of B-CLL cells (patients nos. 2, 4, 6, and 12) cultured in the presence of anti-CD38, IL-2, anti-CD38 + IL-2 or complete medium were measured after 16-hour or 5-day cultures using a sandwich enzyme immunoassay.29 The results (Table 4) indicate that the levels of sCD100 released under these conditions were low and unrelated to the surface expression levels of the molecule, suggesting that only membrane CD100 is responsible for the observed effects.
CD38 ligation in the presence of IL-2 is not followed by sCD100 release
. | 16 h, IU/mL . | . | . | . | 5 d, IU/mL . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | Basal . | Anti-CD38 + IL-2 . | Anti-CD38 . | IL-2 . | Basal . | Anti-CD38 + IL-2 . | Anti-CD38 . | IL-2 . | ||||||
| 2 | 0.923 | 0 | 0 | 0.698 | 0 | 0 | 0 | 0 | ||||||
| 4 | 0 | 1.01 | 0 | 0 | 2.9 | 5.1 | 4.4 | 4.7 | ||||||
| 6 | 12.4 | 9.4 | 8.2 | 12.5 | 2.12 | 0 | 0.8 | 0 | ||||||
| 12 | 0 | 1.41 | 1.6 | 0 | 2.1 | 0 | 0.8 | 0 | ||||||
. | 16 h, IU/mL . | . | . | . | 5 d, IU/mL . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | Basal . | Anti-CD38 + IL-2 . | Anti-CD38 . | IL-2 . | Basal . | Anti-CD38 + IL-2 . | Anti-CD38 . | IL-2 . | ||||||
| 2 | 0.923 | 0 | 0 | 0.698 | 0 | 0 | 0 | 0 | ||||||
| 4 | 0 | 1.01 | 0 | 0 | 2.9 | 5.1 | 4.4 | 4.7 | ||||||
| 6 | 12.4 | 9.4 | 8.2 | 12.5 | 2.12 | 0 | 0.8 | 0 | ||||||
| 12 | 0 | 1.41 | 1.6 | 0 | 2.1 | 0 | 0.8 | 0 | ||||||
Quantification of sCD100 in culture supernatants from 4 different patients harvested after 16 hours or 5 days, respectively.
Nurselike cells obtained from B-CLL patients express high levels of CD31 and plexin-B1
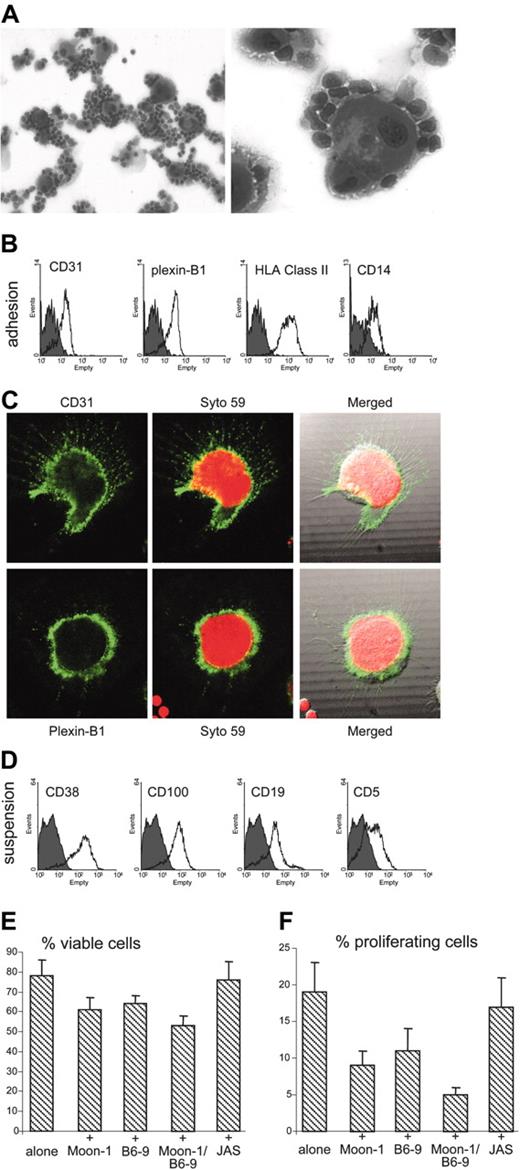
These data identify CD38/CD31 and CD100/plexin-B1 as 2 receptor/ligand systems cooperating in sustaining growth and survival of B-CLL cells. We then asked whether CD31 and plexin-B1 might be expressed by cells playing a key role in the life economy of B-CLL. Attention was focused on a subset of blood mononuclear cells, obtained exclusively from B-CLL patients, and that can differentiate in vitro into nurselike cells.34 This population is reported to protect B-CLL cells from apoptosis through cell-cell contacts and the production of stromal cell-derived factor-1 (SDF-1).25 Giemsa staining of peripheral blood mononuclear cells from 4 different B-CLL patients (nos. 7, 8, 13, and 14) cultured for 2 weeks in complete medium (representative patient no. 14 shown in Figure 7A) highlights the appearance of a population with the hallmarks of nurselike cells (ie, a large, round, and sometimes binucleate morphology and a strong membrane positivity for HLA class II, CD14, and CD45 molecules). This cell subset is also highly CD31+ and plexin-B1+, as shown by cytophluorographic analysis performed on cells detached from the plastic well (Figure 7B) and confirmed by in situ immunofluorescence stainings (Figure 7C). Both molecules display a strong membrane expression in all the samples analyzed. Conversely, the neoplastic B cells, which remained viable after 2-week cultures, display high levels of CD38 and CD100 (Figure 7D), while being CD31– and plexin-B1–.
Nurselike cells obtained from B-CLL patients express high levels of functional CD31 and plexin-B1. Nurselike cells were obtained by culturing peripheral blood mononuclear cells from 4 patients with B-CLL in complete medium for 2 weeks. The appearance of the cell culture is represented in panel A at × 20 (left) and × 40 (right) magnification. A population of cells morphologically similar to nurselike cells is apparent. These cells express CD31 and plexin-B1, as shown by cytofluorography (B) and in situ immunofluorescence (C). Cells in panel C were counterstained with Syto 59 and observed using an Olympus 1 × 71 confocal microscope (oil immersion × 60 objective), and the images were acquired using the FluoView software. (D) Cells in suspension comprise CD19+/CD5+ B lymphocytes that are highly positive for CD38 and CD100. Gray profiles in panels B and D were obtained using an irrelevant isotype-matched mAb. CD31 and plexin-B1 deliver proliferation/survival signals to CD38+/CD100+ B-CLL cells, as inferred after annexin-V (E) and PI (F) stainings performed after 4-day cocultures with autologous purified B-CLL cells. The specificity of the interaction was confirmed using blocking anti-CD31 and anti–plexin-B1 mAbs. Data are the mean values obtained using B-CLL from 4 patients in 2 separate experiments; error bars represent the SD.
Nurselike cells obtained from B-CLL patients express high levels of functional CD31 and plexin-B1. Nurselike cells were obtained by culturing peripheral blood mononuclear cells from 4 patients with B-CLL in complete medium for 2 weeks. The appearance of the cell culture is represented in panel A at × 20 (left) and × 40 (right) magnification. A population of cells morphologically similar to nurselike cells is apparent. These cells express CD31 and plexin-B1, as shown by cytofluorography (B) and in situ immunofluorescence (C). Cells in panel C were counterstained with Syto 59 and observed using an Olympus 1 × 71 confocal microscope (oil immersion × 60 objective), and the images were acquired using the FluoView software. (D) Cells in suspension comprise CD19+/CD5+ B lymphocytes that are highly positive for CD38 and CD100. Gray profiles in panels B and D were obtained using an irrelevant isotype-matched mAb. CD31 and plexin-B1 deliver proliferation/survival signals to CD38+/CD100+ B-CLL cells, as inferred after annexin-V (E) and PI (F) stainings performed after 4-day cocultures with autologous purified B-CLL cells. The specificity of the interaction was confirmed using blocking anti-CD31 and anti–plexin-B1 mAbs. Data are the mean values obtained using B-CLL from 4 patients in 2 separate experiments; error bars represent the SD.
CD38/CD31 and CD100/plexin-B1 crosstalks cooperate in inducing survival of B-CLL cells cocultured with nurselike cells
After showing that nurselike cells express CD31 and plexin-B1, we investigated whether the ligands are active in delivering growth and survival signals. To this aim, autologous B-CLL cells (patient nos. 7, 8, 13, and 14), purified at the time of blood collection and stored frozen, were thawed and cultured alone or on a layer of nurselike cells. As expected, coculture resulted in prolonged growth and survival, as determined after Annexin-V and PI stainings performed after 4-day cocultures. Addition of blocking anti-CD31 and anti–plexin-B1 mAbs partially inhibited proliferation and partially reduced the protection from apoptosis (Figure 7E), implying that these interactions are relevant in vivo. Block of both pathways achieved by simultaneous use of anti-CD31 and anti–plexin-B1 mAbs provided the most significant inhibition of growth and survival.
Discussion
The clinical behavior of B-CLL cases ranges from a stable condition not requiring treatment to a rapidly progressive disease unresponsive to therapy.33-36 Efforts to define markers capable of discriminating between these 2 extremes culminated with the identification of 2 types of B-CLL according to the mutational status of the IgVH genes.37 The general consensus is that indolent and aggressive B-CLL constitute 2 variants of the same disease and are distinguishable on the basis of relatively few genes.38 CD38 is one of these genes: its presence on the surface of B-CLL cells represents a significant risk for the patient of succumbing earlier to this incurable leukemia.
The present work originated with an attempt to use B-CLL as a convenient model for deriving information about the functions of CD38, which still elude full characterization despite the impressive bulk of data available.3 In this context, the clinical observations mentioned were re-read from the perspective of basic scientists: the working hypotheses were whether the presence of the molecule is a simple epiphenomenon reflecting the differentiation step at the moment of the neoplastic transformation or whether CD38 may exert pathogenetic roles. A third hypothesis is that CD38 is not directly linked to the neoplastic transformation, although its presence may increase the growth kinetics of the B-CLL clone.
The evidence supporting the third hypothesis is that CD38+ B-CLL cells bind to murine fibroblasts transfected with the CD31 ligand, with an increased growth and survival. Further, CD38/CD31 contacts implement an up-regulation of the survival receptor CD100 and a concomitant loss of CD72, a low-affinity CD100 ligand, and a negative regulator of immune responses. And last, the model is indirectly confirmed by the evidence that nurselike cells derived from B-CLL patients express high levels of functional CD31 and plexin-B1. The translational relevance is that CD38/CD31 crosstalk occurring in several body districts may be capable of switching on an activation pathway critical for B-CLL proliferation and survival. The signals mediated by CD38 include modulation of CD100, a semaphorin family member involved in sustaining B-CLL growth and survival.30,31 Protein analysis clearly indicates that CD100 is up-modulated exclusively in the blast population of B-CLL cells obtained upon CD38 ligation either by an agonistic mAb or by the CD31+ transfectants. The importance of these observations is confirmed by the finding of a stepwise cooperation between CD38 and CD100 in determining the final effects. Indeed, interaction of CD100 with its plexin-B1 ligand extends the life span of B-CLL cells preactivated by combined CD38 and IL-2 signals. The absence of soluble CD100 suggests that the effects observed are exerted through direct interaction between membrane CD100 and plexin-B1.
Nurselike cells, derived from a subset of the blood mononuclear pool of patients with B-CLL, protect leukemic clones from apoptosis through cell-cell contacts. We added to the analysis of the phenotype of these cells by showing that they express high levels of CD31 and plexin-B1. Worthy of further investigation is the finding of CD157 expression (S.D. and T.V., manuscript in preparation): this molecule is the CD38 paralog and was previously reported as present on nurselike cells obtained from patients with rheumatoid arthritis.39 Further, CD38+/CD100+ B-CLL cells interact with CD31/plexin-B1+ nurselike cells with the final result of a significant improvement of their growth potential.
The experimental results of the present work are in line with our third hypothesis that CD38 may be an occasional bystander of the neoplastic transformation of B-CLL, but not an innocent one. This implies that the CD38/CD31 crosstalk occurring in several body districts may induce B-CLL cells to switch on activation processes that result in cells responding poorly to conventional treatments and, consequently, acting as a tumor reservoir. We suggest that the lymph nodes provide an ideal microenvironment and the appropriate set of receptors and signals for this scenario. Independent results showed that surface CD38 expression in the lymph nodes is significantly higher than in peripheral blood or BM neoplastic B cells.40 However, the present evidence obtained using nurselike cells may extend the validity of the model to the circulating pool of neoplastic B cells.
In conclusion, CD38 signaling in B-CLL may be initiated by close interaction with CD31, giving origin to a signal that induces overexpression of CD100. The successive interplay of CD100 with the plexin-B1 ligand accounts for a second set of signals adding to cell survival and proliferation. Each of these signals is per se a normal component of the life economy of B cells41 ; however, their presence in a pathological situation represents an additional unfavorable element, which triggers detrimental activation/survival pathways. The coexistence of several or all of these mechanisms in a B-CLL cell may lead to the implementation and exasperation of signaling pathways ultimately causing Richter syndrome.
Occasional expression of a normal molecule marking normal differentiation steps thus represents the starting point of events leading to the poor prognosis of a subset of B-CLL patients. An analogous instance of harmful effects exerted by ectopic expression of CD38 may be found in the pathogenesis of retinoic acid syndrome in patients with acute promyelocytic leukemia. Indeed, retinoic acid treatment induces granulocytes differentiated from leukemic blasts (normally CD38–) to express CD38 and to adhere to CD31+ endothelial cells lining pulmonary vessels: the abherrant interactions are followed by acute respiratory distress, the hallmark of the syndrome.42
To be completed, the model requires further investigation on the contribution of soluble factors and, more important, of genetic polymorphisms of the molecules involved.43 This is the focus of our current investigation.
Prepublished online as Blood First Edition Paper, December 21, 2004; DOI 10.1182/blood-2004-10-3873.
Supported by grants from Associazione Italiana Ricerca Cancro (AIRC), Fondo Investimenti Ricerca di Base (FIRB), Ministero Istruzione Universitaria e Ricerca (MIUR), and Progetto Ricerca di Rilevante Interesse Nazionale (PRIN) projects and from the 2002 Health Strategic Research Project (Ministry of Health). The Fondazione Internazionale Ricerche Medicina Sperimentale (FIRMS), Compagnia di SanPaolo, and Regione Piemonte provided financial contributions. T.V. is a student of the PhD Program “Radioimmunolocalization of Human Tumors,” University of Torino Medical School, and L. Bonello is a FIRMS Fellow.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal