Abstract
T-cell interaction with antigen-presenting cells (APCs) results in activation and clonal expansion of naive T cells. CD80 expression/acquisition in T cells has been implicated in disease processes in patients with rheumatoid arthritis and multiple myeloma and patients infected with HIV. Our previous data indicate that antigen-specific activation of naive T cells results in T-cell acquisition of CD80 molecules from APCs. However, the functional relevance of the acquired CD80 by T cells in signal pathways has remained unresolved. This study aims to define for the first time the role of acquired CD80 in T-cell clonal expansion. We demonstrate the following: (1) T cells, upon CD80 acquisition, sustain their proliferative response in the absence of APCs; (2) T cells that acquire CD80 sustain the activity of transcriptional factors such as nuclear factor-κB (NFκB) and activator protein-1 (AP1) for 24 hours after separation from APCs and up-regulate signal transducer and activator of transcription-5 (Stat5) in the absence of APCs or exogenous signal 1; and (3) maintenance of these signals results in unique cytokine production. Collectively, our data support the unique concept that naive T cells sustain their activation by removing “antigen presentasome” (APS; eg, antigen-presenting complex) from APCs, thereby releasing the constraint of APC requirement for further activation. (Blood. 2005;105: 3238-3246)
Introduction
Recognition of major histocompatibility complex (MHC)/peptide on antigen-presenting cells (APCs) by T-cell receptors (TCRs) is essential in triggering T-cell activation.1 However, the interaction of TCRs with MHC is insufficient to achieve an adequate response of T cells.2 To be effectively and promptly activated, T cells require and conjugate with additional costimulatory molecules such as CD80 on APCs.3,4
Several studies have reported that CD80 can be detected on human T cells in patients with rheumatoid arthritis and multiple myeloma. Moreover, the presence of these T cells that have acquired CD80 has been implicated in these disease processes.5,6 It has also been suggested that T cells that express CD80 and MHC in HIV-infected patients may act as APCs.7 Wolthers et al have demonstrated that T cells with increased levels of CD80 expression on both CD4+ and CD8+ cells in HIV-infected patients can costimulate the proliferation of responder T cells from healthy donors.8 To date, the expression of CD80 has been attributed to de novo up-regulation of CD80; however, it has been shown in a number of studies by our group and others that T cells from mice (using B7.1/B7.2 double knock-out [KO] and CD28 KO mice along with reverse transcriptase polymerase chain reaction [RT-PCR] and confocal studies) and humans are clearly able to obtain/acquire CD80 and MHC determinants from APCs.9-12 The significance of this CD80 acquisition by T cells has remained elusive.
In this study, we have investigated the role of acquired CD80 in the maintenance of intracellular signals leading to continued proliferation, using CD4+CD80acq T cells (CD4+ T cells that had acquired CD80). Our results indicate that there is sustained activation of nuclear factor-κB (NFκB) and activator protein-1 (AP1) in CD4+CD80acq T cells and the activation of these transcription factors is directly related to the slow disappearance of MHC class I-IEk and CD80. Also, these events lead to a continued proliferation of CD4+CD80acq T cells. Up-regulation of signal transducer and activator of transcription-5 (Stat5) was observed during the proliferation of CD4+CD80acq T cells. Removal of acquired CD80 by pronase treatment leads to the reduction of CD4+ T-cell proliferation and abolishment of Stat5 activity. In vivo studies demonstrate that CD4+CD80acq T cells undergo rapid proliferation in vivo. Taken together, our data strongly indicate a robust expansion of T cells as a result of CD80/MHC class II acquisition and the critical role of acquired molecules (antigen presentasome [APS]) containing CD80/MHC class II in the process of T-cell activation as a regulator of proliferative signals in activated T cells after acquisition.
Materials and methods
Animals
Pigeon cytochrome c88-104-transgenic (PCC/TCR-Tg) mice, which are TCR transgenic for PCC, were obtained from Taconic Farms (Germantown, NY) and bred in the National Cancer Institute (Bethesda, MD) animal facility under pathogen-free conditions.
Peptide and APCs
The IEk-restricted PCC peptide was synthesized by American Peptide (Sunnyvale, CA). Fibroblast cell lines P13.9 and DCEK were kindly provided by Dr R. Germain (National Institutes of Health [NIH], Bethesda, MD). P13.9 and DCEK cell lines were derived from the same fibroblast cell line and were stably transfected with IEk and CD80 expression vectors. P13.9 and DCEK fibroblasts, with the same level of IEk (MHC class II), express high and low levels of CD80, respectively. Macrophages were generated as described previously and used as APCs.13
Antibodies and flow cytometry
The levels of CD80, IEk, CD25, and CD69 expression on the naive CD4+ and CD4+CD80acq T cells were assessed by staining with anti-CD80-fluorescein isothiocyanate/CD4+-peridinin chlorophyll protein/anti-IEk-phycoerythrin (anti-CD80-FITC/CD4+-PerCP/anti-IEk-PE), anti-CD25-FITC/CD4+-PerCP, and anti-CD69-PE/CD4+-PerCP antibodies (Abs) (Becton Dickinson, Mountain View, CA). All stained samples were further analyzed by flow cytometry using a FACSCalibur and CellQuest software (both Becton Dickinson).
Generation of CD4+CD80acq hi and CD4+CD80acq low T cells
Purified CD4+ T cells (negatively selected according to the manufacturer's instructions; Miltenyi Biotec, Auburn, CA) were cocultured with either PCC-pulsed 13.9 or PCC-pulsed DCEK APCs at a ratio of 10:1 for 20 hours in complete RPMI 1640 medium. After the treatment, CD4+ T cells (CD4+CD80acq T cells) were separated from the APCs.
[3H]thymidine uptake assay
CD4+CD80acq hi T cells were plated at a density of 3 × 105 cells per well and pulsed with [3H]thymidine (1 μCi [0.0037 MBq] per well) for 6 or 24 hours. The incorporated radioactivity was measured in a liquid scintillation counter.
RNase protection assay
Total RNA was isolated from naive and CD4+CD80acq hi T cells using STAT-60 reagent (TEL-TEST, Friendswood, TX). Messenger RNA of cytokines was detected using RiboQuant multiple-probe RNase protection assay system (probe templates: mCK-1b; BD PharMingen, San Diego, CA).
Enzyme-linked immunosorbent assay (ELISA)
Supernatant mediums were collected and analyzed for interleukin-2 (IL-2), IL-9, and interferon-γ (IFN-γ) (Pierce, Rockford, IL). Each result represents the mean from duplicate wells.
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts of CD4+CD80acq hi and CD4+CD80acq low T cells were prepared, and EMSA was performed as described previously.14
Pronase treatment
CD4+CD80acq hi T cells that were separated from APCs were washed 3 times with cold Hanks balanced salt solution (HBSS) and further stripped with 0.01% pronase in the HBSS buffer at 37°C for 12 or 30 minutes.
Adoptive transfer studies in vivo
CD4+CD80acq hi T cells were stained with either PKH-26 dye according to the manufacturer's protocol (Sigma, St Louis, MO) or carboxyfluorescein diacetate succinimidyl ester (CFSE). A total of 1.5 × 107 PKH-26-stained cells or 5 × 106 CFSE-stained cells were injected intravenously into PCC/TCR-Tg mice. CD4+ T cells were analyzed by multicolor fluorescence-activated cell sorter (FACS) analysis.
Results
Kinetics of CD80 (B7.1) and IEk (MHC class II) of CD4+CD80acq T cells
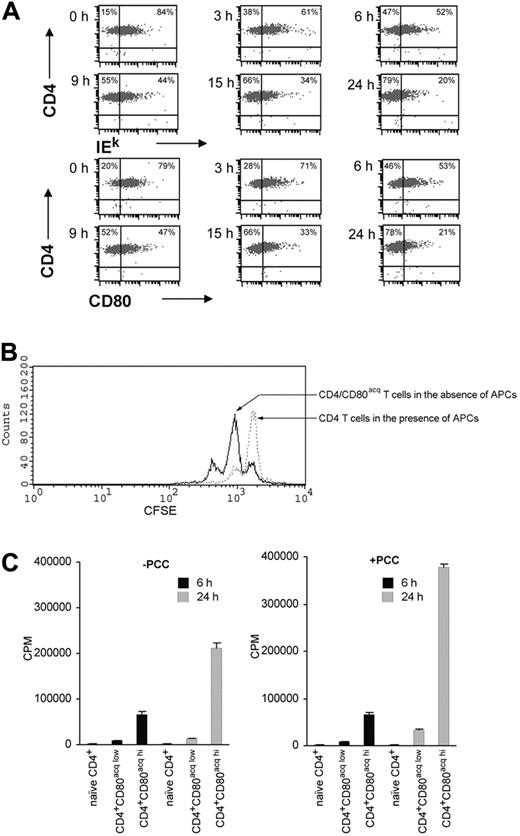
Previously we demonstrated that naive CD4+ T cells can acquire CD80 and MHC class II.10,11 FACS and PCR analysis of naive CD4 T cells that had acquired CD80 demonstrated that these molecules were actively acquired by T cells and that their expression on T cells was not due to APC contamination or de novo up-regulation of these molecules (ie, sensitivity of detection of CD80 in PCR was 1 ng or less of cDNA from APCs, translating to 1% or fewer contaminating cells; Figure S1A [see the Supplemental Figures link at the top of the online article on the Blood website].10 To investigate the fate of MHC class II (IEk) and CD80 upon acquisition by T cells, naive PCC/TCR-Tg CD4+ T cells were incubated with APCs (P13.9 fibroblasts pulsed with 10 μg/mL PCC peptide for 3 days) for 20 hours. To remove all APCs, fibroblasts were fed with Dynal beads to enable us to magnetically separate them from T cells, thus freeing the CD4+CD80acq T cells of contaminating APCs (98% to 99% purity; Figures S1B and S2). After the separation from APCs, there was a significant acquisition of IEk (84%) and CD80 (79%) on the surface of the CD4+ T cells. Following separation and incubation of CD4+ T cells that had acquired CD80, the levels of IEk and CD80 gradually decreased within 24 hours (Figure 1A). In comparison, basic expression of IEk and CD80 on naive CD4+ T cells that were cultured for 24 hours in the absence of APCs was negligible (0% to 4%; data not shown).
Kinetic change in disappearance of CD80 and IEk, continued proliferation of CD4+CD80acq T cells, and acquired CD80-dependent proliferative response. (A) Naive CD4+ T cells were incubated with PCC-pulsed P13.9 APCs for 20 hours to acquire MHC class II-IEk and CD80. CD4+CD80acq hi T cells were cultured in the absence of APCs and PCC peptide at various time points, and acquired IEk and CD80 on these T cells were assessed by using multicolor FACS analysis. (B) CFSE-stained naive CD4+ T cells were stimulated with PCC-pulsed P13.9 APCs for 20 hours. The cells were further cultured in the presence or absence of APCs for an additional 24 hours. Proliferative response of the cells in the presence or absence of APCs was examined by the FACS analysis. (C) Naive CD4+ T cells were cocultured with either PCC-pulsed APCs expressing low levels of CD80low (DCEK) or PCC-pulsed APCs expressing high levels of CD80hi (P13.9) for 20 hours. After the separation from DCEK and P13.9 APCs, both CD4+CD80acq low and CD4+CD80acq hi T cells were incubated in the absence or presence of an additional 1 μg/mL PCC without APCs for 6 (▪) or 24 hours ( ). Proliferation of CD4+CD80acq low and CD4+CD80acq hi T cells (3 × 105 cells per well, 96-well plate) was examined using 3H uptake assay. Data represent mean ± SE of 11 different experiments. Percentages are the percent positive cells in the respective quadrant.
). Proliferation of CD4+CD80acq low and CD4+CD80acq hi T cells (3 × 105 cells per well, 96-well plate) was examined using 3H uptake assay. Data represent mean ± SE of 11 different experiments. Percentages are the percent positive cells in the respective quadrant.
Kinetic change in disappearance of CD80 and IEk, continued proliferation of CD4+CD80acq T cells, and acquired CD80-dependent proliferative response. (A) Naive CD4+ T cells were incubated with PCC-pulsed P13.9 APCs for 20 hours to acquire MHC class II-IEk and CD80. CD4+CD80acq hi T cells were cultured in the absence of APCs and PCC peptide at various time points, and acquired IEk and CD80 on these T cells were assessed by using multicolor FACS analysis. (B) CFSE-stained naive CD4+ T cells were stimulated with PCC-pulsed P13.9 APCs for 20 hours. The cells were further cultured in the presence or absence of APCs for an additional 24 hours. Proliferative response of the cells in the presence or absence of APCs was examined by the FACS analysis. (C) Naive CD4+ T cells were cocultured with either PCC-pulsed APCs expressing low levels of CD80low (DCEK) or PCC-pulsed APCs expressing high levels of CD80hi (P13.9) for 20 hours. After the separation from DCEK and P13.9 APCs, both CD4+CD80acq low and CD4+CD80acq hi T cells were incubated in the absence or presence of an additional 1 μg/mL PCC without APCs for 6 (▪) or 24 hours ( ). Proliferation of CD4+CD80acq low and CD4+CD80acq hi T cells (3 × 105 cells per well, 96-well plate) was examined using 3H uptake assay. Data represent mean ± SE of 11 different experiments. Percentages are the percent positive cells in the respective quadrant.
). Proliferation of CD4+CD80acq low and CD4+CD80acq hi T cells (3 × 105 cells per well, 96-well plate) was examined using 3H uptake assay. Data represent mean ± SE of 11 different experiments. Percentages are the percent positive cells in the respective quadrant.
Continued proliferation of CD4+CD80acq T cells in the absence of APCs
To further investigate the effect of CD80 acquisition on CD4+ T-cell proliferation after separation from APCs, CFSE-labeled naive CD4+ T cells were incubated with PCC peptide-pulsed APCs for 20 hours; half of the T cells were then depleted of APCs by bead separation as described in the preceding paragraph, while the other half remained in the presence of APCs loaded with PCC peptide for an additional 24 hours. As depicted in Figure 1B, CD4+CD80acq T cells in the absence of APCs proliferated more rapidly than those left in culture with APCs and peptide. To control the levels of CD80 transferred onto T cells, 2 fibroblast cell lines (APCs) expressing similar levels of MHC class II-IEk, but different levels of CD80 (P13.9 express more CD80 than DCEK; Figure S3A), were cultured with CD4+ T cells for 20 hours. Coculture of CD4+ T cells with these APCs resulted in different levels of CD80 acquisition (Figure S3B). Almost 80% of T cells acquired CD80 following coculture with P13.9, whereas only 10% of T cells acquired CD80 after coculture with DCEK. Higher CD80 acquisition enabled these CD4+ T cells to proliferate more robustly (measured at 6 or 24 hours; Figure 1C). Interestingly, the addition of exogenous PCC peptide further enhanced their proliferative response regardless of the levels of CD80 acquisition, suggesting that the increased signal 1 in CD4+CD80acq T cells augments their proliferative response. Most of the CD4+ cells that were stimulated with PCC-pulsed DCEK for 20 hours expressed high levels of CD25 and CD69 (Figure S4A). These data further demonstrated that despite the small percentage of cells that acquired CD80, most of the CD4+ T cells that were stimulated with DCEK were activated and expressed CD25 and CD69 (early activation markers). We further stimulated CD4+ T cells with PCC-pulsed DCEK APCs mixed with a graded amount of PCC-pulsed P13.9 APCs (Figure S4A) and found that CD4+ T cells acquired CD80 in a proportional manner. Moreover, the proliferative response of CD4+CD80acq T cells upon separation from APCs was proportional to the amount of CD80 they had acquired (Figure S4B).
We next asked if the continued proliferation of these cells required the persistent presence of CD80 and MHC class II molecules. To this end, these molecules were stripped off T-cell membranes by pronase treatment. As shown in Figure 2A, 12 minutes of pronase treatment resulted in complete stripping of CD4, and approximately 50% of CD80 and IEk remained on the cell surface. Longer (30 minutes) pronase treatment further increased the efficiency of stripping. Six hours of incubation after the termination of the stripping allowed the restored expression of CD4 but not that of CD80 and IEk, suggesting that intercellular transfer, but not de novo synthesis, accounted for the expression of CD80/IEk on the surface of CD4+ T cells (Figure 2B). Additionally, the stripped CD4+CD80acq T cells displayed reduced proliferation compared with unstripped CD4+CD80acq T cells (Figure 2C). Interestingly, a longer pronase stripping (30 minutes) led to the removal of more CD80/MHC class II-IEk molecules, accompanied by a reduced proliferation of the T cells. Because no further CD4 stripping was observed in these cells with prolonged pronase treatment, we concluded that CD80/MHC class II, but not CD4, molecules directly modulate proliferation of CD4 T cells under these circumstances. As control, phorbol myristate acetate (PMA)/ionomycin-activated CD4+ T cells were treated with pronase. As shown in Figure 2D, 12 minutes of pronase treatment resulted in complete stripping of CD4 and reduced proliferative response of the CD4+ T cells slightly (15%). Longer pronase treatment (30 minutes) of PMA/ionomycin-activated CD4+ T cells, however, failed to further reduce the proliferative response of the T cells.
Effect of pronase treatment on the proliferative response of CD4+CD80acq hi T cells, expression of IEk and CD80 on P13.9, function of P13.9 APCs, and impact of possibly contaminated splenic APCs on the proliferation of T cells. (A) Naive CD4+ T cells were cocultured with PCC-pulsed P13.9 (CD80high) APCs for 20 hours. After the separation from the APCs, CD4+CD80acq hi T cells were further stripped with 0.01% pronase for 12 or 30 minutes. Expression of CD4, CD80, and IEk on the unstripped or pronase-stripped cells was analyzed by 3-color FACS analysis. (B) Level of IEk, CD80, and CD4 on unstripped or pronase-stripped (treatment for 12 and 30 minutes) CD4+CD80acq hi T cells, which had been further cultured in the absence of APCs and PCC peptide for 6 hours, was analyzed by 3-color FACS analysis. (C) CD4+CD80acq hi T cells that were not stripped (□) or stripped with pronase for 12 minutes (▪) or 30 minutes (▦) were cultured in the absence of APCs and PCC peptide for 6 hours. Proliferative response of these T cells was examined using 3H uptake assay. (D) Naive CD4+ T cells were stimulated with PMA (5 ng/mL) plus ionomycin (250 ng/mL) for 20 hours and further treated with 0.01% pronase for 12 minutes (▪) or 30 minutes (▦). □ represents unstripped. Proliferative response of these T cells was examined using 3H uptake assay. (E) P13.9 APCs were treated with 0.01% pronase for 30 minutes, and IEk and CD80 expression on P13.9 APCs was analyzed by FACS analysis. (F) Naive CD4+ T cells were stimulated with either PCC-pulsed P13.9 APCs or pronase-treated PCC-pulsed APCs for 24 hours. Proliferative response of these T cells was examined using 3H uptake assay. CD4+: naive T cells only; P13.9: P13.9 APCs pulsed with PCC peptide; pronase-treated APCs: P13.9 APCs pulsed with PCC peptide were further treated with 0.01% pronase for 30 minutes; CD4 + P13.9: naive CD4+ T cells were stimulated with P13.9 APCs; CD4+ pronase-treated P13.9: naive CD4+ T cells were stimulated with pronase-treated P13.9 APCs. (G) Purified naive CD4+ T cells were treated with different concentrations (100 ng/mL to 1 pg/mL) of PCC peptide for 24 hours. Proliferative response of purified CD4+ T cells was examined using 3H uptake assay. Percentages are the percent positive cells in the respective quadrant. Error bars represent mean ± standard deviation (SD).
Effect of pronase treatment on the proliferative response of CD4+CD80acq hi T cells, expression of IEk and CD80 on P13.9, function of P13.9 APCs, and impact of possibly contaminated splenic APCs on the proliferation of T cells. (A) Naive CD4+ T cells were cocultured with PCC-pulsed P13.9 (CD80high) APCs for 20 hours. After the separation from the APCs, CD4+CD80acq hi T cells were further stripped with 0.01% pronase for 12 or 30 minutes. Expression of CD4, CD80, and IEk on the unstripped or pronase-stripped cells was analyzed by 3-color FACS analysis. (B) Level of IEk, CD80, and CD4 on unstripped or pronase-stripped (treatment for 12 and 30 minutes) CD4+CD80acq hi T cells, which had been further cultured in the absence of APCs and PCC peptide for 6 hours, was analyzed by 3-color FACS analysis. (C) CD4+CD80acq hi T cells that were not stripped (□) or stripped with pronase for 12 minutes (▪) or 30 minutes (▦) were cultured in the absence of APCs and PCC peptide for 6 hours. Proliferative response of these T cells was examined using 3H uptake assay. (D) Naive CD4+ T cells were stimulated with PMA (5 ng/mL) plus ionomycin (250 ng/mL) for 20 hours and further treated with 0.01% pronase for 12 minutes (▪) or 30 minutes (▦). □ represents unstripped. Proliferative response of these T cells was examined using 3H uptake assay. (E) P13.9 APCs were treated with 0.01% pronase for 30 minutes, and IEk and CD80 expression on P13.9 APCs was analyzed by FACS analysis. (F) Naive CD4+ T cells were stimulated with either PCC-pulsed P13.9 APCs or pronase-treated PCC-pulsed APCs for 24 hours. Proliferative response of these T cells was examined using 3H uptake assay. CD4+: naive T cells only; P13.9: P13.9 APCs pulsed with PCC peptide; pronase-treated APCs: P13.9 APCs pulsed with PCC peptide were further treated with 0.01% pronase for 30 minutes; CD4 + P13.9: naive CD4+ T cells were stimulated with P13.9 APCs; CD4+ pronase-treated P13.9: naive CD4+ T cells were stimulated with pronase-treated P13.9 APCs. (G) Purified naive CD4+ T cells were treated with different concentrations (100 ng/mL to 1 pg/mL) of PCC peptide for 24 hours. Proliferative response of purified CD4+ T cells was examined using 3H uptake assay. Percentages are the percent positive cells in the respective quadrant. Error bars represent mean ± standard deviation (SD).
To conclude that the intercellular transfer of CD80/MHC class II, but not the contaminating APCs in the culture, played a key role in our observation, we needed to rule out the presence of a small number of APCs left in our system following the separation of CD4+CD80acq T cells and APCs. We observed that even though the pronase treatment stripped more than half of CD80 and IEk molecules off the T-cell surface, it did not alter the expression of CD80 and IEk on fibroblast APCs or the function of the fibroblast APCs (Figure 2E-F). Thus we postulate that the pronase treatment should not have reduced the proliferation of CD4+ T cells if the contaminating APCs were the source of costimulation in our experimental system. We then examined the consequences of intentional contamination of a small number of splenocytes (1% to 2%) into the purified CD4+CD80acq T cells. CD4+ T cells were cultured in the presence of various concentrations of PCC peptide (100 ng/mL to 1 pg/mL). We chose a seemingly low dose of the peptide based upon the hypothesis that the only source of PCC peptide for the 1% to 2% contaminated splenocytes was carried over from the previous coculture of purified CD4+ T cells with peptide-loaded artificial fibroblast APCs. As depicted in Figure 2G, there was no significant proliferation of CD4+ T cells above background at 24 hours. Collectively, these data argue against the possibility that contaminating APCs cause the continued proliferative responses observed. In addition, treatment of CD4+CD80acq hi T cells with a saturating amount of anti-IEk and CD80 Abs exerted no effect on proliferative response at 24 hours (data not shown). This observation further demonstrates that CD4+CD80acq hi T cells are free of APCs or the impact of the small number of APCs is negligible. Furthermore, as shown in Figure 1B, CD4+CD80acq T cells in the absence of APCs proliferate more rapidly as compared with T cells that are continuously cultured with APCs. This further suggests that sustained proliferation of these cells cannot be attributed to the presence of APCs.
Cytokine secretion by CD4+CD80acq T cells
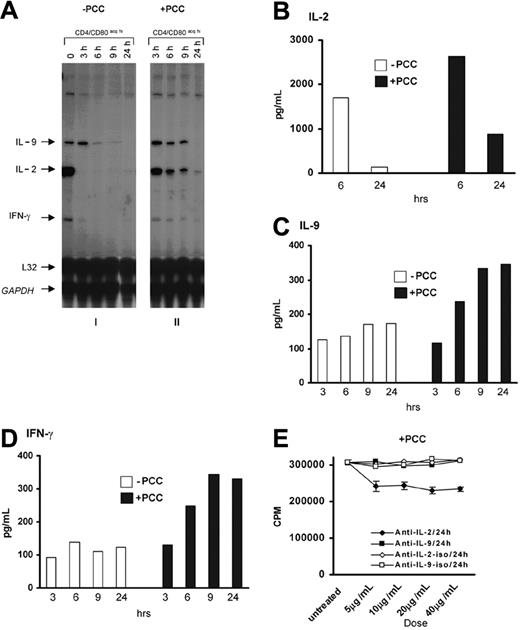
To investigate the cytokine production profile of CD4+CD80acq T cells, the CD4+ T cells that had acquired CD80 were separated from APCs and further cultured with or without 1 μg/mL PCC peptide for 24 hours. As shown in Figure 3A (panel 1), the CD4+ T cells that acquired CD80 expressed mRNA for IL-2, IL-9, and IFN-γ genes at the time of separation from APCs. However, expression of IL-2 and IFN-γ mRNA diminished rapidly following separation from APCs, whereas IL-9 mRNA expression was sustained up to 3 hours in the absence of the antigen and APCs (Figure 3A, panel 1; Figure S5). The addition of exogenous peptide (1 μg/mL PCC) led to the sustained expression of IL-2, IFN-γ, and IL-9 mRNAs up to 9 hours after separation of CD4+CD80acq hi T cells from APCs (Figure 3A, panel 2; Figure S5). Furthermore, the expression of IL-2, IL-9, and IFN-γ proteins was confirmed by ELISA (Figure 3B-D). As described in the legend to Figure 3B, IL-2 was produced in great quantities by CD4+CD80acq hi T cells at 6 hours after separation from APCs and was consumed by 24 hours. In contrast, IL-9 and IFN-γ protein levels remained consistent in the absence of the antigen after separation of CD4+CD80acq hi T cells from APCs (Figure 3C-D). The addition of the PCC peptide (1 μg/mL) in the culture medium increased the secretion of IL-2, IL-9, and IFN-γ over the time periods after separation from APCs (Figure 3B-D). Neutralizing anti-IL-2 Ab partially decreased the proliferative response of CD4+CD80acq hi T cells in the presence of 1 μg/mL PCC peptide in 24 hours, whereas neutralizing anti-IL-9 antibody had no effect (Figure 3E).
Production of IL-2, IL-9, and IFN-γ and the role of IL-2 and IL-9 in the proliferation of CD4+CD80acq hi T cells. (A) Cytokine expression in CD4+CD80acq hi T cells was examined using RPA when these T cells were incubated in the absence (i) or presence (ii) of 1 μg/mL PCC without APCs at various time points. Subpanels i and ii are originally from the same blot. (B-D) Secretion of IL-2, IL-9, and IFN-γ in the culture medium of CD4+CD80acq hi T cells was examined using ELISA. □ indicates without PCC; ▪, with PCC. (E) CD4+CD80acq hi T cells, cultured in the presence of exogenous PCC peptide (1 μg/mL) without APCs, were treated with neutralizing anti-IL-2 (5 to 40 μg/mL) Ab, neutralizing anti-IL-9 Ab (5 to 40 μg/mL), or IgG isotype Abs for 24 hours. The effects of anti-IL-2 and anti-IL-9 Abs on 3H uptake of CD4+CD80acq hi T cells were assessed. CD4+CD80acq hi T cells treated with anti-IL-2 Ab in the presence of PCC peptide (1 μg/mL) (♦); CD4+CD80acq hi T cells treated with anti-IL-9 Ab in the presence of PCC peptide (1 μg/mL) (▪); CD4+CD80acq hi T cells treated with anti-IL-2 IgG isotype Ab in the presence of PCC peptide (1 μg/mL) (⋄); CD4+CD80acq hi T cells treated with anti-IL-9 IgG isotype Ab in the presence of PCC peptide (1 μg/mL) (□). Error bars represent mean ± SD.
Production of IL-2, IL-9, and IFN-γ and the role of IL-2 and IL-9 in the proliferation of CD4+CD80acq hi T cells. (A) Cytokine expression in CD4+CD80acq hi T cells was examined using RPA when these T cells were incubated in the absence (i) or presence (ii) of 1 μg/mL PCC without APCs at various time points. Subpanels i and ii are originally from the same blot. (B-D) Secretion of IL-2, IL-9, and IFN-γ in the culture medium of CD4+CD80acq hi T cells was examined using ELISA. □ indicates without PCC; ▪, with PCC. (E) CD4+CD80acq hi T cells, cultured in the presence of exogenous PCC peptide (1 μg/mL) without APCs, were treated with neutralizing anti-IL-2 (5 to 40 μg/mL) Ab, neutralizing anti-IL-9 Ab (5 to 40 μg/mL), or IgG isotype Abs for 24 hours. The effects of anti-IL-2 and anti-IL-9 Abs on 3H uptake of CD4+CD80acq hi T cells were assessed. CD4+CD80acq hi T cells treated with anti-IL-2 Ab in the presence of PCC peptide (1 μg/mL) (♦); CD4+CD80acq hi T cells treated with anti-IL-9 Ab in the presence of PCC peptide (1 μg/mL) (▪); CD4+CD80acq hi T cells treated with anti-IL-2 IgG isotype Ab in the presence of PCC peptide (1 μg/mL) (⋄); CD4+CD80acq hi T cells treated with anti-IL-9 IgG isotype Ab in the presence of PCC peptide (1 μg/mL) (□). Error bars represent mean ± SD.
Sustained signal transduction pathways upon acquisition of CD80 by CD4+ T cells
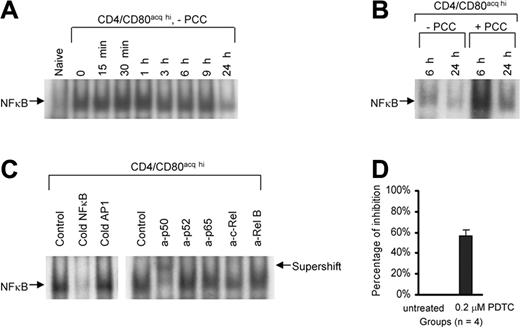
To investigate whether the acquisition of CD80 can lead to the maintenance of signals in T cells, naive PCC/TCR-Tg CD4+ T cells were cocultured with PCC-pulsed P13.9 fibroblast to acquire CD80 and then T cells were separated from APCs. T cells that acquired CD80 and IEk were further cultured in the absence or presence of 1 μg/mL PCC peptide (without APCs) for various times. Using EMSA, NFκB activity of CD4+CD80acq hi T cells was examined at various time points following the separation of the T cells from APCs (termed “0 hour”) up to 24 hours after separation. NFκB binding activity in CD4+CD80acq hi T cells upon separation from APCs was highly elevated, and it sustained its activity up to 24 hours after separation from APCs (Figure 4A). A competitive binding assay confirmed that this binding is specific (Figure 4B, left panel). Furthermore, the supershift assay showed that p50/p50 homodimers are the major subunits in this NFκB complex (Figure 4B, right panel). We then asked if the addition of exogenous PCC peptide (1 μg/mL) would modulate NFκB activity in CD4+CD80acq hi T cells. The addition of PCC peptide to CD4+CD80acq hi T cells further increased the NFκB activity of these T cells at 6 hours and 24 hours (Figure 4C). An NFκB inhibitor (pyrrolidine dithiocarbamate [PDTC]) suppressed proliferation of CD4+CD80acq hi T cells by 60% at 0.2 μM (Figure 4D), demonstrating that NFκB is at least one of the major players in the sustained proliferative response of these cells. Higher concentrations of PDTC affected the viability of CD4+CD80acq hi T cells (data not shown).
The role of NFκB in the proliferation of CD4+CD80acq hi T cells. (A) Naive CD4+ T cells were cocultured with PCC-pulsed P13.9 fibroblasts for 20 hours. After the separation from P13.9 APCs, NFκB activity of nuclear extracts from either naive CD4+ or CD4+CD80acq hi T cells was investigated using EMSA. CD4+CD80acq hi T cells were separated from PCC-pulsed P13.9 fibroblasts (0 hour) and further cultured at various time points in the absence of APCs and PCC peptide, and NFκB activity in these cells was examined. (B) CD4+CD80acq hi T cells were cultured in the absence or presence of PCC peptide (1 μg/mL) without APCs, and NFκB activity in these cells was measured at 6 or 24 hours. (C) NFκB binding band in the cells mentioned in the discussion of Figure 4A was analyzed using competitive and supershift assays. In competitive assay (left), 50-fold of cold NFκB and AP1 oligmers were used to compete with labeled NFκB probe. In the supershift assay (right), a set of anti-NFκB antibodies was applied to detect NFκB subunits. (D) CD4+CD80acq hi T cells were treated with 0.2 μM PDTC (an NFκB inhibitor) in the absence of APCs and PCC peptide for 6 hours. The effect of PDTC on 3H uptake of CD4+CD80acq hi T cells was assessed. Data are expressed as average percentage inhibition of proliferation. This result is the average of 4 experiments. The CD4+CD80acq hi T cells exposed to PDTC were further stained with propidium iodide. Viability of the CD4+CD80acq hi T cells was determined by FACS analysis (data not shown). Error bars represent mean ± SD.
The role of NFκB in the proliferation of CD4+CD80acq hi T cells. (A) Naive CD4+ T cells were cocultured with PCC-pulsed P13.9 fibroblasts for 20 hours. After the separation from P13.9 APCs, NFκB activity of nuclear extracts from either naive CD4+ or CD4+CD80acq hi T cells was investigated using EMSA. CD4+CD80acq hi T cells were separated from PCC-pulsed P13.9 fibroblasts (0 hour) and further cultured at various time points in the absence of APCs and PCC peptide, and NFκB activity in these cells was examined. (B) CD4+CD80acq hi T cells were cultured in the absence or presence of PCC peptide (1 μg/mL) without APCs, and NFκB activity in these cells was measured at 6 or 24 hours. (C) NFκB binding band in the cells mentioned in the discussion of Figure 4A was analyzed using competitive and supershift assays. In competitive assay (left), 50-fold of cold NFκB and AP1 oligmers were used to compete with labeled NFκB probe. In the supershift assay (right), a set of anti-NFκB antibodies was applied to detect NFκB subunits. (D) CD4+CD80acq hi T cells were treated with 0.2 μM PDTC (an NFκB inhibitor) in the absence of APCs and PCC peptide for 6 hours. The effect of PDTC on 3H uptake of CD4+CD80acq hi T cells was assessed. Data are expressed as average percentage inhibition of proliferation. This result is the average of 4 experiments. The CD4+CD80acq hi T cells exposed to PDTC were further stained with propidium iodide. Viability of the CD4+CD80acq hi T cells was determined by FACS analysis (data not shown). Error bars represent mean ± SD.
Similarly, the activity of AP1 was investigated. After incubation with APCs for 20 hours, CD4+CD80acq hi T cells that were just separated from APCs exhibited AP1 binding activity (Figure 5A, indicated at 0 hour). AP1 binding activity of CD4+CD80acq hi T cells kept rising 1 hour thereafter and maintained itself up to 24 hours after separation from APCs in the absence of additional exogenous PCC peptide (Figure 5A). This AP1 binding is specific (Figure 5B, left panel). Anti-Jun B antibody abolished the AP1 binding activity (Figure 5B, right panel), indicating that Jun B is the major component in the AP1 binding complex. However, AP1 activity in CD4+CD80acq hi T cells was markedly enhanced by the addition of exogenous PCC peptide (1 μg/mL) at 6 hours (Figure 5C).
Activity of AP1 and Stat5 in naive CD4+ and CD4+CD80acq hi T cells and effect of endogenous IL-2 on NFκB, AP1, and Stat5. AP1 and Stat5 activity in nuclear extracts from CD4+CD80acq hi T cells was investigated using EMSA. (A) The CD4+CD80acq hi T cells were separated from PCC-pulsed P13.9 APCs (0 hour) and further cultured at various time points in the absence of APCs and PCC peptide, and AP1 activity in these cells was observed. (B) Binding band of AP1 in the cells mentioned in the discussion of Figure 5A was analyzed using competitive and supershift assays. In the competitive assay (left), 50-fold of cold AP1 and NFκB oligmers were used to compete with labeled AP1 probe. In the supershift assay (right), different anti-AP1 Abs were used to determine the major subunit of AP1. (C) CD4+CD80acq hi T cells were cultured in the absence or presence of exogenous PCC (1 μg/mL) without APCs for 6 or 24 hours. Binding activity of AP1 was measured. (D) CD4+CD80acq hi T cells were separated from PCC-pulsed P13.9 fibroblasts (0 hour) and further cultured in the absence of APCs and PCC peptide or in the presence of 1 μg/mL PCC peptide at various times, and Stat5 activity in these cells was determined. (E) Stat5 binding band was identified using supershift assay with anti-Stat5 Ab. (F) Naive CD4+ T cells were cocultured with PCC-pulsed P13.9 fibroblasts for 20 hours. After the separation from P13.9 APCs, CD4+CD80acq T cells were cultured in fresh medium in the absence or presence of anti-IL-2 neutralizing Abs (10 μg/mL) at various time points. Activity of NFκB, AP1, and Stat5 in CD4+CD80acq T cells was measured using EMSA.
Activity of AP1 and Stat5 in naive CD4+ and CD4+CD80acq hi T cells and effect of endogenous IL-2 on NFκB, AP1, and Stat5. AP1 and Stat5 activity in nuclear extracts from CD4+CD80acq hi T cells was investigated using EMSA. (A) The CD4+CD80acq hi T cells were separated from PCC-pulsed P13.9 APCs (0 hour) and further cultured at various time points in the absence of APCs and PCC peptide, and AP1 activity in these cells was observed. (B) Binding band of AP1 in the cells mentioned in the discussion of Figure 5A was analyzed using competitive and supershift assays. In the competitive assay (left), 50-fold of cold AP1 and NFκB oligmers were used to compete with labeled AP1 probe. In the supershift assay (right), different anti-AP1 Abs were used to determine the major subunit of AP1. (C) CD4+CD80acq hi T cells were cultured in the absence or presence of exogenous PCC (1 μg/mL) without APCs for 6 or 24 hours. Binding activity of AP1 was measured. (D) CD4+CD80acq hi T cells were separated from PCC-pulsed P13.9 fibroblasts (0 hour) and further cultured in the absence of APCs and PCC peptide or in the presence of 1 μg/mL PCC peptide at various times, and Stat5 activity in these cells was determined. (E) Stat5 binding band was identified using supershift assay with anti-Stat5 Ab. (F) Naive CD4+ T cells were cocultured with PCC-pulsed P13.9 fibroblasts for 20 hours. After the separation from P13.9 APCs, CD4+CD80acq T cells were cultured in fresh medium in the absence or presence of anti-IL-2 neutralizing Abs (10 μg/mL) at various time points. Activity of NFκB, AP1, and Stat5 in CD4+CD80acq T cells was measured using EMSA.
The role of Stat5 in the TCR signals at an early activation period remains somewhat controversial.15,16 To investigate Stat5 status in the activation of CD4+CD80acq T cells after separation from APCs, naive CD4+ cells were cocultured with PCC-pulsed P13.9 APCs to acquire CD80. A very low level of Stat5 was induced upon coincubation with APCs after 20 hours (Figure 5D). Stat5 was greatly up-regulated between 30 minutes and 1 hour after CD4+CD80acq hi T cells were separated from APCs and then reduced significantly in 3 hours after separation from APCs (Figure 5D). In the presence of exogenous peptide (1 μg/mL PCC), Stat5 in CD4+CD80acq hi T cells was slightly enhanced (Figure 5D) and Stat5 supershift was observed with anti-Stat5 Ab but not with an irrelevant isotype-matched immunoglobulin G (IgG) (Figure 5E). In addition, an enhanced level of Stat5 in CD4+CD80acq hi T cells was supported by sustained mRNA expression of CIS, a Stat5-inducible gene (data not shown).
The role of IL-2 in the activation of NFκB, AP1, and Stat5 in CD4+CD80acq T cells was investigated. CD4+CD80acq T cells were cultured in fresh medium in the absence or presence of 10 μg/mL anti-IL-2 neutralizing antibody for various time points. The binding activity of NFκB, AP1, and Stat5 was examined. As depicted in Figure 5F, anti-IL-2 neutralizing antibody had no effect on the binding activity of NFκB and AP1; however, this antibody partially reduced the levels of Stat5.
To further address whether the sustained signaling of CD4+CD80acq hi T cells shows quantitative correlation with the amount of acquired CD80 on the cell surface, naive CD4+ T cells were incubated with APCs expressing high CD80 (PCC-pulsed P13.9CD80 hi APCs, Figure S3A) or APCs with low CD80 (PCC-pulsed DCEKCD80 low APCs, Figure S3A) for 20 hours. After the separation from APCs, CD4+CD80acq hi T cells (70% to 90% of the cells acquired CD80, Figure S3B) that had been incubated with PCC-pulsed P13.9 APCs exerted higher levels and pro-longed binding activity of NFκB, AP1, and Stat 5 compared with the CD4+CD80acq low T cells (incubated with PCC-pulsed DCEK APCs; 10% to 14% of the T cells acquired CD80, Figures S3B and 6A).
To further investigate if acquisition of CD80 by CD4+ T cells from classical APCs will lead to similar activation pathways, naive CD4+ T cells were cocultured with PCC-pulsed macrophages (Figure S6) for 20 hours. After separation from macrophages, CD4+ T cells acquired both CD80 and MHC class II (IEk). These molecules were acquired at lower levels as compared with the T cells that were activated with fibroblast APCs (macrophages express lower levels of CD80 and MHC class II as compared with transfected fibroblast cell line 13.9; Figure S7). Treatment of these CD4+CD80acq T cells with pronase further demonstrated that continued proliferation of these cells also required the persistent presence of CD80 and IEk (Figure S7). Furthermore, the signaling events in the macrophage-activated CD4+CD80acq T cells were investigated using EMSA. The CD4+CD80acq T cells were cultured in fresh medium for various times. As shown in Figure 6B, binding activities of NFκB and AP1 were induced at the time of separation of the T cells from macrophages, and their activity was sustained up to 6 hours. Stat5 levels were also up-regulated 2 hours after separation. These data further support the premise that acquisition of CD80 and MHC molecules from APCs (either transfected or physiological APCs) will lead to the activation of similar signaling pathways.
Activities of NFκB, AP1, and Stat5 directly associated with the acquisition of CD80 by T cells. (A) Naive CD4+ T cells were cultured with either PCC-pulsed P13.9 fibroblasts (expressing high CD80) or PCC-pulsed DCEK fibroblasts (expressing low CD80) for 20 hours. Upon separation from APCs (0 hour), the T cells that acquired a high amount of CD80 (CD4+CD80acq hi, left) or the T cells that acquired a low amount of CD80 (CD4+CD80acq low, right) were further cultured in the absence of APCs and PCC peptide at various time points. Activity of NFκB, AP1, and Stat5 in CD4+CD80acq hi (left) and CD4+CD80acq low (right) T cells was measured using EMSA. (B) CD4+ T cells were stimulated with PCC peptide-pulsed macrophages for 20 hours. After the separation from macrophages, the activities of NFκB, AP1, and Stat5 in the CD4+CD80acq T cells were measured using EMSA. (C) Naive CD4+ T cells were activated with either PCC-pulsed P13.9 (APCs) or anti-CD3 (10 μg/mL)/anti-CD28 (2 μg/mL) Abs for 20 hours. Upon separation from APCs or anti-CD3/anti-CD28 Abs (0 hour), these activated cells were further cultured in the absence of APCs, PCC peptide, or anti-CD3/anti-CD28 at various time points. The NFκB activity in these cells was analyzed using EMSA.
Activities of NFκB, AP1, and Stat5 directly associated with the acquisition of CD80 by T cells. (A) Naive CD4+ T cells were cultured with either PCC-pulsed P13.9 fibroblasts (expressing high CD80) or PCC-pulsed DCEK fibroblasts (expressing low CD80) for 20 hours. Upon separation from APCs (0 hour), the T cells that acquired a high amount of CD80 (CD4+CD80acq hi, left) or the T cells that acquired a low amount of CD80 (CD4+CD80acq low, right) were further cultured in the absence of APCs and PCC peptide at various time points. Activity of NFκB, AP1, and Stat5 in CD4+CD80acq hi (left) and CD4+CD80acq low (right) T cells was measured using EMSA. (B) CD4+ T cells were stimulated with PCC peptide-pulsed macrophages for 20 hours. After the separation from macrophages, the activities of NFκB, AP1, and Stat5 in the CD4+CD80acq T cells were measured using EMSA. (C) Naive CD4+ T cells were activated with either PCC-pulsed P13.9 (APCs) or anti-CD3 (10 μg/mL)/anti-CD28 (2 μg/mL) Abs for 20 hours. Upon separation from APCs or anti-CD3/anti-CD28 Abs (0 hour), these activated cells were further cultured in the absence of APCs, PCC peptide, or anti-CD3/anti-CD28 at various time points. The NFκB activity in these cells was analyzed using EMSA.
Furthermore, to rule out the notion that these sustained signals are a mere reminiscence of the activation, naive CD4+ T cells were stimulated with either PCC-pulsed APCs or immobilized anti-CD3 (10 μg/mL)/anti-CD28 (2 μg/mL) Abs. The kinetics of NFκB activity was studied. As depicted in Figure 6C, NFκB activity was induced in CD4+ T cells that had been activated with either P13.9 APCs or anti-CD3/anti-CD28 Abs after 20 hours of treatment and removed from APCs or anti-CD3/anti-CD28 Abs (0 hour). CD4+ T cells that had acquired CD80 demonstrated sustained NFκB activity after separation from APCs up to 24 hours (Figure 6C, left panel). However, NFκB activity in the CD4+ T cells, which were collected after stimulation with anti-CD3/anti-CD28 Abs for 20 hours and further incubated in culture medium (in the absence of anti-CD3/anti-CD28 Abs), remarkably decreased in 9 hours and was almost undetectable at 24 hours (Figure 6C, right panel). Further studies using PMA (5 ng/mL)/ionomycin (250 ng/mL) to activate the CD4+ T cells demonstrated that NFκB activity in the CD4+ T cells was not sustained upon withdrawal of PMA/ionomycin following the activation of CD4+ T cells by these reagents (data not shown). These results collectively argue against the supposition that sustained signals observed in CD4+CD80acq T cells are attributable to early primary activation. Similar experiments conducted on AP1 activity generated similar results (data not shown). Taken together, these data strongly suggest that the activity of NFκB and AP1 is sustained in CD4+CD80acq T cells.
CD80 acquisition by CD4+ T cells and its physiological consequences in T-cell activation in vivo
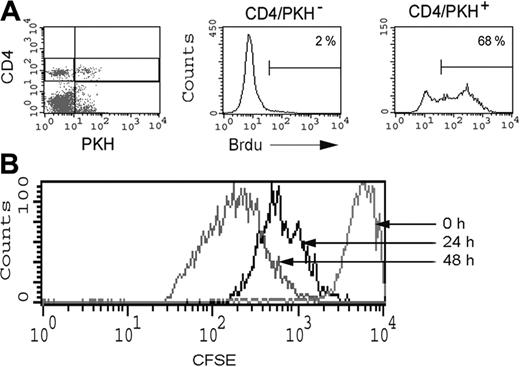
To further demonstrate that the acquisition of CD80 by T cells does lead to sustained proliferation of these cells in vivo, CD4+CD80acq hi T cells were labeled with PKH in vitro (see “Materials and methods”) and adoptively transferred to mice that received bromodeoxyuridine (BrdU) in water. As depicted in Figure 7A, 68% of PKH-stained CD4+CD80acq hi T cells from spleen showed BrdU incorporation at 48 hours, thus demonstrating the proliferation of CD4+CD80acq hi/PKH-positive T cells in vivo. In contrast, 2% of the host CD4+/PKH-negative T cells were BrdU positive (Figure 7A). To further investigate what percentage of T cells proliferate, and to demonstrate the cycle of division of these cells in vivo, CD4+CD80acq hi T cells were labeled with CFSE and adoptively transferred to mice. As depicted in Figure 7B, all CD4+CD80acq T cells entered cell cycle and underwent several rounds of proliferation both at 24 and 48 hours.
Physiological consequence of CD80 acquisition by T cells in vivo. (A) PKH-stained CD4+CD80acq hi T cells were adoptively transferred into PCC/TCR-Tg mice, and the proliferative response of these cells was measured by BrdU incorporation 2 days after adoptive transfer of these cells. PKH-positive and -negative cells were analyzed for BrdU incorporation using 3-color FACS analysis. (B) A total of 6 × 106 CFSE-stained CD4+CD80acq hi T cells were adoptively transferred into PCC/TCR-Tg mice. Proliferative response of CFSE-stained CD4+CD80acq hi T cells was examined 24 and 48 hours after adoptive transfer using FACS analysis. Percentages are the percent positive Brd U positive T cells.
Physiological consequence of CD80 acquisition by T cells in vivo. (A) PKH-stained CD4+CD80acq hi T cells were adoptively transferred into PCC/TCR-Tg mice, and the proliferative response of these cells was measured by BrdU incorporation 2 days after adoptive transfer of these cells. PKH-positive and -negative cells were analyzed for BrdU incorporation using 3-color FACS analysis. (B) A total of 6 × 106 CFSE-stained CD4+CD80acq hi T cells were adoptively transferred into PCC/TCR-Tg mice. Proliferative response of CFSE-stained CD4+CD80acq hi T cells was examined 24 and 48 hours after adoptive transfer using FACS analysis. Percentages are the percent positive Brd U positive T cells.
Discussion
The physiological relevance of T cells acquiring MHC and costimulatory molecules in various diseases such as HIV or cancer remains an area of active investigation. Wolthers et al have shown high expression levels of CD80, CD86, and CD70 on activated CD8 from HIV-infected individuals. They suggested that enhanced stimulatory potential of these nonprofessional APCs may contribute to persistently high levels of immune activation in HIV infection related to disease progression.8
Kochli et al have demonstrated that CD80 and CD86 were present on both circulating CD4+ and CD8+ cells of HIV-infected individuals and were associated with HLA-DR expression. They concluded that it is likely that the expression of costimulatory molecules is a reflection of the chronicity of the T-cell activation during HIV infection. They suggested that during HIV infection, T cells themselves develop an antigen-presenting phenotype due to expression of CD80, HLA-DR, and CD86 molecules.7
Furthermore, Giguere et al demonstrated that CD80 and CD86 are efficiently acquired by HIV-1 virus.17 They demonstrated for the first time that the insertion of CD80 in HIV-1 increases virus infectivity by facilitating the attachment and the entry process due to interactions with CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) ligands. Moreover, they demonstrated that NFκB is induced by CD80-bearing virions when they are combined with the engagement of the TCR/CD3 complex. They suggested that HIV-1 may attach to engaged cells via interactions between cell-derived molecules incorporated into virions and their natural ligands. They assumed that HIV-1 that expresses CD80 in combination with MHC-II molecules can activate T cells. The establishment of the activated state can reveal the physiological significance of the pathogenesis of HIV infection. This process can promote virus transcription if the responding T cells are infected with HIV-1. In addition, cell activation can create a cellular microenvironment that is more favorable for a productive HIV infection.
A study by Verwilghen et al demonstrated that T cells found in the synovial membrane and synovial fluid but not peripheral blood from rheumatoid arthritis patients expressed CD80 in addition to high levels of MHC-II antigen.5 They concluded that rheumatoid synovial T cells that expressed CD80 have been activated in vivo and that these observations further support the hypothesis that in chronic inflammatory arthropathies, in contrast to degenerative arthropathies, T-cell activation is present in the synovial membrane. Their observation that synovial T cells expressed CD80 and HLA-DR may indicate that synovial T cells can activate autologous T cells, thus contributing to the persistent T-cell activation of rheumatoid synovitis.
As demonstrated by Brown et al, CD80+ T cells are common in patients with myeloma and can be either CD4 or CD8 and tend to be associated with stable disease and are polyclonal memory T cells.6 They showed that CD80 mRNA expression was not present in T cells that expressed CD80. They concluded that CD80 expression is common on T cells of patients with myeloma, but this is acquired rather than endogenously produced. They suggested that CD80+CD45RO+ T cells constitute a population of memory T cells chronically exposed to antigen. They hypothesized that expression of CD80+ on T cells may lead to continuous activation of these cells, rendering them either to apoptotic or tolerized. Therefore, they suggest that perhaps most high-affinity idiotype-specific T cells (tumor-specific T cells) have been deleted even by the time of diagnosis although the range of low-affinity unresponsive T cells may exist. They suggest that CD80+ cells in their studies represent the residual nonresponsive T cells in these patients.
Finally, a recent study has demonstrated that other molecules such as CD40L and OX40L can also be acquired by B-chronic lymphocytic leukemia (B-CLL) cells from fibroblasts and can be used in the generation of CD4+/CD8+ cytotoxic T-lymphocytes (CTLs).18 The concept of transferring costimulatory molecules into B-CLL to be used in the clinical setting is further indicative of the physiological importance of this phenomenon. One can speculate that the sustained signaling in CD4+CD80acq T cells can lead to enhanced activation of these cells and bystander T cells. In HIV, this phenomenon may lead to viral replication, which requires activated T cells, and the progression of the disease. In contrast, in cancer settings, the sustained signaling in T cells through the acquisition of costimulatory molecules may lead to the activation of T cells and potentiation of immune responses to tumor antigens. Taken together, the above previous observations and the observations reported here help define the role of acquisition of costimulatory molecules by T cells in disease processes.
In this study, we demonstrate that the acquisition of CD80/MHC molecules by CD4+ T cells from syngeneic APCs in vitro as well as in vivo leads to sustained activation and signaling in these cells. We propose that these acquired molecules can act as an activating unit that in turn can be called “antigen presentasome” (APS). Interestingly, the period of sustained signals corresponds well with the rate of disappearance of CD80 and MHC class II. The acquisition of CD80 by T cells leads to enhanced activity of NFκB and AP1, and the activity of these transcriptional factors correlates directly with the level of CD80 acquired by T cells. Moreover, our results demonstrate that the acquisition of CD80 by T cells results in the activation of Stat5 pathway, which had not been documented previously. We establish that potent activation of T cells with both anti-CD3/anti-CD28 Abs and PMA/ionomycin fails to sustain the NFκB and AP1 signal pathways for a significant amount of time, whereas the cells that had acquired CD80 retain signals for up to 24 hours, demonstrating that sustained signals are not due to the general activation of T cells. We also demonstrate that the sustained signaling upon acquisition of CD80 leads to continued proliferation and unique cytokine production (IL-2, IL-9, and IFN-γ) by these T cells at least up to 24 hours after separation from APCs. The removal of the acquired molecules (APS) from the surface of T cells quickly turns off their activation and proliferation associated with the down-regulation of NFκB, AP1, and Stat5 (data not shown) in these cells. Furthermore, in vivo adoptive transfer studies suggest that CD80 and IEk acquisition by T cells leads to continued proliferation of these cells.
Previously, we have demonstrated that T cells, upon interaction with APCs, can acquire CD80 through CD28 mediation. We speculated that the physical contact between CD80 and CD28 on T cells after separation from APCs might lead to stabilization and amplification of signals and persistent activation of T cells. Indeed, our current studies demonstrate that CD28-mediated acquisition of CD80 by T cells leads to sustained activity of NFκB and AP1 as well as up-regulation of Stat5 in the T cells after separation from APCs. The addition of PCC peptide as signal 1 to CD4+CD80acq T cells (T cells that had also acquired MHC class II) further increases the activities of NFκB, AP1, and Stat5 in the absence of APCs. This may indicate that the interaction of TCRs with MHC can be further stabilized and enhance the signals in CD4+CD80acq T cells, leading to their activation and proliferation.
The concept we propose dictates that the physical contact between APCs and T cells is necessary for the onset of T-cell activation but is not necessarily required for the prolongation of this activation. Thus it is imperative that we eliminate the possibility that a small number of contaminating APCs, rather than the transferred APS from APCs to T cells, are responsible for the observations described. We use numerous criteria to address this issue: (1) Our FACS analysis demonstrated that CD4+CD80acq T cells show 99% purity for CD4+ cells (Figure S1). (2) RT-PCR further demonstrates lack of CD80 mRNA expression in CD4+CD80acq T cells (sensitivity of detection of CD80 in PCR was 1 ng or less of cDNA from APCs, translating to less than 1% contaminating APCs), therefore ruling out APC contamination in our CD4+CD80acq T-cell population (Figure S1).10 (3) Treatment of CD4+CD80acq T cells with pronase allows for the dispersal of these molecules from T-cell surfaces and at the same time prevents the sustained activation of T cells, confirming the need for the physical presence of acquired CD80 for this mode of T-cell activation. (4) Pronase treatment, on the other hand, does not strip off the CD80/MHC class II molecules from the surface of APCs; nor did these APCs lose their ability to present antigen to T cells following the pronase treatment. Nevertheless, pronase treatment of CD4+CD80acq T cells results in further loss of sustained T-cell activation. (5) Inclusion of anti-IEk or CD80 antibodies has no effect on the proliferation of CD4+CD80acq T cells, whereas these antibodies efficiently block the antigen presentation mediated by APCs. Even PMA/ionomycin stimulation, a very potent T-cell activator, does not lead to the sustained activation of transcription factors such as NFκB and AP1. Collectively these results support our proposal that sustained activation is the hallmark of CD4+ T cells following the acquisition of CD80/MHC class II and that the cross-linking of molecules of the antigen receptor complexes on CD4+ T cells leads to prolonged T-cell activation even in the absence of APCs. In addition, we observe a dose-response relationship between the levels of acquired CD80 and the magnitude of the activity of these transcriptional factors.
The role of Stat5 during TCR signaling at an early stage of stimulation is controversial.15,16 Here, stimulation of CD4+ T cells with APCs and peptide results only in a very low level of Stat5 activation. Intriguingly, Stat5 is increased in CD4+CD80acq hi T cells after separation from APCs. This suggests that the Stat5 pathway might be activated upon sustained signaling through both TCR and CD28 as the result of CD80/MHC class II acquisition from APCs. Alternatively, some cytokine produced by these CD4+ cells might have activated the Stat5 molecules in an autologous manner. Our data using the anti-IL-2 Ab study suggest that IL-2 production by CD4+CD80acq T cells might be partially responsible for up-regulation of Stat5. Stat3 was expressed at the time of separation from APCs, but it was diminished in 1 hour, while other Stat molecules (Stat1, Stat4, and Stat6) were not detectable (data not shown). Moreover, removal of CD80/MHC class II by pronase resulted in the concomitant reduction of T-cell proliferation and Stat5 (data not shown). The maintenance of signal pathways upon acquisition of APS by T cells can further lead to enhanced proliferation of these cells. Our pronase results suggest that enhanced proliferation of CD4+CD80acq hi T cells was associated with the sustained signals as the result of the acquisition of CD80 and MHC class II. A previous study demonstrated that pronase can remove CD4 and MHC molecules from the T-cell surface.19 We now demonstrate that both acquired CD80 and MHC class II-IEk can be stripped from the surface of CD4+CD80acq hi T cells and, unlike CD4 molecules, do not reappear on the cell surface. This observation does not exclude the possibility of pronase stripping other molecules, which might also play a role in the sustained proliferative response of these cells.
Our in vitro and in vivo results indicate that upon encountering professional APCs, naive T cells can acquire the necessary molecules (APS) to sustain activation after they have lost contact with APCs. This will enable their continuous activation and interaction with naive T cells. The acquisition of molecules (APS) by T cells might be a mechanism to achieve maximum activation of T cells via the continued proliferation and the interaction with neighboring naive T cells, despite the limited duration of their contact with APCs for optimal and faster clonal expansion in vivo.
Prepublished online as Blood First Edition Paper, January 6, 2005; DOI 10.1182/blood-2004-08-3236.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr Dinah Singer, Dr Alfred Singer, and Dr Richard Hodes, Experimental Immunology Branch, and Dr Scott Abrams, Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, NIH, for reading the manuscript critically and for their suggestions and input. The authors thank Debra Weingarten for her editorial assistance in the preparation of this manuscript.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal