Abstract
To investigate underlying mechanisms of thrombocytopenia in myelodysplastic syndrome (MDS), radiolabeled platelet studies were performed in 30 MDS patients with platelet counts less than 100 × 109/L. Furthermore, plasma thrombopoietin and glycocalicin index (a parameter of platelet or megakaryocyte destruction) were determined. Mean platelet life (MPL), corrected for the degree of thrombocytopenia, was reduced in 15 of 30 patients (4.3 ± 0.9 days [mean ± SD] vs 6.0 ± 1.3, P = .0003). Platelet production rate (PPR) was reduced in 25 of 30 patients (68 ± 34 × 109/d vs 220 ± 65, P < .0001). Thrombopoietin levels were not significantly correlated with the PPR. However, the glycocalicin index was significantly higher compared with controls (15 ± 16 vs 0.7 ± 0.2, P = .001) and significantly correlated with the PPR (P = .02, r = -0.5), but not with the MPL (P = 1.8). Ultrastructural studies demonstrated necrosis-like programmed cell death (PCD) in mature and immature megakaryocytes (n = 9). Immunohistochemistry of the bone marrow biopsies demonstrated no positive staining of MDS megakaryocytes for activated caspase-3 (n = 24) or cathepsin D (n = 21), while activated caspase-8 was demonstrated in a subgroup of patients (5/21) in less than 10% of megakaryocytes. These results indicate that the main cause of thrombocytopenia in MDS is caspase-3–independent necrosis-like PCD resulting in a decreased PPR in conjunction with an increased glycocalicin index.
Introduction
Myelodysplastic syndromes (MDSs) are clonal hematologic disorders characterized by ineffective hematopoiesis resulting in one or more cytopenias in the peripheral blood, despite a normo- or hypercellular bone marrow. Anemia is the most common cytopenia, however thrombocytopenia occurs frequently whereby bleeding complications are often noticed.1,2
Thrombocytopenia in MDS is caused mainly by ineffective platelet production due to a disturbed proliferation and differentiation process of megakaryocytes or their precursors. The cause of the altered megakaryocytopoiesis in MDS has not been elucidated, but recent studies indicate that an increased apoptosis of early progenitors and inhibitory effects of cytokines might contribute to this process.3-7 However, thrombocytopenia in MDS might also be related to other causes such as concurrent bone marrow fibrosis8 and immune-mediated mechanisms.9-11 This notion is supported by findings that immunomodulatory agents, such as danazol, antithymocyte globulin (ATG), and cyclosporin A, are effective in some patients with MDS with thrombocytopenia.12-15 Furthermore, a reduced platelet life span, measured with radiolabeled platelets, has been found in about 15% of MDS patients with thrombocytopenia. Some of these patients had a recovery of their platelet count after splenectomy.16
To discriminate further between an increased destruction of platelets in the peripheral blood and an impaired production by the bone marrow megakaryocytes, plasma levels of glycocalicin (GC) and thrombopoietin (TPO) and ultrastructural study of megakaryocytes may be of relevance.
GC is a fragment of the platelet membrane glycoprotein Ib. The glycocalicin index (GCI), the plasma GC level corrected for the platelet count, is considered to be a parameter of peripheral platelet turnover.17 Recently, it was demonstrated in a subgroup of patients with idiopathic thrombocytopenic purpura (ITP) that a decreased platelet production rate, determined with platelet kinetic studies, was associated with an elevated GCI, reflecting an increased destruction of platelets or megakaryocytes in the bone marrow.18 In addition, the majority of bone marrow megakaryocytes in these patients show ultrastructural features of apoptosis and paraapoptosis, a form of apoptosis-like programmed cell death (PCD). In these cases the GCI was significantly correlated with the proportion of ITP megakaryocytes showing these ultrastructural abnormalities.19
PCD is often considered to be a synonym of apoptosis, a form of PCD that is caspase dependent. However, recently, numerous reports have stated that PCD can occur independently of caspase activation.20,21 Therefore PCD can better be defined as cell death dependent on signals or activities within the dying cells. Morphologic criteria, especially the presence and configuration of chromatin condensation in the nucleus, can be used to divide these alternative forms into apoptosis-like or necrosis-like PCD and to distinguish them from classical apoptosis.20 It has become clear that all these forms of PCD are closely intertwined with each other. Several apoptotic features, including cytoplasmic shrinkage, blebbing, and phosphatidylserine exposure, can be present in cells undergoing apoptosis-like and necrosis-like PCD.22 Furthermore, by inhibition of caspase activities cells can switch from apoptotic to caspase-independent PCD, and depending on the stimulus the same death receptor can trigger either caspase-dependent or caspase-independent PCD.20 Mitochondrial proteins released through membrane permeabilization often play a regulatory role in these forms of PCD, but sometimes proteases released from other organelles, including lysosomes (releasing cathepsins) and endoplasmic reticulum, determine the type of PCD.20,22
To investigate the underlying defects of thrombocytopenia in MDS in more detail, we especially focused on the question of whether the thrombocytopenia is the result of a reduced life span or due to impaired production of platelets by dysfunctional megakaryocytes. The results demonstrate a significant inverse correlation between platelet production rate, determined by platelet kinetic studies, and GCI, suggesting increased intramedullary destruction of platelets and/or megakaryocytes in these patients. Electron microscopy and immunohistochemistry of bone marrow megakaryocytes provide evidence that this increased destruction is predominantly a result of necrosis-like programmed cell death, which is caspase-3 independent.
Patients, materials, and methods
Patients
A total of 35 patients with MDS, with platelet counts less than 100 × 109/L (n = 32) and more than 100 × 109/L (n = 3), were studied. The institutional review board of the University Hospital Groningen approved the study protocol, and all patients gave informed consent.
Although the World Health Organization (WHO) classification has recently superseded it,23 this work was carried out using the widely accepted French-American-British (FAB) classification. By studying bone marrow aspiration smears, the number of megakaryocytes in the bone marrow were rated as decreased (1 megakaryocyte per 5 to 10 low-power fields [× 16 objective]), normal (1 megakaryocyte per 1 to 3 low-power fields), or increased (> 2 megakaryocytes per low-power field).24
Megakaryocytes were studied with regard to size, nuclear segmentation, and granularity. The presence of abnormal megakaryocytes, including micromegakaryocytes, large mononuclear forms, megakaryocytes with multiple small nuclei, and hypogranular megakaryocytes, was considered as dysmegakaryocytopoiesis.
When cytogenetic information was available, patients were classified using the international prognostic scoring system (IPSS) in low risk, intermediate risk-1, intermediate risk-2, and high risk.2
Platelet kinetic studies
Determination of platelet life span and platelet production rate was performed as previously described.24 Briefly, following the recommendations of the International Committee on Standardization in Hematology (ICSH),25 autologous blood collected in acid-citrate-dextrose solution was centrifuged at 200g at room temperature. After harvesting and acidifying (pH 6.5) platelet-rich plasma, platelets were pelleted by centrifugation. The platelet pellet was resuspended in autologous platelet-poor, nonacidified plasma and labeled with 10 MBq 111Indium-tropolonate. After establishing labeling efficiency (usually > 90%), a dose of approximately 7 MBq radiolabeled platelets was injected into the patient.
From whole blood samples taken at intervals of 1, 3, and 5 hours after injection and twice a day for 4 days thereafter, initial platelet recovery, mean platelet life, and platelet production rate were calculated. If reduced platelet life was expected, additional blood samples were drawn on the first day.
Initial platelet recovery is defined as the percentage of circulating labeled platelets at one hour after injection. Initial platelet recovery is calculated from the total blood activity extrapolated to zero time as the fraction of the injected radioactivity.
Mean platelet life (MPL) was calculated using a gamma function model, following the recommendations of the ICSH.25 Blood volume in liters was estimated from height and weight.
Platelet production rate (PPR) was defined as the number of platelets entering the circulation to maintain the platelet count. This is not necessarily identical to platelet production, because production in the bone marrow without release of platelets in the circulation may occur (this is considered as ineffective production by the bone marrow). PPR was calculated from MPL, platelet count (counted manually when less than 20 × 109/L on the automatic counter), blood volume, and initial platelet recovery.
Normal values (mean ± SD) are for MPL, 9.2 ± 1.4 days; for PPR, 220 ± 65 × 109/d; and for initial platelet recovery, 60 ± 12%.26
Plasma TPO and glycocalicin
Plasma TPO concentrations were determined by a commercially available enzyme-linked immunosorbent assay (ELISA), which uses a monoclonal antibody directed against recombinant human TPO (Quantikine; R&D Systems, Minneapolis, MN), according to the manufacturer's instructions (normal values: 118 ± 33 pg/mL). TPO was performed in 18 patients.
Plasma GC concentrations were measured by enzyme immunoassay (EIA) (Takara Shuzo, Ohtsu, Japan). Citrate-anticoagulated blood was processed within 2 hours after blood collection. Since GC levels are dependent on the platelet count, the GC index (GCI) was calculated using the following formula: [GC (μg/mL) × (250 × 109/L)]/individual platelet count (109/L).17 The normal value of GCI is 0.7 ± 0.2. The GC assay was performed in 20 MDS patients.
Electron microscopy of bone marrow megakaryocytes
Fresh bone marrow cells were washed in RPMI 1640 (BioWhittaker Europe, Verviers, Belgium), pelleted, and subsequently fixed in 2% glutaraldehyde, in 0.1 M phosphate buffer for 24 hours at 4°C. Cells were dehydrated, osmicated, and embedded in Epon 812 according to routine procedures as described earlier.19 Semithin sections (1-0.5 μm) were inspected with a light microscope to select megakaryocytes. To examine the ultrastructure in detail, electron microscopic (Philips 201, Eindhoven, the Netherlands) analysis was performed. The major criteria for classifying cells as megakaryocytes are the size of the cells; the quality and quantity of cytoplasm, in particular the presence of α-granules and demarcation membrane system; and the size, lobulation, and chromatin pattern of the nucleus. A further distinction was made in immature and mature megakaryocytes. The immature megakaryocyte (megakaryoblast) is characterized by a large round, indented, or bilobed nucleus; prominent nucleoli and cytoplasm containing scattered mitochondria; variable amounts of rough endoplasmic reticulum; a small Golgi complex; a few α-granules; and rudiments of the demarcation membrane system. The mature megakaryocyte contains a lobulated nucleus in which nucleoli are absent. Granular cytoplasm is abundant. The well-developed demarcation membrane system divides the cytoplasm into platelet fields. The Golgi complex and rough endoplasmic reticulum are greatly reduced.27 A total of 30 megakaryocytes per sample were examined.
Ultrastructural characteristics of programmed cell death
The ultrastructure of megakaryocytes was examined for the presence of programmed cell death (PCD). The following characteristics were examined for making a distinction into 3 subtypes: apoptosis, and apoptosis-like or necrosis-like programmed cell death. Ultrastructurally apoptosis is characterized by margination of condensed chromatin (stage II chromatin condensation), nuclear fragmentation, and the formation of apoptotic bodies.28 In apoptosis-like PCD, chromatin condensation is less compact and lumpier (stage 1 chromatin condensation). In necrosis-like PCD no chromatin condensation or some chromatin clustering (loose speckles) is found.20
Immunohistochemistry
For immunohistochemical staining, serial 3-μm-thick sections were cut from paraffin-embedded bone marrow biopsies and mounted on aminopropyl-ethoxy-silan (APES; Sigma-Aldrich, Diesenhofen, Germany)–coated glass slides. After deparaffinization in xylene, antigen retrieval was performed using microwave heating at 700 W for 10 minutes in EDTA (ethylenediaminetetraacetic acid) buffer. Following blocking of endogenous peroxidase with 3% hydrogen peroxide for 30 minutes, the primary antibody was applied for one hour at room temperature.
To identify activated caspase-3, immunostaining with a rabbit polyclonal antibody (1:100; New England Biolabs, Beverly MA) was used. Subsequently, the slides were incubated for 30 minutes with appropriate secondary and tertiary antibodies with streptavidin-conjugated peroxidase (DAKO, Glostrup, Denmark). Peroxidase activity was visualized with diaminobenzidine. Slides were counterstained with hematoxylin. A sample of colorectal carcinoma and bone marrow megakaryocytes from an ITP patient served as positive controls.19 As negative controls, slides were immunostained in the absence of the primary antibody.
For the detection of activated caspase-8, the mouse monoclonal antibody 1C12 (catalog no. 9746; Cell Signaling Technology, Beverly, MA) was used, dilution 1:50. Antigen retrieval was performed by autoclave heating. A 2-step detection system (rabbit anti–mouse peroxidase [RAMPO]/goat anti–rabbit peroxidase [GARPO]) was used. Diaminobenzidine was used as chromagen, and sections were counterstained with hematoxylin. Positive control was breast carcinoma; negative control, replacement of the primary antibody with phosphate-buffered saline (PBS).
For the detection of cathepsin D, we used the mouse monoclonal antibody clone C5 (Monosan; Sanbio BV, Uden, The Netherlands), dilution 1:200. Antigen retrieval was performed by autoclave heating. Incubation was done using a 2-step technique (RAMPO/GARPO). Breast carcinoma served as normal control, and bone marrow granulocytes, as internal control, replacing the primary antibody with PBS as a negative control. A granular staining pattern in the cytoplasm is expected when cathepsins are located in the lysosomes. Previous studies29,30 have shown that a translocation of cathepsins from lysosomes to the cytosol is required before they can trigger PCD. Therefore, the immunofluorescent detection of cathepsins after the release from lysosomes results in a diffuse staining pattern in the cytosol.
Evaluation of the staining pattern was performed by light microscopy. Slides were evaluated by at least 2 independent investigators who did not have knowledge of the clinical data. If the evaluations did not agree, they were re-evaluated under a multiheaded microscope. Samples were scored as negative (ie, absence of detectable cytoplasmic staining) or positive. A total of 30 megakaryocytes per sample were examined.
Statistical and mathematic analysis
Data are reported as a mean ± SD. Statistical analysis was performed using Kruskal-Wallis nonparametric analysis of variances and the Wilcoxon 2 sample test.
Spearman rank correlation was used for assessing correlations between continuous variables. P less than .05 was considered statistically significant, and all tests were 2-sided.
Since the MPL is dependent on the platelet count, we also used a previously described model31,32 to determine whether the shortening of the mean platelet life observed in our patients could be explained solely by a fixed daily platelet requirement for the maintenance of a normal covering of the endothelium or whether additional mechanisms of platelet removal were involved.
Results
Patients
A total of 35 patients with MDS were studied, included those with refractory anemia (RA; n = 22), RA with ringed sideroblasts (RARS; n = 1), RA with excess blasts (RAEB; n = 9), and chronic myelomonocytic leukemia (CMML; n = 3). There were 32 patients with a platelet count less than 100 × 109/L, and 3 patients with a platelet count more than 100 × 109/L. Tables 1 and 2 show the patient characteristics.
Patient characteristics
. | Platelet counts . | . | . | |
|---|---|---|---|---|
. | Fewer than 100 × 109/L . | 100 × 109/L or more . | Normal values . | |
| No. patients | 32 | 3 | - | |
| No. male/female | 23/9 | 2/1 | - | |
| Age, y | 64 ± 15 | 66 ± 9 | - | |
| Hb, mM | 6.5 ± 1.2 | 6.2 ± 1.2 | 7.4-10 (F)/8.6-11 (M) | |
| Leukocytes, × 109/L | 4.9 ± 2.7 | 3.7 ± 2.1 | 4.0-10 | |
| Platelets, × 109/L | 49 ± 23 | 176 ± 109 | 150-350 | |
| Megakaryocytes in bone marrow, % | ||||
| Normal or increased | 81 | 100 | - | |
| Decreased | 19 | 0 | - | |
| Mean platelet life, d | 5.1 ± 1.4 | ND | 9.2 ± 1.4 | |
| Platelet production rate, × 109/d | 87 ± 54 | ND | 220 ± 65 | |
. | Platelet counts . | . | . | |
|---|---|---|---|---|
. | Fewer than 100 × 109/L . | 100 × 109/L or more . | Normal values . | |
| No. patients | 32 | 3 | - | |
| No. male/female | 23/9 | 2/1 | - | |
| Age, y | 64 ± 15 | 66 ± 9 | - | |
| Hb, mM | 6.5 ± 1.2 | 6.2 ± 1.2 | 7.4-10 (F)/8.6-11 (M) | |
| Leukocytes, × 109/L | 4.9 ± 2.7 | 3.7 ± 2.1 | 4.0-10 | |
| Platelets, × 109/L | 49 ± 23 | 176 ± 109 | 150-350 | |
| Megakaryocytes in bone marrow, % | ||||
| Normal or increased | 81 | 100 | - | |
| Decreased | 19 | 0 | - | |
| Mean platelet life, d | 5.1 ± 1.4 | ND | 9.2 ± 1.4 | |
| Platelet production rate, × 109/d | 87 ± 54 | ND | 220 ± 65 | |
Results are expressed as mean ± SD
F indicates female; M, male; -, not applicable; and ND, not done.
Clinical and laboratory characteristics of the studied patients according to FAB classification
Patient . | FAB . | Hb, mM . | WBC, × 109/L . | Platelets, × 109/L . | Megakaryocytes in bone marrow . | MPL, d . | PPR, × 109/d . | GCI . | TPO, pg/mL . | Karyotype . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | RA | 7.2 | 8.0 | 70 | Increased | 5.3 | 150 | ND | ND | 46XY |
| 2 | RA | 7.0 | 6.3 | 71 | Normal | 7.4 | 45 | ND | ND | 46XX |
| 3 | RA | 4.8 | 5.5 | 88 | Normal | 5.3 | 160 | ND | ND | ND |
| 4 | RA | 6.5 | 2.8 | 23 | Increased | 5.0 | 50 | 41.0 | 118 | 46XY |
| 5 | RA | 5.8 | 4.4 | 33 | Normal | 4.0 | 63 | ND | ND | 47XY, +8 |
| 6 | RA | 6.5 | 0.8 | 50 | Decreased | 7.0 | 95 | ND | ND | 47XX, +5 |
| 7 | RA | 6.5 | 4.7 | 28 | Increased | 3.3 | 100 | ND | ND | ND |
| 8 | RA | 6.8 | 4.1 | 50 | Increased | 3.7 | 155 | ND | ND | ND |
| 9 | RA | 7.1 | 5.7 | 40 | Normal | 4.2 | 55 | ND | ND | ND |
| 10 | RA | 7.7 | 7.9 | 50 | Increased | 4.1 | 45 | ND | ND | 46XX |
| 11 | RA | 5.9 | 1.4 | 71 | Normal | 6.0 | 50 | 4.5 | 131 | ND |
| 12 | RA | 5.8 | 3.0 | 36 | Increased | 5.4 | 45 | 15.3 | 190 | 46XX, -7, +8 |
| 13 | RA | 7.4 | 2.7 | 88 | Normal | 6.3 | 95 | 5.2 | 138 | 46XY |
| 14 | RA | 6.7 | 2.9 | 77 | Normal | 4.4 | 130 | 5.2 | 102 | 46XX |
| 15 | RA | 6.5 | 2.2 | 55 | Normal | 6.1 | 55 | 8.7 | 256 | 46XX |
| 16 | RA | 4.8 | 4.0 | 13 | Decreased | 5.8 | 18 | 34.0 | 370 | 45X, -Y |
| 17 | RA | 4.8 | 5.4 | 54 | Normal | 4.2 | 110 | 3.9 | 361 | 46XY |
| 18 | RA | 4.0 | 5.9 | 29 | Increased | 4.3 | 105 | 15.0 | 384 | 46XY, i(17)(q10) |
| 19 | RA | 6.4 | 2.0 | 36 | Decreased | 6.2 | 55 | 5.9 | 130 | Multiple |
| 20 | RA | 8.6 | 4.2 | 29 | Normal | 8.0 | 40 | 7.3 | 135 | 46XY |
| 21 | RA | 7.5 | 2.2 | 37 | Increased | ND | ND | ND | ND | 46XX, del20p |
| 22 | RA | 5.2 | 1.4 | 110 | Normal | ND | ND | ND | ND | 46XX |
| 23 | RARS | 7.6 | 4.2 | 115 | Increased | ND | ND | ND | ND | 45X, -Y |
| 24 | RAEB | 5.7 | 8.8 | 75 | Normal | 3.5 | 210 | ND | ND | ND |
| 25 | RAEB | 6.0 | 4.2 | 65 | Normal | 5.5 | 75 | 14.5 | 95 | 46XX |
| 26 | RAEB | 5.8 | 2.4 | 46 | Decreased | 3.3 | 100 | 4.2 | 869 | 45XY, -7 |
| 27 | RAEB | 5.9 | 1.6 | 52 | Increased | 7.8 | 60 | 3.4 | 140 | 46XY |
| 28 | RAEB | 5.8 | 3.0 | 26 | Normal | 4.6 | 60 | 21.7 | 180 | 46XY |
| 29 | RAEB | 5.8 | 3.7 | 78 | Normal | 3.0 | 175 | 5.2 | ND | 46XY |
| 30 | RAEB | 8.8 | 9.0 | 14 | Decreased | 3.7 | 45 | 17.1 | ND | ND |
| 31 | RAEB | 5.9 | 5.6 | 302 | Normal | ND | ND | ND | ND | 45X, -Y |
| 32 | RAEB | 5.6 | 8.0 | 50 | Decreased | ND | ND | ND | ND | Multiple |
| 33 | CMML | 9.1 | 12.6 | 17 | Increased | 6.0 | 15 | 64.4 | 44 | ND |
| 34 | CMML | 8.2 | 7.4 | 30 | Normal | 5.7 | 35 | 13.4 | 49 | ND |
| 35 | CMML | 6.8 | 5.5 | 98 | Normal | 4.1 | 210 | 2.7 | 315 | 46XY |
Patient . | FAB . | Hb, mM . | WBC, × 109/L . | Platelets, × 109/L . | Megakaryocytes in bone marrow . | MPL, d . | PPR, × 109/d . | GCI . | TPO, pg/mL . | Karyotype . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | RA | 7.2 | 8.0 | 70 | Increased | 5.3 | 150 | ND | ND | 46XY |
| 2 | RA | 7.0 | 6.3 | 71 | Normal | 7.4 | 45 | ND | ND | 46XX |
| 3 | RA | 4.8 | 5.5 | 88 | Normal | 5.3 | 160 | ND | ND | ND |
| 4 | RA | 6.5 | 2.8 | 23 | Increased | 5.0 | 50 | 41.0 | 118 | 46XY |
| 5 | RA | 5.8 | 4.4 | 33 | Normal | 4.0 | 63 | ND | ND | 47XY, +8 |
| 6 | RA | 6.5 | 0.8 | 50 | Decreased | 7.0 | 95 | ND | ND | 47XX, +5 |
| 7 | RA | 6.5 | 4.7 | 28 | Increased | 3.3 | 100 | ND | ND | ND |
| 8 | RA | 6.8 | 4.1 | 50 | Increased | 3.7 | 155 | ND | ND | ND |
| 9 | RA | 7.1 | 5.7 | 40 | Normal | 4.2 | 55 | ND | ND | ND |
| 10 | RA | 7.7 | 7.9 | 50 | Increased | 4.1 | 45 | ND | ND | 46XX |
| 11 | RA | 5.9 | 1.4 | 71 | Normal | 6.0 | 50 | 4.5 | 131 | ND |
| 12 | RA | 5.8 | 3.0 | 36 | Increased | 5.4 | 45 | 15.3 | 190 | 46XX, -7, +8 |
| 13 | RA | 7.4 | 2.7 | 88 | Normal | 6.3 | 95 | 5.2 | 138 | 46XY |
| 14 | RA | 6.7 | 2.9 | 77 | Normal | 4.4 | 130 | 5.2 | 102 | 46XX |
| 15 | RA | 6.5 | 2.2 | 55 | Normal | 6.1 | 55 | 8.7 | 256 | 46XX |
| 16 | RA | 4.8 | 4.0 | 13 | Decreased | 5.8 | 18 | 34.0 | 370 | 45X, -Y |
| 17 | RA | 4.8 | 5.4 | 54 | Normal | 4.2 | 110 | 3.9 | 361 | 46XY |
| 18 | RA | 4.0 | 5.9 | 29 | Increased | 4.3 | 105 | 15.0 | 384 | 46XY, i(17)(q10) |
| 19 | RA | 6.4 | 2.0 | 36 | Decreased | 6.2 | 55 | 5.9 | 130 | Multiple |
| 20 | RA | 8.6 | 4.2 | 29 | Normal | 8.0 | 40 | 7.3 | 135 | 46XY |
| 21 | RA | 7.5 | 2.2 | 37 | Increased | ND | ND | ND | ND | 46XX, del20p |
| 22 | RA | 5.2 | 1.4 | 110 | Normal | ND | ND | ND | ND | 46XX |
| 23 | RARS | 7.6 | 4.2 | 115 | Increased | ND | ND | ND | ND | 45X, -Y |
| 24 | RAEB | 5.7 | 8.8 | 75 | Normal | 3.5 | 210 | ND | ND | ND |
| 25 | RAEB | 6.0 | 4.2 | 65 | Normal | 5.5 | 75 | 14.5 | 95 | 46XX |
| 26 | RAEB | 5.8 | 2.4 | 46 | Decreased | 3.3 | 100 | 4.2 | 869 | 45XY, -7 |
| 27 | RAEB | 5.9 | 1.6 | 52 | Increased | 7.8 | 60 | 3.4 | 140 | 46XY |
| 28 | RAEB | 5.8 | 3.0 | 26 | Normal | 4.6 | 60 | 21.7 | 180 | 46XY |
| 29 | RAEB | 5.8 | 3.7 | 78 | Normal | 3.0 | 175 | 5.2 | ND | 46XY |
| 30 | RAEB | 8.8 | 9.0 | 14 | Decreased | 3.7 | 45 | 17.1 | ND | ND |
| 31 | RAEB | 5.9 | 5.6 | 302 | Normal | ND | ND | ND | ND | 45X, -Y |
| 32 | RAEB | 5.6 | 8.0 | 50 | Decreased | ND | ND | ND | ND | Multiple |
| 33 | CMML | 9.1 | 12.6 | 17 | Increased | 6.0 | 15 | 64.4 | 44 | ND |
| 34 | CMML | 8.2 | 7.4 | 30 | Normal | 5.7 | 35 | 13.4 | 49 | ND |
| 35 | CMML | 6.8 | 5.5 | 98 | Normal | 4.1 | 210 | 2.7 | 315 | 46XY |
FAB indicates French-American-British; Hb, hemoglobin; WBC, white blood cell; MPL, mean platelet life; PPR, platelet production rate; GCI, glycocalicin index; TPO, thrombopoietin; Multiple, 3 or more abnormalities; and ND, not done. Normal values: MPL, 9.2 ± 1.4 days; PPR, 220 ± 65 × 109/day; GCI, 0.7 ± 0.2; and TPO, 118 ± 33 pg/mL.
In all patients (including those with platelet count > 100 × 109/L), signs of dysmegakaryocytopoiesis, such as micromegakaryocytes, large mononuclear and hypogranular megakaryocytes, and megakaryocytes with multiple small dispersed nuclear lobes, were observed. In the bone marrow aspirates of all patients, more than 50% of studied megakaryocytes were showing one or more of these dysplastic characteristics, irrespective of the platelet count.
IPSS could be scored in 25 patients: low risk (n = 7), intermediate risk-1 (n = 10), intermediate risk-2 (n = 6), and high risk (n = 2).
Platelet kinetic studies
Platelet kinetic studies were performed in 30 patients with platelet count less than 100 × 109/L. Initial platelet recovery was normal, 60 ± 17%. Mean platelet life was 5.1 ± 1.4 days, which was significantly different from healthy controls (P < .0001). However a shortened MPL does not always imply an increased destruction of platelets in the peripheral blood. Hanson and Slichter31 developed a model that predicts whether the MPL is shortened as a result of a fixed rate of platelet use necessary to support vascular integrity (ie, the reduction in MPL is in agreement with the degree of the thrombocytopenia) or whether platelet survival is significantly shorter than expected based on the platelet count, indicating that additional extrinsic mechanisms induce an accelerated destruction of platelets. Using this model, in 50% of the patients the MPL was in accordance to the degree of thrombocytopenia, whereas in the residual patients a significant shortening of the MPL was observed (6.0 ± 1.3 vs 4.3 ± 0.9 days, P = .0003).
The platelet production rate (PPR) was 87 ± 54 × 109/d and significantly lower than in healthy controls (P < .0001). The PPR was normal in 5 patients and reduced in 25 patients (83%).
The number of megakaryocytes in the bone marrow was normal (n = 16) or increased (n = 9) in 83% of the patients. Of the patients with a decreased platelet production rate (n = 25), only 20% demonstrated a reduced number of megakaryocytes (n = 5). There were no differences in numbers of megakaryocytes between the groups with a thrombocytopenia due to normal or increased peripheral platelet destruction.
Thrombopoietin
The plasma TPO level was measured in 18 patients. The median TPO level was significantly higher compared with controls (223 ± 194 vs 118 ± 33 pg/mL, P = .04). The plasma TPO level was not significantly correlated with the number of megakaryocytes in the bone marrow (P = .1, r = -0.4), the platelet count (P = .9, r = 0.0007), or the PPR (P = .2, r = 0.3).
Plasma glycocalicin index
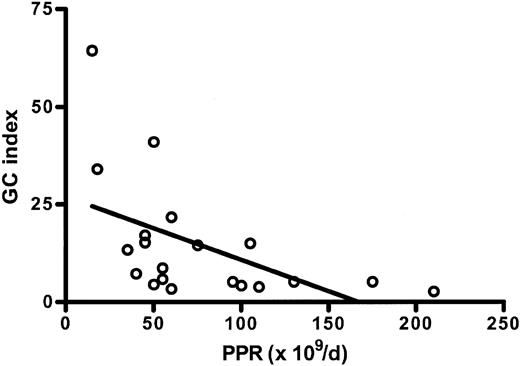
The plasma GCI level was studied in 20 patients. The GCI (15 ± 16) was significantly elevated compared with healthy controls (P = .001). No significant correlation was shown with the number of megakaryocytes in the bone marrow (P = .2, r = 0.3), the MPL (P = .8, r = 0.07), and the plasma TPO level (P = .3, r = -0.2). There was a significant inverse correlation between GCI and PPR (P = .02, r = -0.5; Figure 1).
Correlation between the glycocalicin (GC) index and the platelet production rate (PPR). The GC index is significantly and inversely correlated with the PPR in MDS patients (r = -0.5, P = .02).
Correlation between the glycocalicin (GC) index and the platelet production rate (PPR). The GC index is significantly and inversely correlated with the PPR in MDS patients (r = -0.5, P = .02).
Electron microscopy of bone marrow megakaryocytes
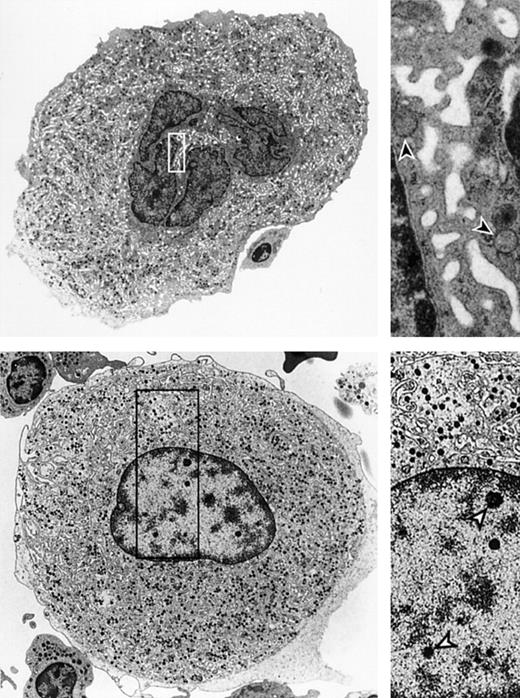
Bone marrow megakaryocyte ultrastructure was studied in 9 MDS patients, 6 patients with platelet counts less than 100 × 109/L, and 3 patients with platelet counts more than 100 × 109/L. The results were compared with bone marrow from healthy controls (n = 4). In all 6 MDS patients (nos. 19-21, 29, 32, 35; Table 2), the majority of megakaryocytes (66 ± 14%; immature and mature taken together) showed ultrastructural features of necrosis-like PCD (Figure 2B), including scanty, diffusely distributed, noncondensed chromatin, partly lying in clear, spherical speckles or small clumps. Nuclei showed predominantly smooth nonlobulated outlines, and nucleoli were absent. In these megakaryocytes, cytoplasmic abnormalities consisted of a disordered demarcation membrane system. In normal megakaryocytes, the varicose segments of the demarcation membrane system form a tubular system with widened and distended areas and local constrictions (Figure 2A). In MDS patients, megakaryocytes show a characteristic demarcation membrane system with smooth and parallel membranes that may form circular loops and lack the varicose segments (Figure 2B). Furthermore, in MDS megakaryocytes hypogranularity is found. The granulae appear dispersed in the cytoplasm and do not show clustering in dense and mature aggregates that are normally present. In 2 of 6 patients (nos. 19 and 20), a proportion of the mature megakaryocytes (38% and 25%, respectively) demonstrated extensive cytosolic vacuolization containing lysosomes with membrane inclusions, suggestive of cytoplasmic membrane breakdown most likely originating from the demarcation membrane system (not illustrated). Mitochondria appeared normal in all of the studied immature and mature megakaryocytes. Characteristics of classic apoptosis or apoptosis-like PCD according to nuclear morphology were found in none of the megakaryocytes. Although cell size cannot be determined reliably with electron microscopy, it was repeatedly found that the majority of MDS megakaryocytes in all samples appeared to be smaller in size when compared with healthy controls.
Ultrastructure of megakaryocytes from a healthy bone marrow donor and an MDS patient. (A) Ultrastructure of a megakaryocyte from a healthy bone marrow donor demonstrating structural features characteristic for normal megakaryocytes. The right panel shows a higher magnification of the cytoplasm highlighting the demarcation membrane system (arrowheads). Original magnification × 3200 (left); and × 20 000 (right). (B) Ultrastructure of an MDS megakaryocyte showing chromatin speckles in the nucleus that has smooth outlines. The right panel shows the speckles (open arrowheads) and the poorly developed circular loops in the demarcation membrane system. Original magnification × 10 000 (left); and × 20 000 (right). Images were acquired using a Philips EM-201 electron microscope (Philips, Eindhoven, The Netherlands), diaphragm 30 μm; images were photographed directly by the microscope using 35-mm film from Eastman Kodak (New York, NY) and were subsequently scanned with a Heidelberg Topaz scanner using Linocolor 6.0 software (Heidelberger Druckmaschinen, Heidelberg, Germany). Images were further processed with Adobe Photoshop CS and Illustrator CS (Adobe, San Jose, CA).
Ultrastructure of megakaryocytes from a healthy bone marrow donor and an MDS patient. (A) Ultrastructure of a megakaryocyte from a healthy bone marrow donor demonstrating structural features characteristic for normal megakaryocytes. The right panel shows a higher magnification of the cytoplasm highlighting the demarcation membrane system (arrowheads). Original magnification × 3200 (left); and × 20 000 (right). (B) Ultrastructure of an MDS megakaryocyte showing chromatin speckles in the nucleus that has smooth outlines. The right panel shows the speckles (open arrowheads) and the poorly developed circular loops in the demarcation membrane system. Original magnification × 10 000 (left); and × 20 000 (right). Images were acquired using a Philips EM-201 electron microscope (Philips, Eindhoven, The Netherlands), diaphragm 30 μm; images were photographed directly by the microscope using 35-mm film from Eastman Kodak (New York, NY) and were subsequently scanned with a Heidelberg Topaz scanner using Linocolor 6.0 software (Heidelberger Druckmaschinen, Heidelberg, Germany). Images were further processed with Adobe Photoshop CS and Illustrator CS (Adobe, San Jose, CA).
Because of the extensive ultrastructural damage, megakaryocytes could be classified in only 2 stages: immature and mature (31 ± 11% vs 69 ± 11%). Of the immature megakaryocytes, 69 ± 27% showed normal morphology. Of the mature megakaryocytes, 12 ± 8% displayed normal nuclear morphology, whereas discrete cytosolic alterations, such as a locally disordered demarcation membrane system (DMS), the presence of myeline structures, and cell surface blebbing, were found in all these cells.
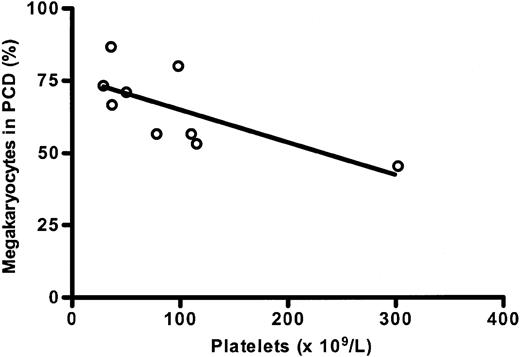
The ultrastructural studies performed in 3 MDS patients (nos. 22, 23, and 31) with platelet counts more than 100 × 109/L demonstrated ultrastructural alterations similar to those found in MDS patients with platelet counts less than 100 × 109/L, but in a lower proportion of all megakaryocytes (49 ± 4%). Taking the results of all MDS patients (n = 9) together, there was a significant and inverse correlation between the number of platelets and the proportion of megakaryocytes showing necrosis-like PCD (r = -0.7, P = .035; Figure 3).
Correlation between the percentage of MDS megakaryocytes in programmed cell death (PCD) and the number of platelets. The proportion of megakaryocytes showing ultrastructural characteristics of necrosis-like programmed cell death is inversely and significantly correlated with the number of platelets in MDS patients (r = -0.7; P = .035).
Correlation between the percentage of MDS megakaryocytes in programmed cell death (PCD) and the number of platelets. The proportion of megakaryocytes showing ultrastructural characteristics of necrosis-like programmed cell death is inversely and significantly correlated with the number of platelets in MDS patients (r = -0.7; P = .035).
No correlation was found between the percentage of megakaryocytes showing PCD ultrastructurally and the proportion of megakaryocytes showing dysplastic abnormalities in light microscopy. This finding applied for the percentage of the total number of megakaryocytes as well as for the percentage of the number of mature megakaryocytes.
Immunohistochemistry of bone marrow megakaryocytes
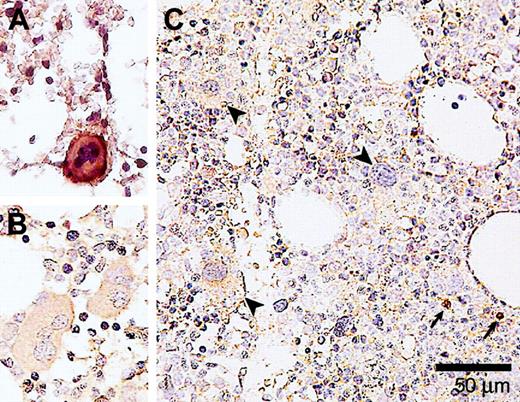
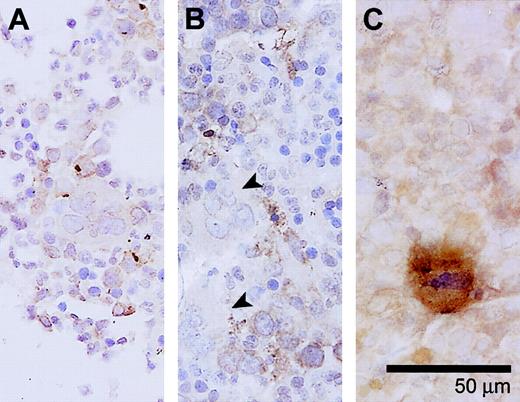
To further examine whether the ultrastructural abnormalities occur in the absence or presence of caspase-3 and caspase-8 activation, immunohistochemical staining was performed with a monoclonal antibody against activated caspase-3 and caspase-8. Activated caspase-3 could not be observed in any of the stained bone marrow samples (n = 24; Figure 4C). This was not due to an improper technique since bone marrow samples of ITP patients (Figure 4A) were positive for activated caspase-3.
Immunohistochemical detection of activated caspase-3 in megakaryocytes from a healthy donor, and MDS and ITP patients. (A) A bone marrow biopsy from an ITP patient showing a caspase-3 immunopositive megakaryocyte. (B) Bone marrow of a healthy donor has been immunostained in the same way. Note that the megakaryocytes are free of caspase-3 reactivity. (C) A bone marrow sample of an MDS patient showing megakaryocytes (indicated by arrowheads) that are negative for activated caspase-3. Small arrows indicate caspase-3 immunopositive labeling in normoblasts. Magnification × 400. Slides were observed with an Olympus BX50 microscope (Paes Nederland, Zoeterwoude, The Netherlands) using a 40×/0.75 numerical objective lens. Images were captured with an Olympus DP70 camera (Paes Nederland) and image analysis software (SIS, Münster, Germany). Images were further processed using Adobe Photoshop CS and Illustrator CS (Adobe).
Immunohistochemical detection of activated caspase-3 in megakaryocytes from a healthy donor, and MDS and ITP patients. (A) A bone marrow biopsy from an ITP patient showing a caspase-3 immunopositive megakaryocyte. (B) Bone marrow of a healthy donor has been immunostained in the same way. Note that the megakaryocytes are free of caspase-3 reactivity. (C) A bone marrow sample of an MDS patient showing megakaryocytes (indicated by arrowheads) that are negative for activated caspase-3. Small arrows indicate caspase-3 immunopositive labeling in normoblasts. Magnification × 400. Slides were observed with an Olympus BX50 microscope (Paes Nederland, Zoeterwoude, The Netherlands) using a 40×/0.75 numerical objective lens. Images were captured with an Olympus DP70 camera (Paes Nederland) and image analysis software (SIS, Münster, Germany). Images were further processed using Adobe Photoshop CS and Illustrator CS (Adobe).
Activated caspase-8 (n = 21) was not detected in bone marrow megakaryocytes from 16 MDS patients. In 5 patients (nos. 18, 20, 26, 31, 32), a fraction (8 ± 3.8%) of megakaryocytes stained positive for activated caspase-8 (Figure 5). These findings did not coincide with detection of activated caspase-3. Normal bone marrow megakaryocytes stained negative in all cases (n = 4).
Activated caspase-8 in megakaryocytes from a healthy donor and MDS patients. (A) A bone marrow sample from a healthy control. No caspase-8 activity is detected in the megakaryocytes. (B) Bone marrow of an MDS patient showing activated caspase-8 immunonegative megakaryocytes (detected in 5/21 samples, in < 10% of megakaryocytes; indicated by arrowheads). (C) Bone marrow sample of another MDS patient showing a megakaryocyte that is positive for activated caspase-8. Magnification × 400. Slides were observed with an Olympus BX50 microscope (Paes Nederland, Zoeterwoude, The Netherlands) using a 40×/0.75 numerical objective lens. Images were captured with an Olympus DP70 camera (Paes Nederland) and image analysis software (SIS, Münster, Germany). Images were further processed using Adobe Photoshop CS and Illustrator CS (Adobe).
Activated caspase-8 in megakaryocytes from a healthy donor and MDS patients. (A) A bone marrow sample from a healthy control. No caspase-8 activity is detected in the megakaryocytes. (B) Bone marrow of an MDS patient showing activated caspase-8 immunonegative megakaryocytes (detected in 5/21 samples, in < 10% of megakaryocytes; indicated by arrowheads). (C) Bone marrow sample of another MDS patient showing a megakaryocyte that is positive for activated caspase-8. Magnification × 400. Slides were observed with an Olympus BX50 microscope (Paes Nederland, Zoeterwoude, The Netherlands) using a 40×/0.75 numerical objective lens. Images were captured with an Olympus DP70 camera (Paes Nederland) and image analysis software (SIS, Münster, Germany). Images were further processed using Adobe Photoshop CS and Illustrator CS (Adobe).
To investigate mediators of caspase-(in)dependent programmed cell death, immunohistochemical staining for cathepsin D was performed. Neither diffuse nor granular cytoplasmic staining for cathepsin D was detected in bone marrow megakaryocytes from both MDS patients (n = 21) and healthy controls (n = 4), while neutrophils in the same bone marrow samples demonstrated stained positive for cathepsin D (granular cytoplasmic staining pattern; data not illustrated).
Discussion
Platelet kinetic studies in MDS have shown divergent results with regard to mean platelet life (MPL). Some studies demonstrate a normal MPL,33-35 while other studies revealed a shortened MPL in all patients irrespective of the platelet count.36 The present study demonstrates a normal MPL in 50% of the patients when MPL was adjusted for the platelet count, and a reduced MPL in the additional patients. This indicates increased peripheral platelet destruction in the latter group, which might be due to antibodies directed to platelet-specific antigens or be the result of abnormal platelets derived from perturbed platelet production. The variability in the results of platelet kinetic studies in patients with MDS might be related to the technical procedure and patient characteristics. The composition of the studied patient population is probably the most important aspect. The effects of ATG and danazol have especially been noticed in RA, suggesting that in this subgroup immune-mediated phenomena might be more prominent.13,14
Besides the reduced MPL in a subgroup of patients, a reduced PPR was noticed in 83% of patients. The reduced PPR reflects a diminished release of platelets into the circulation due to a decreased production or as a result of an increased destruction of platelets in the bone marrow as a sign of ineffective thrombopoiesis. The combination of a low PPR with a normal or increased number of megakaryocytes in the bone marrow and the increased plasma GCI in a subgroup of patients support the concept of ineffective thrombopoiesis. An increased plasma GCI is the result of an increased release of membrane glycoprotein Ib complex in the circulation due to the destruction of bone marrow megakaryocytes and platelets. An increase of GCI due to an increased peripheral platelet destruction, should be visible in a shortening of the mean platelet life. The fact that no significant correlation was found between GCI and MPL further supports the conclusion of ineffective thrombopoiesis. Moreover, the plasma TPO levels are only slightly elevated in the patients with a strongly reduced PPR. These findings indicate that the total mass of TPO-receptor bearing cells is only slightly reduced in this group of MDS patients. Similar results were recently observed in ITP patients. In these patients, a reduced PPR was significantly associated with an elevated GCI, while TPO plasma levels were almost normal.18 In addition, the GCI was significantly correlated with the proportion of ITP megakaryocytes showing ultrastructural features of apoptotic and para-apoptotic cell death.19 In the present study, ultrastructural and immunohistochemical examinations were distinct from ITP and revealed necrosis-like PCD in bone marrow megakaryocytes from MDS patients.
Ultrastructural studies of bone marrow cells in MDS patients are scarce and concern predominantly the erythroid and myeloid lineages.37-40 Cohen et al41 described some salient ultrastructural abnormalities of megakaryocytes in MDS megakaryocytes, such as scanty heterochromatin, cytoplasmic microvacuoles, and a shift toward immaturity, that show resemblance with our ultrastructural findings of necrosis-like PCD.
The present results are in contrast with findings of previous studies that enhanced intramedullary apoptosis might be present in MDS.37,42-45 This discrepancy cannot be ascribed to methodologic limitations, since with the same techniques we could detect apoptosis in ITP megakaryocytes.19 A number of reports on increased spontaneous apoptosis in MDS bone marrow cells have been based on findings from in vitro cell culture assays. Some studies have shown apoptosis when cells of MDS patients were cultured in vitro, whereas no apoptosis was found in the same patients when fresh bone marrow samples were studied with TUNEL (terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick end labeling) technique.46,47 It is therefore questionable whether the cell behavior in these in vitro culture conditions reflects the in vivo situation. It is conceivable that the detachment of the hematopoietic cells from their bone marrow microenvironment in conjunction with the observed intracellular abnormalities will lead to an increased susceptibility to apoptosis. Furthermore, others have found increased apoptosis only in bone marrow cells of MDS with trisomy 8, but not in cells with other cytogenetic abnormalities.48 In addition, treatment with antiapoptotic agents, such as erythropoietin and granulocyte colony-stimulating factor (G-CSF), shows limited durable effects in MDS.49,50
The Fas/Fas ligand (FasL) system is thought to play an important role in inducing intramedullary apoptosis in MDS.51 However, many studies have reported that stimulation of Fas and other death receptors, such as the tumor necrosis factor (TNF)–receptor 1, can also trigger caspase-independent pathways that lead to necrosis-like PCD.52-54 Crucial in Fas-mediated signaling to either apoptotic or necrosis-like PCD is the presence of the Fas-associated death domain (FADD).55 When caspases are inhibited, signaling via FADD leads to necrosis-like PCD. Alternatively, FADD-induced necrotic PCD can be reverted to apoptosis by degradation of receptor interacting protein 1 (RIP1).20,56 Thus, since it is the cellular context that determines whether stimulation of Fas triggers apoptotic or necrosis-like PCD, increased Fas/FasL expression in bone marrow cells of MDS patients might also be related to the presence of necrosis-like PCD. A similar switch in PCD might occur dependent on in vivo or in vitro conditions. In addition, it has been reported57 that stimulation of Fas can lead to necrosis in a manner dependent on activation of caspase-8, but not caspase-3. The cell death is thought to be mediated by delayed Fas-dependent production of ceramide, a sphingolipid derivative, which has been found to be capable of inducing caspase-dependent and -independent apoptosis.58 Ceramide can induce mitochondrial dysfunction, either directly59 or indirectly by activation of cathepsin D.60 These findings might explain why in the present study activated caspase-8 was detected in a fraction of the bone marrow megakaryocytes from a subgroup of the MDS patients, while the staining for activated caspase-3 was negative.
Recently, Ichikawa et al61 reported that bone marrow from mice deficient for transcription factor AML-1 (acute myeloid leukemia-1) contains abnormal megakaryocytes resembling micromegakaryocytes found in MDS. The ultrastructure of these megakaryocytes demonstrated a poorly developed demarcation membrane system and nuclei with a low level of polyploidy, which shows similarity to the ultrastructural results of the present study. Of interest is that mutations in the AML1 gene have been observed in MDS.62
In summary, the present results demonstrate predominantly caspase-3– and caspase-8–independent necrosis-like PCD in bone marrow megakaryocytes of MDS patients. This leads to ineffective thrombopoiesis and an increased intramedullary destruction of megakaryocytes and platelets, visualized in the decreased platelet production rate, which is inversely correlated to the glycocalicin index. It would be of interest to investigate whether agents, such as thrombopoietin, can influence the megakaryopoiesis in MDS either by inhibiting this type of PCD or by stimulating the residual normal megakaryocytes in MDS patients.
Prepublished online as Blood First Edition Paper, November 12, 2004; DOI 10.1182/blood-2004-06-2108.
Supported by a grant from the J. K. de Cock Stichting and the Dutch Cancer Foundation (2003-2920).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Mrs N. Zwart, technical research assistant, Department of Pathology, and Dr J. J. Koornstra, MD, Department of Oncology, University Hospital Groningen, for their assistance in performing immunohistochemistry and to Dr E. van den Berg, MD, for performing cytogenetic studies. We thank Dr B. Vrugt, pathologist, Martini Hospital Groningen, and Dr S. Rosati, pathologist, University Hospital Groningen, for providing bone marrow samples and for critically reviewing the bone marrow slides.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal