Thalidomide, which is clinically recognized as an efficient therapeutic agent for multiple myeloma, has been thought to exert antiangiogenic action through an unknown mechanism. We here show a novel mechanism of thalidomide-induced antiangiogenesis in zebrafish embryos. Thalidomide induces the defect of major blood vessels, which is demonstrated by their morphologic loss and confirmed by the depletion of vascular endothelial growth factor (VEGF) receptors such as neuropilin-1 and Flk-1. Transient increase of ceramide content through activation of neutral sphingomyelinase (nSMase) precedes thalidomide-induced vascular defect in the embryos. Synthetic cell permeable ceramide, N-acetylsphingosine (C2-ceramide) inhibits embryonic angiogenesis as well as thalidomide. The blockade of ceramide generation by antisense morpholino oligonucleotides for nSMase prevents thalidomide-induced ceramide generation and vascular defect. In contrast to ceramide, sphingosine-1-phosphate (S1P) inhibits nSMase-dependent ceramide generation and restores thalidomide-induced embryonic vascular defect with an increase of expression of VEGF receptors. In human umbilical vein endothelial cells (HUVECs), thalidomide-induced inhibition of cell growth, generation of ceramide through nSMase, and depletion of VEGF receptors are restored to the control levels by pretreatment with S1P. These results suggest that thalidomide-induced antiangiogenic action is regulated by the balance between ceramide and S1P signal.
Introduction
In the 1950s, thalidomide came out as a sedative and antinausea medicine, but eventually it was withdrawn from the market as it was found to be a potent teratogen to a human fetus. Prenatal use of thalidomide induced severe developmental defects such as a stunted limb growth named phocomelia.1 It has been postulated that the limb defects caused by thalidomide were secondary to an inhibition of angiogenesis in the developing limb buds.2 In tumorigenesis as well as normal development of fetus, angiogenesis plays a critical role to sustain the nutrient and oxygen supply in tumor growth.3 Several studies have shown that there is a direct correlation between density of tumor microvessels, incidence of metastases, and survival of the patients.4-6 As a possible mechanism of thalidomide to induce antitumor action, it is suggested that antiangiogenic action through inhibition of cytokine-induced nuclear factor κB (NF-κB), tumor necrosis factor-α (TNF-α), vascular endothelial growth factor (VEGF), and prostagrandin synthesis plays a role in vitro.7-10 Thus, these observations suggest a clinical role of thalidomide as an anticancer agent.11 However, the precise mechanism of the antiangiogenic action by thalidomide remains to be clarified.
In vivo, thalidomide was shown to inhibit a basic fibroblast growth factor–induced neovascularization by using a rabbit cornea micropocket assay, but it failed to induce antiangiogenic action in the chicken embryo and the mice implanted with either B16-F10 melanoma or CT-26 colon carcinoma cells.2,12,13 In addition, thalidomide had no teratogenic effect in rodents and unexpectedly promoted the metastasis of prostate adenocarcinoma cells in rats.14 Thus, the establishment of the animal model is worthwhile for understanding the mechanism of thalidomide-induced antiangiogenesis. The zebrafish provides an alternative vertebrate model system,15 since the rapid external development of the transparent embryo permits visual analysis of angiogenesis, and the fish possesses not only a complex circulatory system similar to mammals but also the reasonable counterparts for other mammalian organ systems.16,17 Therefore, we here establish the appropriate zebrafish model for assessing the antiangiogenic action of thalidomide.
Ceramide has been described as a lipid mediator to induce programmed cell death (PCD).18,19 On the other hand, a diverse array of stresses including Fas ligand, oxidative stress, growth factor withdrawal, anticancer drugs, ionizing radiation, heat shock, and ultraviolet light are known to induce an elevation of ceramide level.18,20 Ceramide is generated from sphingomyelin (SM) hydrolysis by acid or neutral sphingomyelinase (aSMase or nSMase), enzymes that are activated, for example, in response to TNF-α and heat shock.18 Generated ceramide is deacylated to sphingosine by ceramidase, and then sphingosine kinase converts sphingosine to sphingosine-1-phosphate (S1P), which has been recognized as an intercellular bioactive lipid for cell migration, neurite retraction, and cell growth.21,22
S1P has been shown as an enhancer of VEGF-induced angiogenesis. For example, S1P induces vascular endothelial cell adherens junction assembly through S1P receptors, endothelial differentiation gene-1 (EDG-1) and EDG-3, and VEGF induces up-regulation of EDG-1 in endothelial cells although S1P alone fails to exert proangiogenic action.23,24 S1P also enhances DNA synthesis in human umbilical vein endothelial cells (HUVECs),25 and endothelial cell–specific deletion of Edg-1 impairs vascular maturation in the conditional targeting mice.26 In contrast, even though synthetic ceramide induces apoptosis in HUVECs,27 and ceramide generation caused by inhibition of neutral ceramidase in zebrafish is reported to be involved in the dysregulation of blood flow,28 the role of ceramide in angiogenesis is not revealed.
Thus, it is not known whether and how thalidomide-induced antiangiogenic action is regulated through sphingolipids, ceramide and S1P in vivo as well as in vitro. Here we show a critical role of nSMase-dependent ceramide generation in thalidomide-induced vascular defect, and a counteraction of S1P against ceramide-mediated thalidomide action through depletion of VEGF receptors such as neuropilin-1 and FLK-1 in zebrafish embryos and HUVECs.
Materials and methods
Cell culture and reagents
Human umbilical vein endothelial cells (HUVECs) were obtained from American Type Culture Collection (ATCC, Manassas, VA; no. CRL-1730). These cells were cultured in MCDB131 medium (Gibco Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (Sigma, St Louis, MO) and 10 ng/mL human basic fibroblast growth factor (hbFGF; Gibco Laboratories), plate in triplicate in a 48-well culture plate at a concentration of 1 × 104 cells/well. Adherent nonconfluent monolayers were treated with thalidomide for 2 days. C6-7-nitro-2-1,3-benzoxadiazol-4-yl (C6-NBD) sphingomyelin, C6-NBD-ceramide, and sphingosine-1-phosphate (S1P) were purchased from Matreya (Pleasant Gap, PA). COS-7 cells for overexpression of zebrafish magnesium-dependent neutral sphingomyelinase (ZNSMase) were cultured in RPMI 1640 medium (Sigma) supplemented with 10% fetal calf serum and 80 ng/mL kanamycin sulfate.
Maintenance of zebrafish and drug treatments
Breeding fishes were maintained at 28.5°C on a 14-hour light/10-hour dark cycle. Embryos were collected by natural spawning, raised in 10% Hanks saline, and staged up to 24 hours, according to Kimmel et al16 ; beyond this point, embryo stage is given as hours post fertilization (hpf). Thalidomide (Tocris Cookson, St Louis, MO) was dissolved in dimethylsulfoxide (DMSO) at a stock concentration of 800 mM. C2-ceramide and S1P were dissolved at 80 mM and 10 mM, respectively, in DMSO, diluted in embryo media,29 and added to zebrafish embryos at the indicated stages. Control embryos were treated with the equivalent amount of DMSO solution. All embryos were incubated at 28.5°C.
MTT assay
Cells (1 × 104/well) were seeded in a 48-well plate in 400 μL MCDB131 supplemented with 5% fetal calf serum and 10 ng/mL hbFGF. At the indicated time points, 40 μL 3-(4,5-dimethyl-thiazoyl-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) stock solution (Nakalai tesque, Kyoto, Japan) was added to each well and incubated at 37°C in 5% CO2 for 3 hours. Subsequently, cells were solubilized by the addition of 400 μL lysis buffer (10% sodium dodecyl sulfate [SDS] in 0.01 M HCl) to each well and incubated at room temperature overnight. The production of the formazan dye was measured using a multiwell plate reader SME 3400 (Iwaki, Tokyo, Japan) at 595 nm absorbance.
Ceramide measurement
Lipids in zebrafish embryos and HUVECs were extracted by the method of Bligh and Dyer,60 and ceramide mass measurement using Escherichia coli diacylglycerol kinase (DGK) was performed as described.30 The solvent system used to separate ceramide-1-phosphate was chloroform/acetone/methanol/acetic acid/H2O (10:4:3:2:1). To calculate ceramide content, the positive spots on thin-layer chromatography (TLC) plates were measured using the BAS III image analyzer system (Fuji, Tokyo, Japan).
Sphingomyelinase assay
The embryos and the cells were lysed by passing through a 27-gauge needle in a lysis buffer containing 10 mM Tris/HCl (pH 7.5), 1 mM EDTA (ethylenediaminetetraacetic acid), 0.1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 10 μg/mL pepstatin A, 0.15 U/mL aprotinin, and 50 μg/mL leupeptin. The lysate was centrifuged at 10 000g for 10 minutes at 4°C. The supernatant was used as an enzyme source. Sixty micrograms of supernatant protein was mixed in a reaction buffer (neutral SMase: 100 mM Tris/HCl [pH 7.5], 10 mM MgCl2, 5 mM dithiothreitol [DTT], 10 μM C6-NBD-sphingomyelin, and 0.1% Triton X-100; acid SMase: 100 mM sodium acetate [pH 5.0], 10 μM C6-NBD-sphingomyelin, and 0.1% Triton X-100) and incubated at 37°C for 1 hour. The reaction was stopped by the addition of 900 μLH2O and 2 mL chloroform and methanol (2:1; vol/vol), mixed well, and centrifuged. Lower phase was collected, and solvent was evaporated. Aliquots were applied to TLC plates. The solvent system used to separate C6-NBD-ceramide and C6-NBD-sphingomylin was chloroform/methanol/12 mM MgCl2 in H2O (65:25:4; vol/vol). C6-NBD-ceramide was visualized by UV irradiation and measured by a TLC scanner with fluorometer (475 nm excitation; 525 nm emission).
Tissue section
Embryos were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer. Frozen sections at 40 μm thickness were cut on a cryostat. Histologic hematoxylin and eosin staining of the sections was subsequently carried out using a standard protocol.
RNA in situ hybridization
Whole-mount zebrafish in situ hybridization using various digoxigenin-labeled antisense RNA probes was preformed using standard methods as previously described.31 RNA probes containing digoxigenin-11-UTP (Roche, Basel, Switzerland) were visualized using the NBT/BCIP alkaline phosphatase substrate (Roche). Embryos were cleared and flat-mounted in 70% glycerol for photography.
Expression cloning of ZNSMase cDNA
Total RNA was isolated from zebrafish embryonic cells using TRIzol regent (Invitrogen, Carlsbad, CA), and poly(A)+ RNA was purified from the total RNA with Oligotex-dt30 mRNA purification kit (Takara-bio, Shiga, Japan). Double-stranded cDNA was synthesized from the poly(A)+-RNA with a Superscript Choice System for cDNA Synthesis (Invitrogen). A cDNA library was prepared with the isopropyl-1-thio-β-D-galactopyranoside (IPTG) inducible expression vector, pET-28a (Novagen), in Escherichia coli BL21(DE3)pLysE cells (Novagen EMD Biosciences, Madison, WI).The library contained 1 × 107 independent clones. Each aliquot containing 1000 E coli clones from the library were inoculated ina4mL Luria-Bertain (LB) broth and grown overnight at 30°C in shaker at 200 rpm. The culture was transferred to 4 mL fresh LB broth with 100 μg/mL ampicillin, and incubation continued until turbidity at 600 nm reached 0.8. A final concentration of 0.5 mM IPTG was added into the culture for a further 4 hours to induce expression of the transgene product. Bacterial cells were collected by centrifugation, and a neutral sphingomyelinase (nSMase) activity was assayed. E coli colonies were incubated on an LB plate from the positive pool having high nSMase activity. Each E coli clone was in culture and its nSMase activity was measured. Finally, the positive clone encoding magnesium-dependent nSMase cDNA from zebrafish was isolated and sequenced.
Determination of nucleotide sequence
The nucleotide sequence was determined by DNA sequencer (ABI 310; Perkin Elmer, Shelton, CT). The nucleotide sequence of the isolated ZNSMase cDNA was deposited to the DNA DataBank of Japan (DDBJ)/GenBank/European Molecular Biology Laboratory (EMBL) database under accession number AB196 165.
Transfection of ZNSMase cDNA into COS-7 cells
The ZNSMase cDNA containing the complete open reading frame (ORF) was amplified by polymerase chain reaction (PCR) with the isolated full-length cDNA as template with sense primer: 5′-TCAGGAGCGGACTGAAGCGGCATCATGGCA-3′ and an antisense primer: 5′-CCGTCGAGTCCTTTCAAACGGGAGGAATAA-3′. PCR was carried out by 25 cycles of denaturation at 94°C for 30 seconds, annealing at 63°C for 30 seconds, and extension at 72°C for 2 minutes. The amplified product was subcloned downstream of the human cytomegalovirus promoter of the pTARGET Mammali Expression Vector (Promega, Madison, WI) according to the manufacturer's instructions. The nucleotide sequence of the PCR product was confirmed by sequence analysis. This vector was named as pTARGET-ZNSMase. At approximately 80% of confluence, each dish of cells was transiently transfected with 5 μg DNA of pTARGET-ZNSMase vector or mock vector with Lipofection Amine 2000 reagent (Invitrogen) according to the manufacturer's instructions. At 24 hours after transfection, cells were washed once with phosphate-buffered saline (PBS) and homogenized in lysis buffer, and nSMase activity was measured.
Morpholino oligonucleotide injection
Morpholino oligonucleotides (MOs) were solubilized in water at the concentration of 50 μg/μL. The resulting stock solution was diluted to a working concentration in water before injection into 1-cell– or 2-cell–stage embryos. Injected embryos were cultured in 10% Hanks saline until use. The sequences of MOs used in this study were based on cDNA cloned by us (T.Y., H.T., Y.T., S.Y., Y.I., T.O., manuscript in preparation; Expression cloning of zebrafish neutral sphingomylinase cDNA by using E coli expression system based on neutral sphingomylinase activity), and as follows: nSMase-invert-morpholino, 5′-CCGTAGTACCGTGGTGTCGTCGGG-3′; and nSMase-antisense-morpholino, 5′-GGGCTGCTGTGGTGCCATGATGGCC-3′.
Detection of human endothelial marker genes by reverse transcription–PCR
The total RNA of the HUVECs was extracted using TRIZOL reagent (Invitrogen) according to the manufacturer's instructions. For the first-strand synthesis of complementary DNA (cDNA), 3 μg messenger RNA (mRNA) used in a 20-μL reaction mixture using a ThermoScript RT-PCR System (Invitrogen) according to the manufacturer's instructions. Resulting reverse transcription (RT) products were diluted 10 times and stored at –20°C until later use. The polymerase chain reaction (PCR) primers of Flk-1, neuropilin-1, neuropilin-2, and β-actin were synthesized as follows: Flk-1; upstream 5′-CTGGCATGGTCTTCTGTGAAGCA-3′, downstream 5′-AATACCAGTGGATGTGATGCGG-3′ (PCR product, 790 base pair [bp]), NRP-1; upstream 5′-AGGACAGAGACTGCAAGTATGAC-3′, downstream 5′-AACATTCAGGACCTCTCTTGA-3′ (PCR product, 209 bp), NRP-2; upstream 5′-AGCACTAATGGAGAGGACTG-3′, downstream 5′-CCGTTTAGGCTGTAGGAGAC-3′ (PCR product, 606 bp), β-actin; upstream 5′-GAGACCTTCAACACCCCAGC-3′, downstream 5′-TCTTCATTGTGCTGGGTGCC-3′ (PCR product, 606 bp). PCR was carried out in a total volume of 40 μL reaction mixture using Premix Taq (Ex Taq Version; Takara). Reaction conditions were 94°C for 2 minutes, followed by 30 cycles (β-actin for 25 cycles), 1 minute denaturation at 94°C, 1 minute annealing at 58°C, 2 minutes of polymerization at 72°C, and 7 minutes extension at 72°C. β-actin was chosen as an internal control. All the cycles chosen were in range of the linear relationship between the PCR products and the number of cycles.
Analysis of statistical significance
The results are shown as means plus or minus 1 standard deviation (SD) of 3 independent experiments, and significance was determined by Student t test or analysis of variance (ANOVA) test.
Results
Vascular defect caused by thalidomide during the development of zebrafish embryo
Zebrafish embryos at the 8-hpf stage were incubated with different concentrations of thalidomide (0, 100, 200, 400, and 800 μM). Thalidomide caused the defects of dorsal artery and posterior cardinal vein at 48 hpf in a concentration-dependent manner (Table 1). Treatment with 800 μM thalidomide for 40 hours caused shortness in length and loss of blood flow in 93% of zebrafish embryos as judged under microscopy (Figure 1A-B, white arrow; Table 1), and a complete defect of blood vessel formation was confirmed by hematoxylin and eosin staining analysis at 48 hpf of embryo (Figure 1C-D). The vascular defect resulted in further complications such as severe pericardial edema and heart failure, and subsequently the embryos died by 5 days of development. Except shortness in length, deleterious effects were not observed on the general morphology of embryonic structures by 30 hpf (Figure 1A-B). The defect of large vessels was assessed by in situ mRNA staining for VEGF receptors at 30 hpf (Figure 1E-J). We demonstrated the vascular defect in the region corresponding to dorsal artery and vein (indicated in the figure by a rectangular shape) by showing the depleted expression of endothelial markers, neuropilin-1 (Figure 1E-F) and FLK-1 (Figure 1G-J).32,33 The higher magnification (×40) clearly showed the loss of Flk-1 expression in the thalidomide-treated embryo (Figure 1H,J).
Thalidomide-induced vascular defect during the development of zebrafish embryos
Concentration . | Dorsal artery presence, % (no.) . | Dorsal artery absence, % (no.) . | No. embryos . |
|---|---|---|---|
| Thalidomide | |||
| Control | 100 (250) | 0 (0) | 250 |
| 100 μM | 100 (240) | 0 (0) | 240 |
| 200 μM | 89 (212) | 11 (27) | 239 |
| 400 μM | 41 (147) | 59 (210) | 357 |
| 800 μM | 7 (49) | 93 (641) | 690 |
| C2-ceramide | |||
| Control | 100 (250) | 0 (0) | 250 |
| 2.5 μM | 80 (187) | 20 (47) | 234 |
| 5.0 μM | 48 (118) | 52 (127) | 245 |
| 10 μM | 27 (95) | 73 (256) | 351 |
| 20 μM | 12 (54) | 88 (397) | 451 |
| 40 μM | 0 (0) | 100 (535) | 535 |
Concentration . | Dorsal artery presence, % (no.) . | Dorsal artery absence, % (no.) . | No. embryos . |
|---|---|---|---|
| Thalidomide | |||
| Control | 100 (250) | 0 (0) | 250 |
| 100 μM | 100 (240) | 0 (0) | 240 |
| 200 μM | 89 (212) | 11 (27) | 239 |
| 400 μM | 41 (147) | 59 (210) | 357 |
| 800 μM | 7 (49) | 93 (641) | 690 |
| C2-ceramide | |||
| Control | 100 (250) | 0 (0) | 250 |
| 2.5 μM | 80 (187) | 20 (47) | 234 |
| 5.0 μM | 48 (118) | 52 (127) | 245 |
| 10 μM | 27 (95) | 73 (256) | 351 |
| 20 μM | 12 (54) | 88 (397) | 451 |
| 40 μM | 0 (0) | 100 (535) | 535 |
Zebrafish embryos were treated at 75% epiboly stage (8 hpf) with various concentrations of thalidomide or C2-ceramide, and then the defect of dorsal artery was determined by assessing the loss of blood flow under microscopy at the 48-hpf stage.
Increase of endogenous ceramide by thalidomide in zebrafish embryos, and mimicry of thalidomide-induced vascular defect by exogenous N-acetylsphingosine (C2-ceramide)
When we examined the content of endogenous ceramide by DGK assay after treatment with thalidomide at 75% epiboly stage (8 hpf), the increase of ceramide peaked at 18 to 24 hours after treatment (26-32 hpf), and then returned to the control level (Figure 2A). Ceramide generation by thalidomide was dose-dependently increased at the 26-hpf stage (Figure 2B). As shown in Figure 2B, the control level of ceramide in zebrafish embryos at 26 hpf was 4.2 pmol/nmol phosphate, and the treatment with 800 μM thalidomide increased ceramide content to approximately 210% of the control level (8.8 pmol/nmol phosphate). Treatment of embryo with N-acetylsphingosine (C2-ceramide) completely blocked the formation of large vessels at a concentration of 40 μM (Figure 2C-D), resulting in severe pericardial edema and heart failure like thalidomide. C2-ceramide–induced antiangiogenic effect as judged by loss of blood flow was increased in a dose-dependent manner (Table 1). C2-ceramide–induced phenotypes such as shortness and vascular defect were almost identical to thalidomide-treated embryos (Figure 1B,D, Figure 2D,F). This distinctive loss of large vessels at 48 hpf was confirmed morphologically by the transverse section after hematoxylin and eosin staining (Figure 2E-F) and biochemically by in situ mRNA hybridization for endothelial markers, neuropilin-1 and FLK-1 (Figure 2G-L).
Thalidomide induced vascular defect during the development of zebrafish embryos. Zebrafish embryos were treated at 75% epiboly stage (8 hpf) without (A,C,E,G) or with 800 μM thalidomide (B,D,F,I) and examined at the indicated hpf stages. (A-D) Lateral view of fixed embryo shows that thalidomide-treated embryos (B) are shorter in length than the control (A), as indicated by white arrows. Hematoxylin and eosin staining for the transverse section of thalidomide-treated embryos shows that the lumens of the dorsal artery (marked “A” in panel C) and posterior cardinal vein (marked “V” in panel C), which are evident in the control embryo (C), are lost in the thalidomide-treated embryo (D). (E-J) In situ mRNA hybridization is performed for endothelial markers neuropilin-1 (E,F) and FLK-1 (G-J). Expressions of neuropilin-1 and FLK-1 are absent at the region corresponding to dorsal artery and vein (indicated by boxes) after treatment with thalidomide (F,I). The defect of FLK-1 expression at large vessels is more evident by a higher magnification (H,J). The results are representative of each experiment. noto indicates notochord; A, dorsal artery; V, posterior cardinal vein; hpf, hours after fertilization. Original magnification of panels A, B, E, F, G, and I is ×40; of C and D, ×150; and of H and J, ×63. The images were visualized using an Olympus BH2 Biological microscope equipped with an SPlan APo20 20×/0.70 objective lens and a C-35AD-2 camera (Olympus, Tokyo, Japan) or a Nikon Stereoscope SMZ1000 microscope equipped with a Plan Apo 1×/0.10 objective lens with zoom system covering the total magnification to 40× and 60×, and an FDX-35 camera (Nikon, Tokyo, Japan). Images were processed with Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA).
Thalidomide induced vascular defect during the development of zebrafish embryos. Zebrafish embryos were treated at 75% epiboly stage (8 hpf) without (A,C,E,G) or with 800 μM thalidomide (B,D,F,I) and examined at the indicated hpf stages. (A-D) Lateral view of fixed embryo shows that thalidomide-treated embryos (B) are shorter in length than the control (A), as indicated by white arrows. Hematoxylin and eosin staining for the transverse section of thalidomide-treated embryos shows that the lumens of the dorsal artery (marked “A” in panel C) and posterior cardinal vein (marked “V” in panel C), which are evident in the control embryo (C), are lost in the thalidomide-treated embryo (D). (E-J) In situ mRNA hybridization is performed for endothelial markers neuropilin-1 (E,F) and FLK-1 (G-J). Expressions of neuropilin-1 and FLK-1 are absent at the region corresponding to dorsal artery and vein (indicated by boxes) after treatment with thalidomide (F,I). The defect of FLK-1 expression at large vessels is more evident by a higher magnification (H,J). The results are representative of each experiment. noto indicates notochord; A, dorsal artery; V, posterior cardinal vein; hpf, hours after fertilization. Original magnification of panels A, B, E, F, G, and I is ×40; of C and D, ×150; and of H and J, ×63. The images were visualized using an Olympus BH2 Biological microscope equipped with an SPlan APo20 20×/0.70 objective lens and a C-35AD-2 camera (Olympus, Tokyo, Japan) or a Nikon Stereoscope SMZ1000 microscope equipped with a Plan Apo 1×/0.10 objective lens with zoom system covering the total magnification to 40× and 60×, and an FDX-35 camera (Nikon, Tokyo, Japan). Images were processed with Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA).
Expression cloning of a cDNA encoding zebrafish magnesium-dependent neutral sphingomyelinase (ZNSMase)
To clone a cDNA encoding magnesium-dependent neutral sphingomyelinase (nSMase) that generates ceramide by enzymatic hydrolysis of sphingomyelin (SM), we used an Escherichia coli expression system based on a nSMase activity as described in “Materials and methods.” Finally, a single clone, ZNSMase, was isolated. ZNSMase was found to contain an insert 1471 bp in length. This clone had a 1260 bp ORF that encoded 420 amino acids with a predicted molecular mass of 46.9 kDa (Table 2). A FASTA search of the SWISS-PROT/PIR protein sequence database revealed that ZNSMase possesses significantly high homology with the human NSMase 1.34 The SOSUI program35 suggested that this peptide carries 2 transmembrane domains in the C-terminal region (Table 3). To confirm the function of cDNA encoding NSMase, we inserted it into a mammalian cell transient expression vector and transfected it into COS-7 cells. Table 3 shows that cells overexpressing NSMase cDNA exhibited about an 11-fold incresase in NSMase activity as compared with cells transfected with mock vector 24 hours after transfection.
Amino-acid sequence of zebrafish magnesium-dependent neutral sphingomylinase (ZNSMase)
MAPQQPGKLR | VFSLNCWGIR | FLSKLCAQRY | EMIGELLGRE | GHDIALLQEV | WSERDFLFLK | 60 |
| RKLSCSHPYT | HYFKSGVIGS | GLAVFSKHRI | QDALLYQYSL | NGYPYMLSHG | DWFGGKAAGL | 120 |
| VIVEVFGLKA | HVYVTHLHAE | YSRAQDGYLP | HRIVQSWELQ | QFVRHTSHGA | DLVILGGDLN | 180 |
| MHPSDLGNRL | LRSHTGLRGC | YTETDKFDGC | EDGHTLIANN | HFTKKQDLIP | FEKGIRIDYT | 240 |
| LMKGSQRVSV | KCESLSTTKG | SVSDKPFPYS | DHEALMADLN | LLSSQDCTDA | PPASDRMDVV | 300 |
| SEARAVVKEG | LGKTEALRDR | SLHLMFAGLL | LLLLLCSSS | FFSFSAAGFL | GAVCVFILLS | 360 |
| GALLYMLFTT | HIKVLKETED | QMMLHSQNLQ | TKLTGCRISG | SSSSDASPEI | QPSSPFKREE | 420 |
MAPQQPGKLR | VFSLNCWGIR | FLSKLCAQRY | EMIGELLGRE | GHDIALLQEV | WSERDFLFLK | 60 |
| RKLSCSHPYT | HYFKSGVIGS | GLAVFSKHRI | QDALLYQYSL | NGYPYMLSHG | DWFGGKAAGL | 120 |
| VIVEVFGLKA | HVYVTHLHAE | YSRAQDGYLP | HRIVQSWELQ | QFVRHTSHGA | DLVILGGDLN | 180 |
| MHPSDLGNRL | LRSHTGLRGC | YTETDKFDGC | EDGHTLIANN | HFTKKQDLIP | FEKGIRIDYT | 240 |
| LMKGSQRVSV | KCESLSTTKG | SVSDKPFPYS | DHEALMADLN | LLSSQDCTDA | PPASDRMDVV | 300 |
| SEARAVVKEG | LGKTEALRDR | SLHLMFAGLL | LLLLLCSSS | FFSFSAAGFL | GAVCVFILLS | 360 |
| GALLYMLFTT | HIKVLKETED | QMMLHSQNLQ | TKLTGCRISG | SSSSDASPEI | QPSSPFKREE | 420 |
Predicted amino acid sequence of ZSMase is shown in the table. The two putative transmembrane domains predicted by the SOSUI program are boldfaced and underlined.
Enzymatic activity of zebrafish magnesium-dependent neutral sphingomylinase (ZNSMase) in overexpressed COS-7 cells
COS-7 cells . | nSMase activity,*pmol/mg per hour . | Standard deviation, ± . |
|---|---|---|
| Untransfected | 120.2 | 2.3 |
| Mock-transfected | 123.3 | 2.4 |
| pTARGET-ZNSMase | 1321.7 | 4.5 |
COS-7 cells . | nSMase activity,*pmol/mg per hour . | Standard deviation, ± . |
|---|---|---|
| Untransfected | 120.2 | 2.3 |
| Mock-transfected | 123.3 | 2.4 |
| pTARGET-ZNSMase | 1321.7 | 4.5 |
A mammalian-cell transient expression vector with ZNSMase cDNA was transfected into COS-7 cells. nSMase activities were compared between untransfected and transfected cells with mock vector or pTRAGET-ZNSMase at 24 hours after transfection.
Values represent the average of 3 independent determinations.
Thalidomide increases endogenous ceramide during the development of zebrafish embryos, and exogenous C2-ceramide mimics thalidomide-induced antiangiogenic action. (A,B) Zebrafish embryos were treated at 75% epiboly stage (8 hpf) with the indicated concentrations of thalidomide, and harvested at various times (A) or 18 hours after treatment (B). Lipids were extracted, and ceramide content was measured by the DGK assay as described in “Materials and methods.” (A) ○ indicates control; ♦, 400 μM thalidomide; and •, 800 μM thalidomide. The results were obtained from 3 independent experiments. The error bars indicate 1 SD. *The difference in ceramide content between the embryos treated with thalidomide and the control embryo is statistically significant at P < .01 by ANOVA test. (C-F) Lateral view of fixed embryo shows that 10 μM C2-ceramide–treated embryos (D) are shorter in length than the control (C), as indicated by white arrows. Hematoxylin and eosin staining for the transverse section of C2-ceramide–treated embryos shows that the lumens of the dorsal artery (marked “A” in panel E) and posterior cardinal vein (marked “V” in panel E), which are evident in the control embryo (E), are lost in the C2-ceramide–treated embryo (F). (G-L) Expressions of neuropilin-1 and FLK-1 mRNA at the region corresponding to dorsal artery and vein are not detected after treatment with C2-ceramide as well as thalidomide (indicated by boxes; H,K). The defect of FLK-1 expression is more evident by the higher magnification (J,L). The results are representative of each experiment. noto indicates notochord; A, dorsal artery; V, posterior cardinal vein; hpf, hours after fertilization. Original magnifications of panels are as follows: C, D, G, I, H, and K, ×40; E and F, ×150; and J and L, ×63. Image acquisition was performed as described for Figure 1.
Thalidomide increases endogenous ceramide during the development of zebrafish embryos, and exogenous C2-ceramide mimics thalidomide-induced antiangiogenic action. (A,B) Zebrafish embryos were treated at 75% epiboly stage (8 hpf) with the indicated concentrations of thalidomide, and harvested at various times (A) or 18 hours after treatment (B). Lipids were extracted, and ceramide content was measured by the DGK assay as described in “Materials and methods.” (A) ○ indicates control; ♦, 400 μM thalidomide; and •, 800 μM thalidomide. The results were obtained from 3 independent experiments. The error bars indicate 1 SD. *The difference in ceramide content between the embryos treated with thalidomide and the control embryo is statistically significant at P < .01 by ANOVA test. (C-F) Lateral view of fixed embryo shows that 10 μM C2-ceramide–treated embryos (D) are shorter in length than the control (C), as indicated by white arrows. Hematoxylin and eosin staining for the transverse section of C2-ceramide–treated embryos shows that the lumens of the dorsal artery (marked “A” in panel E) and posterior cardinal vein (marked “V” in panel E), which are evident in the control embryo (E), are lost in the C2-ceramide–treated embryo (F). (G-L) Expressions of neuropilin-1 and FLK-1 mRNA at the region corresponding to dorsal artery and vein are not detected after treatment with C2-ceramide as well as thalidomide (indicated by boxes; H,K). The defect of FLK-1 expression is more evident by the higher magnification (J,L). The results are representative of each experiment. noto indicates notochord; A, dorsal artery; V, posterior cardinal vein; hpf, hours after fertilization. Original magnifications of panels are as follows: C, D, G, I, H, and K, ×40; E and F, ×150; and J and L, ×63. Image acquisition was performed as described for Figure 1.
Ceramide generation through thalidomide-activated neutral sphingomyelinase (nSMase) in zebrafish embryos, and restoration of thalidomide-induced vascular defect by antisense-morpholino oligonucleotides (MO) for nSMase
We examined whether ceramide-related enzymes such as neutral sphingomyelinase (nSMase), acid sphingomyelinase (aSMase), sphingomyelin synthase (SMS), glucosylceramide synthase (GCS), and ceramidase were involved in an increase of ceramide in thalidomide-treated embryos. We found that thalidomide increased the activity of nSMase in a dose-dependent manner (Figure 3A). However, the activities of aSMase, SMS, GCS, and ceramidase in embryos were not changed by thalidomide (data not shown). Neutral SMase activity was increased from 1.0 to 1.4 nmol/mg protein/hour and 2.2 nmol/mg protein/hour, by 400 μM and 800 μM thalidomide, respectively, at the 26-hpf stage (Figure 3A). We next examined whether ceramide generation by nSMase was prevented by the injection of antisense-morpholino oligonucleotides (MOs) for nSMase. MO has been reported to work as an effective and specific translational inhibitor in zebrafish.36 Indeed, the injection with antisense-MO for nSMase (2 μg/μL) decreased not only basal activity of nSMase from 1.0 nmol/mg per hour to 0.35 nmol/mg per hour but also thalidomide-activated nSMase from 2.2 nmol/mg per hour to 0.38 nmol/mg per hour (Figure 3B). Antisense-MO for nSMase did not affect the basal level of ceramide content (Figure 3C) and the morphologic phenotypes (data not shown), suggesting that ceramide level is regulated at the steady condition by other ceramide-related enzyme(s) different from nSMase. On the other hand, ceramide level, which was increased from 4.3 pmol/nmol to 8.0 pmol/nmol phosphate by the treatment with 800 μM thalidomide, was decreased to 3.8 pmol/nmol phosphate by preinjection with nSMase antisense-MO (Figure 3C). These results indicate that the injection of nSMase antisense-MO is effective to prevent thalidomide-induced ceramide generation through nSMase in zebrafish embryos. The inhibitory effect of nSMase antisense-MO against thalidomide-induced antiangiogenic action is confirmed by a morphologic reconstruction of large vessels (Figure 3D-E) and an induction of endothelial cells as judged by in situ RNA staining for FLK-1 at 30 hpf (Figure 3F-J). At a higher magnification (×40), the restoration of FLK-1 expression by antisense-MO was more evident (Figure 3G,I). As shown in Table 4, the percent of positively FLK-1–expressed embryo was reduced from 100% to 5% by 800 μM thalidomide at the 30-hpf stage, whereas the preinjection of nSMase antisense-MO restored this thalidomide-reduced percent to 70%, which is almost identical to the positive percent in only antisense-MO–injected control embryos. These results suggest that ceramide generation through nSMase is indispensable to thalidomide-induced vascular defect in zebrafish embryos.
Restoration of thalidomide-induced inhibition of FLK-1 expression by antisense-MO for nSMase
Experimental condition . | High FLK-1 expression, % (no.) . | Low FLK-1 expression, % (no.) . | No. embryos . |
|---|---|---|---|
| Uninjected embryos | 100 (41) | 0 (0) | 41 |
| nSMase invert-MO | 95 (40) | 5 (2) | 42 |
| nSMase antisense-MO | 71 (32) | 29 (13) | 45 |
| Uninjected embryos + thalidomide treatment | 5 (2) | 95 (39) | 41 |
| nSMase invert-MO + thalidomide treatment | 13 (5) | 87 (35) | 40 |
| nSMase antisense-MO + thalidomide treatment | 70 (40) | 30 (17) | 57 |
Experimental condition . | High FLK-1 expression, % (no.) . | Low FLK-1 expression, % (no.) . | No. embryos . |
|---|---|---|---|
| Uninjected embryos | 100 (41) | 0 (0) | 41 |
| nSMase invert-MO | 95 (40) | 5 (2) | 42 |
| nSMase antisense-MO | 71 (32) | 29 (13) | 45 |
| Uninjected embryos + thalidomide treatment | 5 (2) | 95 (39) | 41 |
| nSMase invert-MO + thalidomide treatment | 13 (5) | 87 (35) | 40 |
| nSMase antisense-MO + thalidomide treatment | 70 (40) | 30 (17) | 57 |
Whole-mount in situ mRNA hybridization for FLK-1 in trunk blood vessels of embryos was performed at 30 hpf after injection with nSMase antisense- or invert-MO in the presence or absence of 800 μM thalidomide at 8 hpf. The densities of FLK-1 expression showing more than and lower than 75% of average of uninjected control were assessed as high and low expression, respectively.
Ceramide generation through thalidomide-activated nSMase in zebrafish embryos, and restoration of thalidomide-induced antiangiogenic action by antisense-morpholino oligonucleotides (MOs) for nSMase. (A-C) Zebrafish embryos were treated at 75% epiboly stage (8 hpf) with the indicated concentrations of thalidomide in the presence or absence of antisense- or invert-MO for nSMase, and harvested at 18 hours after treatment (26 hpf). Thalidomide-induced increase of ceramide generation through nSMase activity peaked at 12 to 18 hours after treatment (A). Injection of antisense-MO for nSMase into embryos inhibits thalidomide-increased activation of nSMase (B) and ceramide generation (C), but that of invert-MO does not. (B-C) ▪ indicates presence of thalidomide; ▪, absence of thalidomide. The results are obtained from 3 independent experiments. The error bars indicate 1 SD. *Statistical significance of antisense-MO effect as compared with invert-MO effect (P < .01). (D,E) Hematoxylin and eosin staining for the transverse section of antisense-MO–injected 48-hpf embryos shows the restoration of the dorsal artery (marked “A”) and posterior cardinal vein (marked “V”) against thalidomide-induced defects (original magnification, ×150). (F-I) Lateral view of in situ mRNA hybridization for FLK-1 at 30 hpf shows that the injection of antisense-MO rescues thalidomide-inhibited FLK-1 expression, as indicated by a rectangular shape (original magnification, ×40; F,H). The restoration by antisense MO of thalidomide-inhibited FLK-1 expression is more evident by the higher magnification (original magnification, ×63; G,I). The results are representative of each experiment (D-J). noto indicates notochord; A, dorsal artery; V, posterior cardinal vein; hpf, hours after fertilization; MO, morpholino oligonucleotide; nSMase, neutral sphingomylinese. Image acquisition was performed as described for Figure 1.
Ceramide generation through thalidomide-activated nSMase in zebrafish embryos, and restoration of thalidomide-induced antiangiogenic action by antisense-morpholino oligonucleotides (MOs) for nSMase. (A-C) Zebrafish embryos were treated at 75% epiboly stage (8 hpf) with the indicated concentrations of thalidomide in the presence or absence of antisense- or invert-MO for nSMase, and harvested at 18 hours after treatment (26 hpf). Thalidomide-induced increase of ceramide generation through nSMase activity peaked at 12 to 18 hours after treatment (A). Injection of antisense-MO for nSMase into embryos inhibits thalidomide-increased activation of nSMase (B) and ceramide generation (C), but that of invert-MO does not. (B-C) ▪ indicates presence of thalidomide; ▪, absence of thalidomide. The results are obtained from 3 independent experiments. The error bars indicate 1 SD. *Statistical significance of antisense-MO effect as compared with invert-MO effect (P < .01). (D,E) Hematoxylin and eosin staining for the transverse section of antisense-MO–injected 48-hpf embryos shows the restoration of the dorsal artery (marked “A”) and posterior cardinal vein (marked “V”) against thalidomide-induced defects (original magnification, ×150). (F-I) Lateral view of in situ mRNA hybridization for FLK-1 at 30 hpf shows that the injection of antisense-MO rescues thalidomide-inhibited FLK-1 expression, as indicated by a rectangular shape (original magnification, ×40; F,H). The restoration by antisense MO of thalidomide-inhibited FLK-1 expression is more evident by the higher magnification (original magnification, ×63; G,I). The results are representative of each experiment (D-J). noto indicates notochord; A, dorsal artery; V, posterior cardinal vein; hpf, hours after fertilization; MO, morpholino oligonucleotide; nSMase, neutral sphingomylinese. Image acquisition was performed as described for Figure 1.
S1P prevents thalidomide-induced vascular defect by inhibiting ceramide generation through nSMase
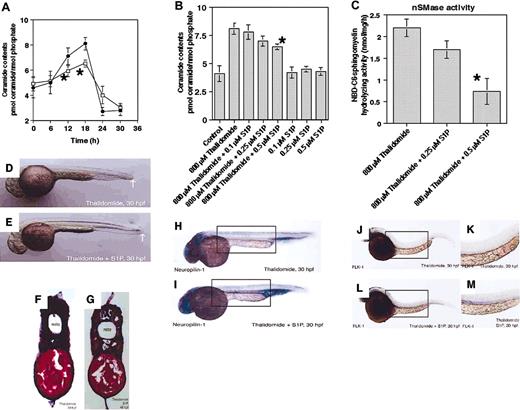
Previous studies showed that S1P protects endothelial cells from C2-ceramide–induced apoptotic cell death.37,38 Therefore, we investigated the involvement of S1P in thalidomide-induced vascular defect in zebrafish embryos. First, ceramide level was measured in zebrafish embryos after S1P was pretreated with or without thalidomide. As shown in Figure 4A, ceramide content-was significantly decreased to 78% and 68% of only thalidomide-treated level, 12 hours and 18 hours, respectively, after preaddition of 0.5 μM S1P. S1P inhibited not only thalidomide-induced ceramide generation, but also nSMase activity in a dose-dependent manner (Figure 4B-C). These results suggest that S1P reduced ceramide generation by inhibiting nSMase in thalidomide-treated embryos. To know whether S1P prevents thalidomide-induced vascular defect, embryos at 60% epiboly stage were pretreated with 0.5 μM S1P for 1 hour before addition of 800 μM thalidomide. The results showed that S1P restored thalidomide-induced shortness in length (Figure 4D-E, white arrow) and vascular endothelial defect in a dose-dependent manner (Figure 5F-G, Table 4). The proangiogenic effect of S1P was further confirmed by a significant restoration of thalidomide-depleted expressions of neuropilin-1 and FLK-1 in the region corresponding to the dorsal artery and posterior cardinal vein at 30 hpf (Figure 4H-M).TBL5
S1P prevents thalidomide-induced antiangiogenic action by inhibition of ceramide generation through nSMase. (A-C) Zebrafish embryos at 60% epiboly stage (7 hpf) were pretreated with the indicated concentrations of S1P for 1 hour before treatment with 800 μM thalidomide, and harvested at the indicated times (A) or 18 hours after treatment (B, C). (A) Thalidomide-induced generation of ceramide (•), peaked 18 hours after treatment, is decreased by pretreatment with S1P (▪). S1P dose-dependently inhibits thalidomide-induced generation of ceramide and activation of nSMase (B,C). The results are obtained from 3 independent experiments. The error bars indicate 1 SD. *Statistically significant inhibition by 0.5 μM S1P of 800 μM thalidomide-induced effects (P < .01). (D,E) Lateral view of a fixed embryo at 30 hpf shows that pretreatment with S1P restores thalidomide-caused shortness of embryo (white arrows). (F,G) Hematoxylin and eosin staining for the transverse section shows that thalidomide-induced defects of the dorsal artery (marked “A”) and vein (marked “V”) are reconstructed by pretreatment with S1P. (H-M) In situ mRNA hybridization was performed for endothelial markers neuropilin-1 (H,I) and FLK-1 (J-M). Thalidomide-caused inhibition of neuropilin-1 and FLK-1 expressions at the large vascular region is restored by pretreatment with S1P, as indicated by boxes (I,L). The restoration by S1P of thalidomide-inhibited FLK-1 expression at large vessels is more evident by the higher magnification (K,M). The results are representative of each experiment. noto indicates notochord; A, dorsal artery; V, posterior cardinal vein; hpf, hours after fertilization. Original magnifications of panels are as follows: D, E, H, I, J, L, ×40; F, G, ×150; and K and M, ×63. Image acquisition was performed as described for Figure 1.
S1P prevents thalidomide-induced antiangiogenic action by inhibition of ceramide generation through nSMase. (A-C) Zebrafish embryos at 60% epiboly stage (7 hpf) were pretreated with the indicated concentrations of S1P for 1 hour before treatment with 800 μM thalidomide, and harvested at the indicated times (A) or 18 hours after treatment (B, C). (A) Thalidomide-induced generation of ceramide (•), peaked 18 hours after treatment, is decreased by pretreatment with S1P (▪). S1P dose-dependently inhibits thalidomide-induced generation of ceramide and activation of nSMase (B,C). The results are obtained from 3 independent experiments. The error bars indicate 1 SD. *Statistically significant inhibition by 0.5 μM S1P of 800 μM thalidomide-induced effects (P < .01). (D,E) Lateral view of a fixed embryo at 30 hpf shows that pretreatment with S1P restores thalidomide-caused shortness of embryo (white arrows). (F,G) Hematoxylin and eosin staining for the transverse section shows that thalidomide-induced defects of the dorsal artery (marked “A”) and vein (marked “V”) are reconstructed by pretreatment with S1P. (H-M) In situ mRNA hybridization was performed for endothelial markers neuropilin-1 (H,I) and FLK-1 (J-M). Thalidomide-caused inhibition of neuropilin-1 and FLK-1 expressions at the large vascular region is restored by pretreatment with S1P, as indicated by boxes (I,L). The restoration by S1P of thalidomide-inhibited FLK-1 expression at large vessels is more evident by the higher magnification (K,M). The results are representative of each experiment. noto indicates notochord; A, dorsal artery; V, posterior cardinal vein; hpf, hours after fertilization. Original magnifications of panels are as follows: D, E, H, I, J, L, ×40; F, G, ×150; and K and M, ×63. Image acquisition was performed as described for Figure 1.
Restoration of thalidomide-induced vascular defect by S1P
Experimental condition . | Dorsal artery presence, % (no.) . | Dorsal artery absence, % (no.) . | No. embryos . |
|---|---|---|---|
| Control | 100 (250) | 0 (0) | 250 |
| 800 μM thalidomide, 0.5 μM | 92 (225) | 8 (20) | 245 |
| S1P treatment | |||
| 800 μM thalidomide, 0.25 μM | 34 (73) | 56 (141) | 214 |
| S1P treatment | |||
| 800 μM thalidomide, 0.1 μM | 0 (211) | 100 (211) | 211 |
| S1P treatment |
Experimental condition . | Dorsal artery presence, % (no.) . | Dorsal artery absence, % (no.) . | No. embryos . |
|---|---|---|---|
| Control | 100 (250) | 0 (0) | 250 |
| 800 μM thalidomide, 0.5 μM | 92 (225) | 8 (20) | 245 |
| S1P treatment | |||
| 800 μM thalidomide, 0.25 μM | 34 (73) | 56 (141) | 214 |
| S1P treatment | |||
| 800 μM thalidomide, 0.1 μM | 0 (211) | 100 (211) | 211 |
| S1P treatment |
Zebrafish embryos were pretreated with various concentrations of S1P for 1 hour before addition of 800 μM thalidomide at 75% epiboly stage (8 hpf), and then the defect of dorsal artery was determined by assessing the loss of blood flow under microscopy at the 48-hpf stage.
S1P prevents thalidomide-induced growth inhibition, increase of ceramide generation through nSMase, and depletion of VEGF receptors in HUVECs
After pretreatment with 0.1 μM S1P 1 hour before addition of 200 μM thalidomide, cell growth, ceramide generation, and nSMase activity were measured in HUVECs. S1P restored not only thalidomide-inhibited HUVEC growth from 73% to 105% of the control MTT assay level, but also returned thalidomide-increased ceramide content (15.2 pmol/nmol phosphate) to the control level (10.8 pmol/nmol phosphate) after 12 hours of treatment (Figure 5A-B). In addition, pretreatment of S1P decreased thalidomide-increased nSMase activity from 165 pmol/mg protein/hour to 109 pmol/mg protein/hour, which is similar to the control level (118 pmol/mg protein/hour) (Figure 5C). These results suggest that S1P inhibits thalidomide-induced increase of ceramide through nSMase in HUVECs as well as zebrafish embryos. We finally investigated whether S1P restores the thalidomide-inhibited expression of VEGF receptors, neuropilin-1, neuropilin-2, and FLK-1 in HUVECs. By RT-PCR analysis, their expression levels were examined with or without S1P in thalidomide- or C2-ceramide–treated HUVECs. As compared with the β-actin mRNA levels, pretreatment with S1P clearly restored thalidomide- or C2-ceramide–induced depletion of mRNA expressions in neuropilin-1, neuropilin-2, and FLK-1 (Figure 5D), suggesting that S1P restores endothelial cell growth by counteracting ceramide-induced depletion of VEGF receptors through inhibition of nSMase in thalidomide-treated HUVECs.
S1P prevents thalidomide-induced growth inhibition, ceramide generation through nSMase, and depletion of VEGF receptor expressions (neuropilin-1, neuropilin-2, and FLK-1) in HUVECs. (A-D) Human umbilical vein endothelial cells (HUVECs) were pretreated without or with 0.1 μM S1P for 1 hour before 200 μM thalidomide, and then harvested at the indicated times (A,B) or 6 hours after thalidomide treatment (C,D). (A-B) ▪ indicates 200 μM thalidomide; ♦, 200 μM thalidomide plus 100 nM S1P; and ○, control. Pretreatment with S1P restores thalidomide-induced inhibition of cell growth (A), generation of ceramide (B), and activation of nSMase (C) to the control levels. S1P also restores thalidomide-inhibited mRNA levels of FLK-1, neuropilin-1, and neuropilin-2 in HUVECs. (D) The expression levels of FLK-1, neuropilin-1, and neuropilin-2 were measured by the RT-PCR–based amplification of mRNA as described in “Materials and methods.” The results are obtained from 3 independent experiments (A-C) or the representative of 3 different experiments (D). The error bars indicate 1 SD. *Statistically significant difference between pretreatment with and without S1P (P < .01).
S1P prevents thalidomide-induced growth inhibition, ceramide generation through nSMase, and depletion of VEGF receptor expressions (neuropilin-1, neuropilin-2, and FLK-1) in HUVECs. (A-D) Human umbilical vein endothelial cells (HUVECs) were pretreated without or with 0.1 μM S1P for 1 hour before 200 μM thalidomide, and then harvested at the indicated times (A,B) or 6 hours after thalidomide treatment (C,D). (A-B) ▪ indicates 200 μM thalidomide; ♦, 200 μM thalidomide plus 100 nM S1P; and ○, control. Pretreatment with S1P restores thalidomide-induced inhibition of cell growth (A), generation of ceramide (B), and activation of nSMase (C) to the control levels. S1P also restores thalidomide-inhibited mRNA levels of FLK-1, neuropilin-1, and neuropilin-2 in HUVECs. (D) The expression levels of FLK-1, neuropilin-1, and neuropilin-2 were measured by the RT-PCR–based amplification of mRNA as described in “Materials and methods.” The results are obtained from 3 independent experiments (A-C) or the representative of 3 different experiments (D). The error bars indicate 1 SD. *Statistically significant difference between pretreatment with and without S1P (P < .01).
Discussion
It is certain that studies knowing the precise molecular mechanism of thalidomide-induced antiangiogenic action are worthwhile for the further development of a novel anticancer agent. There are currently 3 basic approaches to investigate the mechanism of angiogenesis: the first is microcirculatory preparations in animals, the second is vascularization into biocompatible matrix implants, and the third is excision of vascularized tissues.39 Only the first approach includes in vivo assay, and the latter 2 methods require a subsequent in vivo validation. As the in vivo assays are experimentally elaborate and expensive, only chick choriallantoic membrane (CAM) assay has been applied to the vascular biology.40 However, the CAM assay takes time, and the number of compounds that can be tested is limited because of the availability and cost of eggs, whereas assay using zebrafish embryos allows us the quick and inexpensive in vivo evaluation of blood vessels and flow.
We find that thalidomide induces the defects of large artery and vein during the development of zebrafish embryos through nSMase-generated ceramide action. We here treated zebrafish embryos with thalidomide at concentrations of 100 μM to 800 μM (26 μg/mL-206 μg/mL) because 100 μM thalidomide was effective to inhibit angiogenesis in mouse embryoid bodies.41 So far, thalidomide is reported to down-regulate VEGF expression in lung carcinoma cells at 0.6 μg/mL to 6 μg/mL9 and to inhibit retinal epithelial cell proliferation at 50 μg/mL.42 The effective concentrations of thalidomide in zebrafish embryos seem to be higher as compared with the previous in vitro experiments, suggesting the loss of activity by spontaneous metabolism in the water and the requirement of its conversion to active metabolites in zebrafish as well as in the mouse model.43 Synthetic ceramide with a short carbon chain such as C2-ceramide has been employed because of its cell permeability and functional mimicry to physiologic ceramide. Indeed, C2-ceramide induces the identical effect with thalidomide-increased endogenous ceramide in terms of the vascular defect in zebrafish embryos. Recently, C2-ceramide is suggested to exert the effects by increasing long-chain ceramide through activation of SMase. We also find that C2-ceramide increases endogenous ceramide generation in embryo and HUVECs (data not shown). These findings suggest that an increase of endogenous ceramide through nSMase is involved in thalidomide-induced antiangiogenic action.
The simple method for generating a loss-of-function of a particular gene in the zebrafish is usage of antisense morpholino oligonucleotides (MOs) to induce a translational block.36,44 By using this technology, it was examined whether ceramide generation through nSMase is indispensable to thalidomide-induced vascular defect since we succeeded to obtain cDNA for zebrafish magnesium-dependent nSMase by expression cloning method as shown in Table 2. The results clearly show that thalidomide-induced ceramide generation through nSMase activation and vascular defect are inhibited by the injection with antisense MOs for nSMase. Exogenous C2-ceramide mimics, and inhibition of endogenous ceramide generation by nSMase antisense-MO prevents, thalidomide-induced vascular defect, and it is evident that ceramide generation through nSMase plays a critical role in thalidomide-induced antiangiogenic action in zebrafish embryos.
Many compounds such as PTK787/ZK222 584, SU5416, and TNP470 are shown to exert antiangiogenic effects through inactivating VEGF function in the zebrafish model.45,46 In addition, the loss of VEGF caused by its antisense MO inhibits VEGF-dependent angiogenesis in zebrafish embryos,47 and thalidomide is reported to inhibit VEGF-induced endothelial cell growth48 probably by inhibiting the extracellular secretion of VEGF.49 In contrast to VEGF, we here investigate whether VEGF receptors such as FLK-1 and neuropilin-1 are involved in thalidomide-induced vascular defect.50 It is shown that thalidomide or C2-ceramide markedly down-regulates the expression of FLK-1 and neurophilin-1 whereas nSMase antisense-MO prevents thalidomide-induced vascular defect by restoring their expression in zebrafish embryos. Thus, it is suggested that thalidomide-induced antiangiogenic action is based on ceramide-induced depletion of VEGF receptors.
S1P is a biologically active lipid that evokes a variety of cellular responses, including cell proliferation, antiapoptosis, and neurite retraction.51-53 The actions of S1P are mediated via its specific interaction with cell-surface EDG receptors. Recently, 5 mammalian and 2 zebrafish EDG receptors have been cloned as S1P receptors.54-56 In endothelial cells, antisense for EDG-1 blocks the inhibition by S1P of C2-ceramide–induced apoptosis,23 and inhibits S1P-induced survival of endothelial cells.57 Interestingly, when S1P signaling through EDG-1 is blocked in knockout mice, the morphogenesis of limb during development is severely affected.58 This limb defect by the blockade of S1P signal is similar to thalidomide-induced morphologic deformity in the human fetus. We here show that S1P decreases ceramide content by inhibiting nSMase and restores thalidomide-induced vascular defect in the zebrafish embryo by increasing the expression of neuropilin-1 and FLK-1. It should be clarified in the future which EDG receptor is most involved in the inhibitory effect of S1P against thalidomide-induced vascular defect.
Ceramide induces apoptotic cell death in HUVECs, whereas S1P acts as a survival factor. It is reported that S1P counteracted the proapoptotic effects of ceramide through protein kinase C,52 and inhibited apoptosis in macrophages by depressing aSMase.59 Indeed, we find that S1P restores ceramide-mediated cell death in thalidomide-treated HUVECs. However, it remains unclear whether ceramide and S1P exclusively counteract in angiogenesis. Since thalidomide-induced death of zebrafish embryos caused by loss of blood flow is restored by S1P through preventing ceramide-mediated vascular defect, it is suggested that S1P and ceramide exclusively regulate not only embryonic death but also angiogenesis. As a mechanism by which S1P rescues thalidomide-induced vascular defect, we show the inhibition of ceramide generation through nSMase and the restoration of depleted VEGF receptors by S1P. Thus, we here suggest that the balance between ceramide and S1P-mediated signal regulates the angiogenesis through VEGF receptors Flk-1 and neuropilin-1.
Although the in vivo angiogenesis is a highly complex and coordinated process, requiring a diverse array of signaling steps, there is no doubt that VEGFs and their receptors are key molecules to regulate vessel growth and maturation. We here show that ceramide and S1P exclusively play a role in thalidomide-induced vascular defect and endothelial cell death through the regulation of VEGF receptors. These findings may not only lead to the quest for a better and complete understanding of in vivo thalidomide-induced antiangiogenesis, but also shed light on the development of a novel anticancer agent based on the counteracting function of sphingolipids, ceramide, and S1P in angiogenesis.
Prepublished online as Blood First Edition Paper, March 1, 2005; DOI 10.1182/blood-2004-09-3679.
Supported in part by grants to T.O. from the Japan Society of the Promotion of Science and the Ministry of Education, Culture, Sports, Science and Technology of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We appreciate Ms Hitomi Nakabayashi for exclusive technical support for preparing zebrafish samples.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal