Macrophage galactose-type C-type lectins (MGLs), which were recently named CD301, have 2 homologues in mice: MGL1 and MGL2. MGLs are expressed on macrophages and immature dendritic cells. The persistent presence of granulation tissue induced by a protein antigen was observed in wild-type mice but not in mice lacking an endogenous, macrophage-specific, galactose-type calcium-type lectin 1 (MGL1) in an air pouch model. The anti-MGL1 antibody suppressed the granulation tissue formation in wild-type mice. A large number of cells, present only in the pouch of MGL1-deficient mice, were not myeloid or lymphoid lineage cells and the number significantly declined after administration of interleukin 1 α (IL-1α) into the pouch of MGL1-deficient mice. Furthermore, granulation tissue was restored by this treatment and the cells obtained from the pouch of MGL1-deficient mice were incorporated into the granulation tissue when injected with IL-1α. Taken together, MGL1 expressed on a specific subpopulation of macrophages that secrete IL-1α was proposed to regulate specific cellular interactions crucial to granulation tissue formation.
Introduction
Granulation tissue formation is a consequence of chronic cellular immune responses associated with persistent infection or autoimmunity. Granulation tissue and chronic tissue fibrosis are formed and maintained by macrophages and other myelomonocytic cells1-4 that produce cytokines such as interleukin 1 α (IL-1α). These cytokines are thought to increase fibroblast proliferation and collagen production which, in turn, induce tissue remodeling.5-9 Although it is widely noted that fibrosis constitutes an important final stage of the chronic cellular immune response, and the contribution of T cells, macrophages, and fibroblasts has been documented,10 the molecular mechanisms governing the interactions of these cells are still obscure.
Macrophages and related cells are the predominant arm of cellular immunity involved with sensitization, inflammation, and tissue remodeling through cellular interactions and production of soluble factors.11,12 The interactions of macrophages with extracellular milieu are mediated by a variety of cell surface recognition molecules including lectins, the carbohydrate-recognition proteins. Lectins expressed on these cells include galectins, particularly galectin-3; siglecs such as sialoadhesin; and many calcium-type (C-type) lectins.13,14 These lectins are known to be involved in endocytosis,15,16 cell adhesion,17 and cellular trafficking.18,19
The macrophage galactose-type calcium-type lectins (MGLs; CD301) constitute a unique class of C-type lectins because of their specificity for galactose and its structural homologues.20,21 In mice, we have recently found that 2 closely related MGLs, termed MGL1 and MGL2 (CD301a and CD301b), have different carbohydrate specificity, though their distinct biologic roles are unknown.21 MGLs/CD301 are type II transmembrane glycoproteins and are expressed on macrophages and related cells of myeloid origins, particularly immature dendritic cells (DCs).22 Using a monoclonal antibody (mAb) reactive to both MGL1 and MGL2, these lectins were shown to be expressed on connective tissue macrophages, such as those in dermis, subdermic regions of skin, lamina propria in the gastrointestinal tract, bronchiole-associated connective tissues, perivascular regions in a variety of organs and tissues, and lymphoid organs.23-25 Bone marrow–derived DCs and thioglycollate-induced peritoneal exudate cells were previously shown to express both MGL1 and MGL2.21,22 However, the distribution of MGL1-positive cells and MGL1-negative cells within several organs seems to be distinct. For example, MGL1-positive/MGL2-positive cells and MGL1-positive/MGL2-negative cells but not MGL1-negative/MGL2-positive cells were present in dermis. MGL1, having a tyrosine internalization motif, was previously shown to be involved in endocytosis of galactose-terminated glycoconjugates in vitro. However, homozygous MGL1-deficient mice seemed to be healthy and immunologically competent as long as they were maintained under specific pathogen-free conditions.21,26 The possibility that this is due to compensation by MGL2 remains to be elucidated.
MGL1/2-positive cells (reactive with mAb LOM-14) have been shown to be involved in the sensitization phase of antigen-dependent contact hypersensitivity.27-29 Upon epicutaneous sensitization with a hapten, we found that trafficking of MGL1/2-positive macrophages from the topical site of the hapten application to the draining lymph nodes occurred. The extent of the macrophage trafficking appeared to be positively correlated to the efficiency of the sensitization.27 Furthermore, sialoadhesin (siglec-1) has recently been identified as an endogenous ligand of MGL1 in lymph nodes.30 Persistent antigen-specific stimulation within the dorsal air pouch also results in granulation tissue formation and the accumulation of MGL1/2-positive cells in the remodeled tissue.31 Staining with antibodies specific for CD31, a marker for vascular endothelial cells, strongly suggested the presence of chronic granulation tissue. In this experimental model, MGL1/2-positive cells apparently produced and secreted IL-1α and acted through this cytokine.
In the present report, we provide direct evidence that MGL1 is causally involved in granulation tissue formation associated with an antigen-specific cellular immune response. Using an air pouch model, we show that MGL1-deficient mice or mice injected with anti-MGL1 antibody exhibit substantially reduced granulation tissue formation. Furthermore, the role of IL-1α was established by blocking antibodies, or the partial reconstitution of granulation tissue formation in MGL1-deficient mice resulting from the injection of IL-1α. The most likely interpretation of these results is that tissue localization of important inflammatory cells is MGL1 dependent, and, as a consequence, the local delivery of IL-1α was reduced. These observations should lead to an understanding of the molecular mechanism of granulation tissue formation that mimics the late stages of persistent infections and systemic sclerosis.
Materials and methods
Mice
MGL1-deficient mice of C57BL/6 background were used.26 Heterozygotes were bred in the animal facility of the Graduate School of Pharmaceutical Sciences of the University of Tokyo to obtain MGL1-deficient mice and their wild-type control. Nine backcross generations were carried out to C57BL/6. All animal experiments were performed in accordance with the guidelines of the Bioscience Committee of the University of Tokyo and were approved by the Animal Care and Use Committee of the Graduate School of Pharmaceutical Sciences of the University of Tokyo.
Antigen and antibody
Azobenzene arsonate–conjugated acetylated bovine serum albumin (ABA-AcBSA) was prepared as described.32 A rat mAb LOM-14 reactive to both MGL1 and MGL2 and a rat mAb LOM-8.7 reactive only to MGL1 have been described previously.24 Preparation and characterization of rat mAbs specific for MGL2 and URA-1 will be described elsewhere (S. Aida, K. Denda-Nagai, and T.I., manuscript in preparation).
Antigen-induced granulation tissue formation in mouse skin
Mice (wild-type or MGL1-deficient) were subcutaneously immunized with 200 μg ABA-AcBSA emulsified 1:1 in complete Freund adjuvant (CFA; Difco, Detroit, MI) as described previously.31 In brief, mice preimmunized with ABA-AcBSA were repeatedly challenged in dorsal air pouches. Skin samples covering the air pouch were collected on days 4, 11, and 18, and concurrently, serum samples were taken by tail phlebotemy. In some experiments, cells in the pouch fluid were collected on days 1 to 5 and were analyzed for the expression of cell surface markers by flow cytometry, as described in “Flow cytometric analysis.”
Humoral immune response
The total immunoglobulin G (IgG) concentration in serum was determined with the mouse IgG enzyme-linked immunosorbent assay (ELISA) Quantitation Kit (Bethyl Laboratories, Montgomery, TX) according to the supplier's instructions. To determine antigen-specific IgG levels in the serum samples, an ELISA was performed as follows: ABA-AcBSA solutions (100 μg/mL, 100 μL/well) were added and incubated overnight. The wells were blocked with 1% BSA dissolved in 50 mM Tris/HCl buffer (pH 8.0) containing 0.14 M NaCl (Tris-buffered saline [TBS]) before adding serum samples serially diluted in TBS containing 1% BSA and 0.05% Tween 20 (TBS-BSA-Tween). After incubation, horseradish peroxidase (HRP)–conjugated goat anti–mouse IgG-Fc (Bethyl) diluted in TBS-BSA-Tween (1:50 000) was added and antigen-bound IgG was detected by incubation with a substrate solution (3,3′,5,5′-tetramethyl benzidene [TMB]; Kirkegaard & Perry, Gaithersburg, MD). After addition of 50 μL of 2 M H2SO4 to stop reactions, absorbance was determined at 450 nm.
Antigen-specific proliferation of splenocytes
Mice (MGL1-deficient or wild-type mice) were immunized by intradermal injection of ABA-AcBSA in CFA at 200 μg/mouse. Three weeks later, the splenocytes were collected by collagenase D digestion and were cultured in 96-well microculture plates, at a density of 2 × 105 cells/well, in 200 μL RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), in the presence or absence of various concentrations of ABA-AcBSA (3 μg/mL-300 μg/mL) for 5 days. To assess the lymphocyte proliferation, cultures were pulsed with [3H]-thymidine (Amersham, Piscataway, NJ) for the last 16 hours and radioactivity incorporated into insoluble fraction in 5% trichloroacetic acid was measured.
Treatment of anti-MGL1 or anti–IL-1α antibodies in vivo
One hundred microliter solutions of anti–IL-1α mAb (Genzyme, Cambridge, MA; 5 μg/mouse), anti-MGL1 mAb LOM-8.7 (25 μg/mouse), rat IgG (Caltag, Burlingame, CA; 5 μg/mouse), or saline were injected into the air pouch on days 5, 6, and 7. Skin samples from the air pouch were obtained on days 11 and 18, and were analyzed for the thickness of granulation tissue. To examine the number of cells in the granulation tissue in the absence or presence of anti-MGL1 antibody, subdermal granulation tissue that formed after the challenge at the site of the air pouch was peeled from the skin. The tissue samples were cut with scissors into 2-mm cubes, and the fragments were incubated in 0.5% collagenase. Cells released from the tissue were washed and resuspended in phosphate-buffered saline (PBS), and then the number of cells was counted.
Treatment of recombinant mouse IL-1α in vivo
Solutions of recombinant mouse IL-1α (50 μg/mL; 100 μL/mouse) or PBS were injected into the air pouch on day 0. Skin samples from the air pouch were obtained on day 4 and were analyzed for the thickness of granulation tissue. Cells in the pouch were collected on days 3 and 4, washed by centrifugation in saline, and analyzed for their cell surface markers by flow cytometry. In other experiments, 100-μL solutions of IL-1α (5 μg/mouse) or PBS were injected into the air pouch on day 5. Skin samples from the air pouch were obtained on day 11 and analyzed for the thickness of granulation tissue.
In other experiments, the migrated cells from MGL1-deficient mice on day 3 were collected and CD45-negative cells were isolated by magnetic activated cell sorting (MACS) using mAb rat anti–mouse CD45 (30-F11, rat IgG2a; BD Pharmingen, San Diego, CA) and goat anti–rat κ light chain microbeads (Miltenyi, Bergisch Gladbach, Germany) according to the supplier's instructions. The isolated cells were labeled with PKH26 (Sigma Chemical, St Louis, MO) and were injected with 100-μL solutions of IL-1α (5 μg/mouse) or PBS into the air pouch on day 3. Skin samples were obtained on day 6 and were analyzed by immunohistochemistry.
Flow cytometric analysis
Cells (1 × 106) were incubated for 60 minutes on ice with either: biotin rat anti–mouse MGL1/2 mAb LOM-14 (1 μg/mL); biotin-conjugated mAb rat anti–mouse CD11b (M1/70.15, Caltag; 1 μg/mL); fluorescein isothiocyanate (FITC)–conjugated mAb rat anti–mouse CD45 (Pharmingen; 1 μg/mL); F4/80 (Caltag; 1 μg/mL); or moma-2 (1 μg/mL). As controls, purified rat IgG2b (Zymed Laboratories, South San Francisco, CA; 1 μg/mL), biotin-conjugated rat IgG (eBiosciences, San Diego, CA; 1 μg/mL), or FITC-conjugated rat IgG (eBiosciences; 1 μg/mL) were used. After being washed twice with PBS containing 0.1% BSA, 0.1% NaN3 (F-PBS), cells were incubated with FITC-conjugated mAb anti–rat κ and λ light chains (Sigma; 1:100 dilution) or FITC-conjugated streptavidin (Zymed; 1:100 dilution) for 30 minutes on ice. The cells were then treated with propidium iodide (PI; 5 μg/mL in F-PBS) and were analyzed on a flow cytometer (EPICS XL; Beckman Coulter, Fullerton, CA) by collecting data only from PI-unstained cells.
Immunohistochemistry
Cells expressing MGLs were immunohistochemically detected using mAb LOM-14 (1 μg/mL), mAb LOM-8.7 (1 μg/mL), or mAb URA-1 (1 μg/mL). The binding of the mAbs was detected by a biotinylated mouse mAb to rat κ and λ chains and alkaline phosphatase–streptavidin as described previously.25,33 As a negative control, the sections were incubated with rat IgG2b in place of the primary mAb. Some sections were stained with hematoxylin and eosin.
For multicolor immunofluorescence, sections were fixed with acetone and then incubated with the fibroblast marker ER-TR7 for 1 hour at 20°C. The binding of the ER-TR7 was detected by incubation with FITC-conjugated anti–rat IgG (Pharmingen). After each incubation or fixation, the sections were gently washed twice in PBS, then mounted in PermaFlow (Shandon, CA), and observed under a confocal microscope (MRC-1024; Bio-Rad, Herts, United Kingdom).
Statistical analysis
The Dunnet multiple comparison test was used to assess the statistical significance of differences after a one-way analysis of variance; a value of P less than.05 was considered significant.
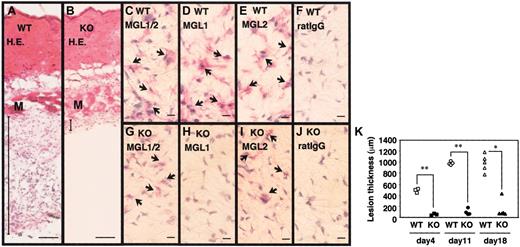
Lack of granulation tissue formation in MGL1-deficient mice. (A-J) Samples of inflamed skin covering the dorsal air pouch were collected on day 4 from wild-type (WT) or MGL1-deficient (KO) mice. Frozen sections were prepared and stained with hematoxylin and eosin (H.E.), with mAb LOM-14 that is reactive with both MGL1 and MGL2, with mAb LOM-8.7 that is reactive only with MGL1, and with mAb URA-1 that is reactive with MGL2, or mouse IgG. The binding of mAbs was immunohistochemically detected using biotin anti–rat κ/λ mAb plus alkaline phosphatase-streptavidin. (K) Specimens of inflamed skin covering the dorsal air pouch were collected on days 4, 11, and 18. The thickness of the granulation tissue (ordinate) was microscopically determined by measuring the hypodermis region between the muscle fiber layer and the inner surface of the air pouch. Each symbol represents the thickness of an individual wild-type mouse (open symbols) or MGL1-deficient mouse (filled symbols). Wild-type mice developed granulation tissues at the site of chronic antigenic stimulation, whereas MGL1-deficient mice did not. M represents muscle fiber layer. Bars represent 100 μm (A,B) or 10 μm (D-J). Arrows indicate positively stained cells with the mAbs. *P < .05, **P < .001.
Lack of granulation tissue formation in MGL1-deficient mice. (A-J) Samples of inflamed skin covering the dorsal air pouch were collected on day 4 from wild-type (WT) or MGL1-deficient (KO) mice. Frozen sections were prepared and stained with hematoxylin and eosin (H.E.), with mAb LOM-14 that is reactive with both MGL1 and MGL2, with mAb LOM-8.7 that is reactive only with MGL1, and with mAb URA-1 that is reactive with MGL2, or mouse IgG. The binding of mAbs was immunohistochemically detected using biotin anti–rat κ/λ mAb plus alkaline phosphatase-streptavidin. (K) Specimens of inflamed skin covering the dorsal air pouch were collected on days 4, 11, and 18. The thickness of the granulation tissue (ordinate) was microscopically determined by measuring the hypodermis region between the muscle fiber layer and the inner surface of the air pouch. Each symbol represents the thickness of an individual wild-type mouse (open symbols) or MGL1-deficient mouse (filled symbols). Wild-type mice developed granulation tissues at the site of chronic antigenic stimulation, whereas MGL1-deficient mice did not. M represents muscle fiber layer. Bars represent 100 μm (A,B) or 10 μm (D-J). Arrows indicate positively stained cells with the mAbs. *P < .05, **P < .001.
Results
Absence of antigen-induced granulation tissue formation in MGL1-deficient mice
Sections were prepared from skin samples derived from the exterior of the air pouch after the antigenic challenge. The sections were histologically compared between wild-type and MGL1-deficient mice. Representative results of the skin obtained from mice on day 4 are shown in Figure 1A-B. Development of granulation tissues in the subdermal areas was evident in wild-type mice, whereas the formation of such tissue was not observed in MGL1-deficient mice. Similar results were obtained with skin samples prepared at days 11 and 18 (data not shown). The thickness between the muscle fiber layer and the inner surface of the air pouch was microscopically determined as described.31 Nine arbitrarily selected fields in 5 sections independently prepared from each mouse were used for each measurement. The mean values of 45 measurements for an individual mouse are plotted in Figure 1K. On day 4, the presence of granulation tissue was evident in wild-type mice but not in MGL1-deficient mice. The thickness of the granulation tissue increased after the second challenge and persisted in wild-type mice. Formation of the granulation tissue was not observed in MGL1-deficient mice even after the second antigenic challenge, except for one mouse that produced a moderate formation of the tissue on day 18. Therefore, we concluded that formation of granulation tissue was severely impaired in MGL1-deficient mice.
The absence of MGL1 in the MGL1-deficient mice was confirmed in the inflamed skin by immunohistochemistry (Figure 1C-J). Cells recognized by mAb LOM-14 (MGL1 and MGL2 cross-reactive) were present in the dermis of wild-type and MGL1-deficient mice. In contrast, cells recognized by mAb LOM-8.7 (MGL1-specific) were seen only in wild-type mice and were absent from MGL1-deficient mice. The results indicated the absence of MGL1 proteins but the presence of related protein MGL2 in the dermis of MGL1-deficient mice, which was also shown by an anti-MGL2 mAb URA-1. Cells recognized by mAb LOM-14 and mAb LOM-8.7 were also observed within the granulation tissue in the wild-type mice. The results strongly suggest that MGL1 is required for granulation tissue formation induced by repeated local antigenic stimulation.
Characterization of cells in the air pouch fluid
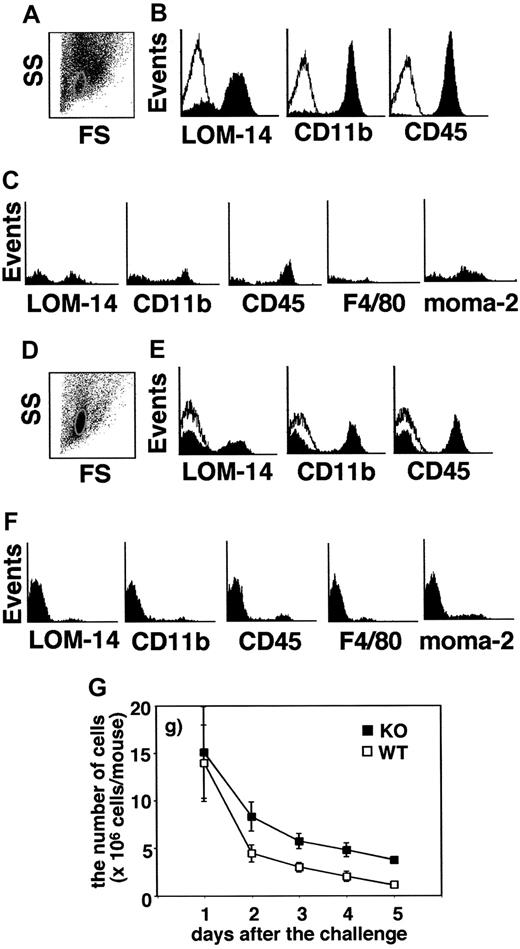
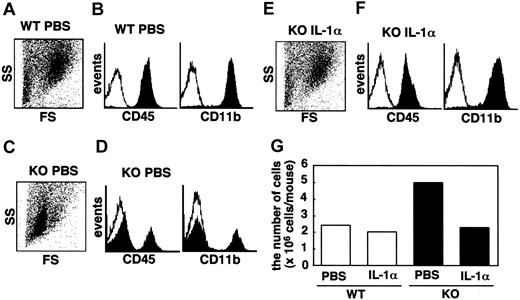
Cells in the pouch fluid were collected from MGL1-deficient and wild-type mice on days 1 to 5, and the numbers of the cells were compared (Figure 2G). On day 1, similar numbers of cells were obtained between wild-type and MGL1-deficient mice. The number of cells gradually decreased, though the decrease was slower in MGL1-deficient mice than in wild-type mice. To characterize these cells, flow cytometric analysis was performed. The predominant population of the cells recovered from the air pouch on day 1 was CD11b-positive/CD45-positive/Ly6G-positive/major histocompatibility complex (MHC) class II–negative in both MGL1-deficient and wild-type mice (data not shown). Thus, the cells that migrated into this compartment on day 1 mainly consisted of neutrophils. There was no difference in the cell numbers between MGL1-deficient and wild-type mice. On days 3 to 5, the cells in wild-type mice expressed CD45 (Figure 2B on day 4) or CD11b (Figure 2B on day 4), and a portion of the cells displayed MGL1/2 (Figure 2B on day 4), moma-2, F4/80, Ly6G, or MHC class II, but not CD11c. We concluded that the cells that migrated into the pouch during this period in wild-type mice consisted of a mixture of macrophages, neutrophils, and leukocytes. In the case of the cells from MGL1-deficient mice, they apparently contained macrophages, neutrophils, and other leukocytes, yet there were cells that were hardly reactive with known markers of cells of myeloid origins (Figure 2E). In the scatter profiles, cells smaller in size than leukocytes were prominent in MGL1-deficient mice (Figure 2A,D). These cells, which were gated in Figure 2D, did not express MGL1/2, CD11b, CD45, F4/80, moma-2 (Figure 2F), Ly6G, MHC class II, or CD3 (data not shown). Cells gated to the same area from wild-type mice, though the number was small, were also examined. As shown in Figure 2C, there were cells expressing CD11b/CD45/MGL1/2. Interestingly, there were cells that were not reactive with any of the antibodies used in both MGL1-deficient and wild-type mice. While CD45 is a common leukocyte antigen, these unstained cells do not seem to correspond to leukocytes or lymphocytes. The proportion of these unstained cells was significantly greater in MGL1-deficient mice than in wild-type mice. CD3-positive T cells were observed on days 3 and 4 in cells from MGL1-deficient and wild-type mice (data not shown) and T cells were supposed to be involved in the formation of granulation tissue since the process was antigen-specific.
Effects of in vivo treatment with anti-MGL1 mAb LOM-8.7 on the formation of granulation tissue in chronic phase
To examine the roles of MGL1 in the granulation tissue, an antibody against MGL1 was repeatedly injected into the air pouch during the secondary antigenic challenge in wild-type mice. As shown in Figure 3A-E, antibody treatment caused formation of granulation tissue to be blocked by approximately 40%. By histologic analysis, areas close to the inner surface of the air pouch seemed to be impaired. Furthermore, the mass of the granulation tissues was significantly thinner in the pouch treated with anti-MGL1 mAb LOM-8.7 than in the pouch treated with saline or control IgG, as observed on days 11 and 18 (Figure 3F). The granulation tissue was removed and the number of cells recovered from the tissue by collagenase digestion was compared. On day 11, 3.3 × 106 and 1.9 × 106 cells per mouse were obtained from the control IgG-treated and anti-MGL1–treated groups, respectively. On day 18, the figures were 4.5 × 106 and 2.7 × 106, respectively. These data confirmed that the number of cells in the granulation tissue was reduced on treatment with anti-MGL1 antibody.
Characterization of the cells in the pouch fluid. The cells in the pouch fluid were collected from both MGL1-deficient (closed square in G) and wild-type (open square in G) mice on days 1 to 5, and the number of cells was counted (G). Using the cells on day 4 from both wild-type (A-C) and MGL1-deficient mice (D-F), the expression of MGL1/2, CD11b, CD45, F4/80, or moma-2 was analyzed by flow cytometry. Flow cytometric scatter profiles of the cells are shown in panels A and D (SS, side scatter; FS, forward scatter). The expressions of MGL1/2, CD11b, or CD45 on the cells in panel A or D are shown in panels B and E. Open histograms in panels B and E represent staining with control mAb. The expressions of MGL1/2, CD11b, CD45, F4/80, or moma-2 on cells in the subpopulation circled in panel A (wild-type) and panel D (MGL1-deficient) are shown in panel C and panel F, respectively. The number of cells recovered from the pouch fluid of MGL1-deficient mice was greater than that recovered from wild-type mice. Furthermore, there was a predominant subpopulation having a unique cell surface phenotype of CD11b-negative/CD45-negative/MGL1/2-negative/F4/80-negative/moma-2–negative in MGL1-deficient mice.
Characterization of the cells in the pouch fluid. The cells in the pouch fluid were collected from both MGL1-deficient (closed square in G) and wild-type (open square in G) mice on days 1 to 5, and the number of cells was counted (G). Using the cells on day 4 from both wild-type (A-C) and MGL1-deficient mice (D-F), the expression of MGL1/2, CD11b, CD45, F4/80, or moma-2 was analyzed by flow cytometry. Flow cytometric scatter profiles of the cells are shown in panels A and D (SS, side scatter; FS, forward scatter). The expressions of MGL1/2, CD11b, or CD45 on the cells in panel A or D are shown in panels B and E. Open histograms in panels B and E represent staining with control mAb. The expressions of MGL1/2, CD11b, CD45, F4/80, or moma-2 on cells in the subpopulation circled in panel A (wild-type) and panel D (MGL1-deficient) are shown in panel C and panel F, respectively. The number of cells recovered from the pouch fluid of MGL1-deficient mice was greater than that recovered from wild-type mice. Furthermore, there was a predominant subpopulation having a unique cell surface phenotype of CD11b-negative/CD45-negative/MGL1/2-negative/F4/80-negative/moma-2–negative in MGL1-deficient mice.
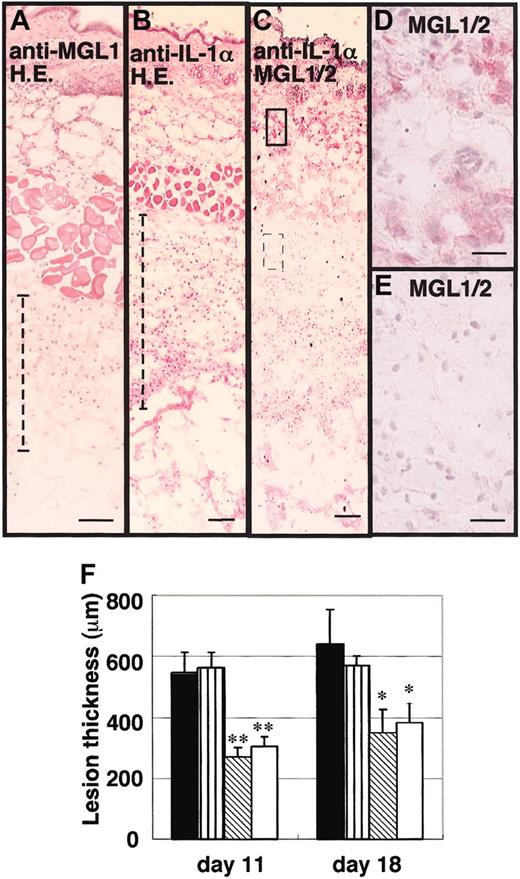
Effects of anti–IL-1α Ab and anti-MGL1 mAb LOM-8.7 on the formation of granulation tissue after the second antigenic challenge in the air pouch. An antigenic challenge was performed in the air pouch on days 0 and 5. Anti–IL-1α mAb, anti-MGL1 mAb LOM-8.7, or saline was subsequently injected into the air pouch on days 5, 6, and 7. Skin samples were collected on day 18 and frozen sections were prepared. (A-E) Tissue stained with hematoxylin and eosin from mAb LOM-8.7–treated mice (A), hematoxylin and eosin–stained tissue obtained from anti–IL-1α–treated mice (B), immunohistochemical staining profiles with mAb LOM-14 (anti-MGL1/2) are shown (C-E). A boxed area and a dotted boxed area in panel C are shown at higher magnifications in D and E, respectively. Very similar profiles were obtained from samples prepared on day 11 (data not shown). Scale bars represent 100 μm (A-C) and 10 μm (D-E). (F) Thickness of the granulation tissue as indicated with dotted lines in panels A and B is shown (ordinate) on days 11 and 18. ▪ represents saline-treated mice; ▥, control rat IgG instead of mAb; ▧, anti-IL-1α–treated mice; and □, anti-MGL1 mAb LOM 8.7. Mean ± standard error (SE; n = 4). Treatment with anti–IL-1α mAb significantly inhibited the formation of granulation tissues as well as the infiltration of MGL1/2-positive cells in the area far from the inner surface of the air pouch. Resident MGL1/2-positive cells in the dermis were still observed. *P < .05, **P < .01.
Effects of anti–IL-1α Ab and anti-MGL1 mAb LOM-8.7 on the formation of granulation tissue after the second antigenic challenge in the air pouch. An antigenic challenge was performed in the air pouch on days 0 and 5. Anti–IL-1α mAb, anti-MGL1 mAb LOM-8.7, or saline was subsequently injected into the air pouch on days 5, 6, and 7. Skin samples were collected on day 18 and frozen sections were prepared. (A-E) Tissue stained with hematoxylin and eosin from mAb LOM-8.7–treated mice (A), hematoxylin and eosin–stained tissue obtained from anti–IL-1α–treated mice (B), immunohistochemical staining profiles with mAb LOM-14 (anti-MGL1/2) are shown (C-E). A boxed area and a dotted boxed area in panel C are shown at higher magnifications in D and E, respectively. Very similar profiles were obtained from samples prepared on day 11 (data not shown). Scale bars represent 100 μm (A-C) and 10 μm (D-E). (F) Thickness of the granulation tissue as indicated with dotted lines in panels A and B is shown (ordinate) on days 11 and 18. ▪ represents saline-treated mice; ▥, control rat IgG instead of mAb; ▧, anti-IL-1α–treated mice; and □, anti-MGL1 mAb LOM 8.7. Mean ± standard error (SE; n = 4). Treatment with anti–IL-1α mAb significantly inhibited the formation of granulation tissues as well as the infiltration of MGL1/2-positive cells in the area far from the inner surface of the air pouch. Resident MGL1/2-positive cells in the dermis were still observed. *P < .05, **P < .01.
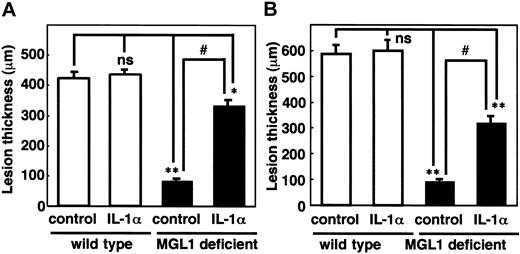
Effects of IL-1α on the formation of antigen-induced granulation tissue in the air pouch. (A) An antigen challenge was performed in the air pouch and recombinant murine IL-1α (5 μg/mouse) was injected into the pouch on day 0. (B) Skin samples from the air pouch were collected on day 4 (A) and day 11 (B) and frozen sections were prepared. The thickness of the granulation tissue is shown. □ indicates wild-type mice; ▪, MGL1-deficient mice. Mean plus or minus SE (n = 3). Treatment with IL-1α induced the formation of the granulation tissues at transient and chronic phases. *P < .05, **P < .001. ns indicates not significant.
Effects of IL-1α on the formation of antigen-induced granulation tissue in the air pouch. (A) An antigen challenge was performed in the air pouch and recombinant murine IL-1α (5 μg/mouse) was injected into the pouch on day 0. (B) Skin samples from the air pouch were collected on day 4 (A) and day 11 (B) and frozen sections were prepared. The thickness of the granulation tissue is shown. □ indicates wild-type mice; ▪, MGL1-deficient mice. Mean plus or minus SE (n = 3). Treatment with IL-1α induced the formation of the granulation tissues at transient and chronic phases. *P < .05, **P < .001. ns indicates not significant.
Effects of in vivo treatment with anti–IL-1α mAb on the formation of granulation tissue in the chronic phase
The cytokine profile of isolated MGL1/2-positive cells from the granulation tissue previously suggested that IL-1α was produced and secreted from MGL-positive cells.31 To study the biologic roles of IL-1α locally produced by MGL1/2-positive cells, an antibody against IL-1α was repeatedly injected into the air pouch during the secondary antigenic challenge. Similar to the anti-MGL1 mAb treatment, the thickness of the granulation tissues was significantly less in the air pouch of mice treated with anti–IL-1α mAb than was the case with those treated with saline, as observed on days 11 and 18 (Figure 3). Immunohistochemical data indicated that MGL1/2-positive cells shown by the binding of mAb LOM-14 almost disappeared from an area distant from the inner surface of the air pouch. In contrast, MGL1/2-positive cells were still observed in the dermis after antibody treatment. In contrast, the number of CD11b-positive cells in the tissue did not decrease after treatment with anti–IL-1α (data not shown). These results suggested that IL-1α plays a significant role in the accumulation of MGL1/2-positive cells, particularly in the chronic phases.
Effects of in vivo treatment with recombinant mouse IL-1α on the formation of granulation tissue
To confirm the involvement of IL-1α in the impairment of the antigen-induced tissue formation in MGL1-deficient mice, recombinant mouse IL-1α was injected into the air pouch on day 0 of the antigenic challenge. Development of granulation tissue was observed by IL-1α treatment in MGL1-deficient mice (71.1% of the wild-type mice), whereas IL-1α did not show any effects in wild-type mice (Figure 4A). In other experiments, recombinant mouse IL-1α was injected along with the secondary antigenic challenge on day 5 in order to confirm the effects of IL-1α on tissue formation in the chronic phase. As shown in Figure 4B, granulation tissue formation in a chronic phase was also induced and the recovery was calculated to be 56.5%. There was no morphologic difference in the granulation tissue between wild-type and MGL1-deficient mice (data not shown). Thus, the effect of MGL1-deficient status on antigen-induced granulation tissue formation was likely to be mediated by the local absence of IL-1α.
Effects of IL-1α on the cells recovered from the pouch. An antigenic challenge was performed and recombinant murine IL-1α (5 μg/mouse) or PBS was injected into the air pouch of MGL1-deficient or wild-type mice on day 0. The cells in the pouch fluid were collected from wild-type mice that were treated with PBS (A-B) and MGL1-deficient mice treated with PBS (C-D) or with IL-1α (E-F) on day 3. To examine the effect of IL-1α, the expression of CD11b or CD45 on these cells was analyzed by flow cytometry (B,D,F). Flow cytometric scatter profiles of these cells are also shown (A,C,E). Open histograms in panels B, D, and F represent the staining with control mAb. The number of cells recovered from the pouch was counted and plotted in panel G. The number of cells in the pouch fluid obtained from MGL1-deficient mice was greater than that from wild-type mice or that from MGL1-deficient mice injected with IL-1α. Injection of IL-1α also reduced the number of CD11b-negative/CD45-negative subpopulations in the air pouch of MGL1-deficient mice to a level similar to that of wild-type mice. Similar data were obtained on day 4.
Effects of IL-1α on the cells recovered from the pouch. An antigenic challenge was performed and recombinant murine IL-1α (5 μg/mouse) or PBS was injected into the air pouch of MGL1-deficient or wild-type mice on day 0. The cells in the pouch fluid were collected from wild-type mice that were treated with PBS (A-B) and MGL1-deficient mice treated with PBS (C-D) or with IL-1α (E-F) on day 3. To examine the effect of IL-1α, the expression of CD11b or CD45 on these cells was analyzed by flow cytometry (B,D,F). Flow cytometric scatter profiles of these cells are also shown (A,C,E). Open histograms in panels B, D, and F represent the staining with control mAb. The number of cells recovered from the pouch was counted and plotted in panel G. The number of cells in the pouch fluid obtained from MGL1-deficient mice was greater than that from wild-type mice or that from MGL1-deficient mice injected with IL-1α. Injection of IL-1α also reduced the number of CD11b-negative/CD45-negative subpopulations in the air pouch of MGL1-deficient mice to a level similar to that of wild-type mice. Similar data were obtained on day 4.
Fate of cells obtained from the pouch of MGL1-deficient mice after injection with recombinant mouse IL-1α
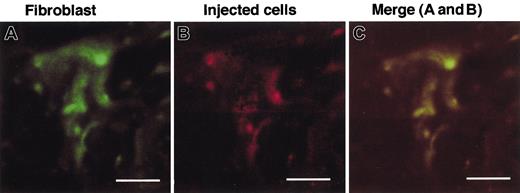
As shown in Figure 2, the number of free cells recovered from the pouch in MGL1-deficient mice was greater than that in wild-type mice. Furthermore, a CD45-negative/CD11b-negative population was predominant only in MGL1-deficient mice. To determine the role of IL-1 in the association of these cells with the granulation tissue, the number and identity of cells isolated from the air pouch were determined after the administration of IL-1α. Injection of IL-1α reduced the number of CD45-negative/CD11b-negative cells to a level similar to that of wild-type mice (Figure 5B,D,F). The size of these cells became similar to that of wild-type mice (Figure 5A,C,E). Total numbers of migrated cells also decreased to a level similar to that found in wild-type mice (Figure 5G). After IL-1α treatment, the percentage of CD45-negative cells (fibroblast-like cell) significantly decreased from 56.3% to 2.5%. The value was similar to the level in wild-type mice (1.8%). CD45-negative/CD11b-negative cells were collected from the pouch fluid in MGL1-deficient mice 3 days after the antigenic challenge. These were labeled with PKH-26 and were injected with or without IL-1α into the pouch of MGL1-deficient mice. Granulation tissue formation was observed only when IL-1α was provided and, as observed by the fluorescence of PKH-26, the distribution of the injected cells overlapped with that of cells stained with mAb ER-TR7–positive fibroblasts (Figure 6). These results suggest that the impairment of migration of the CD45-negative/CD11b-negative pouch cells to the granulation tissue in MGL1-deficient mice was also due to a local shortage in IL-1α and that CD45-negative cells in MGL1-deficient mice represent precursors of fibroblasts.
Examination of the cells recovered from the pouch fluid obtained from MGL1-deficient mice. An antigenic challenge was performed and cells in the pouch were collected on day 3. The CD45-negative cells were separated from the pouch fluid cell suspensions by MACS and were labeled with PHK-26. The labeled cells mixed with IL-1α (50 μg/mL) were injected into the pouch on MGL1-deficient mice on day 3. The injected cells, which were labeled with PKH26 (B), were shown also to be ER-TR7–positive and classified into fibroblasts (A). Bars represent 5 μm.
Examination of the cells recovered from the pouch fluid obtained from MGL1-deficient mice. An antigenic challenge was performed and cells in the pouch were collected on day 3. The CD45-negative cells were separated from the pouch fluid cell suspensions by MACS and were labeled with PHK-26. The labeled cells mixed with IL-1α (50 μg/mL) were injected into the pouch on MGL1-deficient mice on day 3. The injected cells, which were labeled with PKH26 (B), were shown also to be ER-TR7–positive and classified into fibroblasts (A). Bars represent 5 μm.
MGL1-deficient mice can mount an immune response against T-cell–dependent antigen
To determine whether or not the lack of MGL1 could affect initiation of immune responses against ABA-AcBSA, IgG production was compared between wild-type and MGL1-deficient mice. Mice were immunized with a mixture of antigen and CFA, and challenged twice by subcutaneous application of the antigen directly into the dorsal air pouch. During the immunization and the antigenic challenge, total IgG concentration (Table 1) and ABA-AcBSA–specific IgG levels (Figure 7B-C) in sera were determined. After each challenge, serum IgG concentrations increased; no difference showed in the level of total IgG between wild-type and MGL1-deficient mice. Furthermore, the antigen-specific IgG levels increased similarly regardless of whether wild-type or MGL1-deficient mice were used.
IgG by time after first challenge
Mouse genotype . | IgG by time after first challenge, μg/mL, mean ± SEM . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| . | -10 d . | 4 d . | 11 d . | 18 d . | |||
| WT | 102.6 ± 3.4 | 650.0 ± 87.8* | 832.1 ± 97.0* | 860.8 ± 211.4* | |||
| KO | 99.1 ± 3.7 | 694.0 ± 80.2* | 717.7 ± 165.9* | 853.6 ± 231.3* | |||
Mouse genotype . | IgG by time after first challenge, μg/mL, mean ± SEM . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| . | -10 d . | 4 d . | 11 d . | 18 d . | |||
| WT | 102.6 ± 3.4 | 650.0 ± 87.8* | 832.1 ± 97.0* | 860.8 ± 211.4* | |||
| KO | 99.1 ± 3.7 | 694.0 ± 80.2* | 717.7 ± 165.9* | 853.6 ± 231.3* | |||
Total IgG concentration in the serum from individual mice (n = 3) was measured by an ELISA.
Difference is statistically significant (P < .001) compared with the value on day -10. Antigen-specific immune responses between MGL1-deficient mice and wild-type mice were almost identical.
The involvement of MGL1 on antigen-specific T-cell proliferation was also examined. The splenocytes were obtained from the mice that had been intradermally injected with the antigen-CFA mixture 3 weeks earlier. The cells were cultured with various concentrations of antigen for 5 days, and DNA synthesis was assessed by [3H]-thymidine incorporation. There were no differences in proliferative response to the antigen between MGL1-deficient mice and wild-type mice (Figure 7A).
These results indicated that MGL1 is not essential for the initiation of immune responses such as antigen-specific T-cell proliferation and T-cell–dependent IgG production in this system.
Discussion
Many macrophage functions are mediated by lectins. For example, mice deficient in macrophage mannose receptors exhibited decreased clearance of peptide hormones with sulfated N-acetylgalactosamine residues, although these mice showed similar susceptibility to the infections of Candida albicans and Pneumocystis carinii.34-37 Galectin-3–deficient mice showed reduced recruitment of neutrophils into inflamed peritoneal cavities38 ; macrophages from these mice showed suppressed spreading and phagocytic functions and the mice developed glomerulopathy under diabetic conditions.39-41
MGL1 belongs to a large family of calcium-type lectins that include cell surface molecules such as type 2 transmembrane proteins, type 1 multilectins, selectins, humoral components such as collectins,42 and extracellular matrix components such as aggrecan.43 Earlier studies have reported that macrophage functions were not strongly impaired in MGL1-deficient mice.26 There was no abnormality in the controlled T-cell development, allogeneic cytotoxic T lymphocyte (CTL) responses, hematopoiesis, or turnover of red blood cells.26 Lack of phenotypes might be attributed to the presence of other macrophage C-type lectins with similar carbohydrate specificity. Subsequently, MGL2, which potentially substitutes MGL1 function, was identified on similar cell populations.21 However, it is presently not known whether or not these 2 isolectins share similar functions.
Evaluation of antigen-specific immune response in MGL1-deficient mice. (A) Mice (□, wild type; ▪, MGL1-deficient) were intradermally immunized with ABA-AcBSA and the spleen was taken 3 weeks after immunization. Splenocyte proliferation in response to ABA-AcBSA (ordinate) expressed as the ratio of the [3H]-thymidine incorporation count per minute (cpm) given by cells exposed to ABA-AcBSA to the cpm given by unexposed cells. Each value represents mean plus or minus SD(n = 6). (B-D) In other experiments, wild-type mice (B; open symbols) and MGL1-deficient mice (C; closed symbols) were subcutaneously immunized with ABA-AcBSA on day –10, and were subsequently challenged by injecting the antigen into the dorsal air pouch on days 0 and 5. Sera were collected on days –10, 4, 11, and 18. Each serum sample was prepared from 5 mice. The binding of IgG to immobilized ABA-AcBSA (ordinate) was measured for serum samples collected on days –10 (no symbol), 4 (circles), 11 (triangles), and 18 (squares) and plotted to the serum dilution (abscissa) as determined by means of an ELISA.
Evaluation of antigen-specific immune response in MGL1-deficient mice. (A) Mice (□, wild type; ▪, MGL1-deficient) were intradermally immunized with ABA-AcBSA and the spleen was taken 3 weeks after immunization. Splenocyte proliferation in response to ABA-AcBSA (ordinate) expressed as the ratio of the [3H]-thymidine incorporation count per minute (cpm) given by cells exposed to ABA-AcBSA to the cpm given by unexposed cells. Each value represents mean plus or minus SD(n = 6). (B-D) In other experiments, wild-type mice (B; open symbols) and MGL1-deficient mice (C; closed symbols) were subcutaneously immunized with ABA-AcBSA on day –10, and were subsequently challenged by injecting the antigen into the dorsal air pouch on days 0 and 5. Sera were collected on days –10, 4, 11, and 18. Each serum sample was prepared from 5 mice. The binding of IgG to immobilized ABA-AcBSA (ordinate) was measured for serum samples collected on days –10 (no symbol), 4 (circles), 11 (triangles), and 18 (squares) and plotted to the serum dilution (abscissa) as determined by means of an ELISA.
In the present study, we found a remarkable phenotype in MGL1-deficient mice (Figure 1). The tissue remodeling associated with a chronic immune response was almost completely absent in mice deficient in MGL1. This result was reinforced by experiments in which the administration of mAb LOM-8.7 (specific for MGL1) into the air pouch during the second antigenic challenge also suppressed the granulation tissue maintenance (Figure 3). Although the connective tissue macrophages isolated from the dermis covering the air pouch expressed MGL2 (Figure 1), the cells that migrated into the pouch expressed MGL1 but not MGL2 (data not shown). These results and the impairment of granulation tissue formation in the MGL1-deficient mice that express MGL2 suggested that MGL1 plays the primary role in this process. There was no difference in the proliferative response to the antigen ABA-AcBSA or in the synthesis of IgG antibodies specific for the antigen between wild-type and MGL1-deficient mice (Figure 7) after the immunization. As described in “Results,” CD3-positive T cells were identified on days 3 and 4 in the air pouch of MGL1-deficient or wild-type mice. These observations strongly suggest that T cells were recruited to the inflammatory sites, even in the absence of MGL1. Thus, we conclude that the defect in granulation tissue formation in MGL1-deficient mice was not due to a lack of a T-cell–specific immune response.
To understand the process of granulation tissue formation, the involvement of cytokines was examined. MGL1/2-positive macrophages in the granulation tissue produced IL-1α, and this was confirmed by immunohistochemical analysis.31 As demonstrated in this report, the importance of IL-1α as an effector molecule was further confirmed by the use of anti–IL-1α antibody treatment to block granulation tissue formation (Figure 3), and by the use of recombinant IL-1α in MGL1-deficient mice (Figure 4) to “rescue” the response. As shown in Figure 2, flow cytometric analysis of the cells collected from the pouch fluid in MGL1-deficient mice showed that a significant population of the cells were neither myelomonocytic nor lymphocytic based on the expression of CD45, CD11b, Ly6G, or MHC class II (data not shown). Similar cells were obtained from wild-type mice in a much smaller number. The number of these cells declined to a similar level in the wild-type mice after IL-1α administration (Figure 5). Cell entry from pouch fluid to the tissue seemed to be regulated by IL-1α, which was produced by MGL1/2-positive cells. We conclude that the cells from granulation tissue injected with IL-1α are likely to be fibroblasts because they were found to express the mAb ER-TR7 epitope (Figure 6).
Accumulation of fibroblasts and synthesis of collagen by these cells are important events in granulation tissue formation.44 These processes are known to require IL-1α.7,9,45,46 We hypothesize that the lack of IL-1α caused the failure of fibroblasts to activate the synthesis and secretion of collagen and the formation of granulation tissue in the inflamed tissue of MGL1-deficient mice. MGL1 molecule was assumed to regulate trafficking and localization of MGL1-positive cells that produce and secrete IL-1α. The lineage origin and the distribution of MGL1-positive cells are important areas to be investigated. In addition, identification of the ligands for MGL1 in the inflammatory tissue and identification of the cells carrying these ligands will directly prove this hypothesis in the future.
Previous work has shown that MGL1-positive macrophages and DCs develop from bone marrow myeloid cells.22 In humans, MGL-positive macrophages and DCs can be developed from monocytes.47,48 It is likely that dermal MGL-positive cells are derived from these cells. Migration of dermal MGL1/2-positive cells from dermis to draining lymph nodes was shown to be initiated by IL-1.28 In a skin explant model, anti–IL-1α or anti–IL-1β inhibited emigration of MGL1/2-positive cells from the dermis.28 Therefore, IL-1α is also expected to activate macrophage migration and initiate a positive feedback loop in the chronic inflammatory process. Local availability of IL-1α must be severely impaired by the lack of MGL1, due perhaps to deteriorated trafficking of MGL1-positive cells, because this population of macrophages produces IL-1α. Under such conditions, fibroblasts seem to be unable to remodel the surrounding tissue. Granulation tissue formation is also known for its complex regulation through a network of cytokines that includes transforming growth factor β, tumor necrosis factor α, IL-6, interferon γ, and IL-1α/β.49 The involvement of these other cytokines in MGL1-dependent granulation tissue formation will be examined in the future.
Prepublished online as Blood First Edition Paper, March 22, 2005; DOI 10.1182/blood-2004-12-4943.
Supported by grants-in-aid from the Ministry of Education, Science, Sports and Culture of Japan (11 672 162 and 12 307 054); the Research Association for Biotechnology; the Program for Promotion of Basic Research Activities for Innovative Biosciences; and the Program for Promotion of Fundamental Studies in Health Sciences of the Organization for Pharmaceutical Safety and Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Sharon Hermann for her editorial assistance.

![Figure 7. Evaluation of antigen-specific immune response in MGL1-deficient mice. (A) Mice (□, wild type; ▪, MGL1-deficient) were intradermally immunized with ABA-AcBSA and the spleen was taken 3 weeks after immunization. Splenocyte proliferation in response to ABA-AcBSA (ordinate) expressed as the ratio of the [3H]-thymidine incorporation count per minute (cpm) given by cells exposed to ABA-AcBSA to the cpm given by unexposed cells. Each value represents mean plus or minus SD(n = 6). (B-D) In other experiments, wild-type mice (B; open symbols) and MGL1-deficient mice (C; closed symbols) were subcutaneously immunized with ABA-AcBSA on day –10, and were subsequently challenged by injecting the antigen into the dorsal air pouch on days 0 and 5. Sera were collected on days –10, 4, 11, and 18. Each serum sample was prepared from 5 mice. The binding of IgG to immobilized ABA-AcBSA (ordinate) was measured for serum samples collected on days –10 (no symbol), 4 (circles), 11 (triangles), and 18 (squares) and plotted to the serum dilution (abscissa) as determined by means of an ELISA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/1/10.1182_blood-2004-12-4943/4/m_zh80130580560007.jpeg?Expires=1767767545&Signature=KcfozI025XG2SafKeMBEsIbSpKsro3eC~T2bPW-fh25REnBnWMgtbWWb4KIbsU176K3kU0sdrc-k-Tb7TVaTX-CH4TA9SoFpXMHB3RKIwUqtcjAxboEZPrTT7AWBSBrqTEKKxFhdYnGHFndomPiyqtUujd13P-daMqaD9KEVu21uUlYBNM~LadX7EXnCwt5n5diVQ9Vex5xcSSIMT~EJ7WzJZ49hjvgxkHeDO5aUOEJENQ8uWZrvPBHQ6hgSGlCNUyiCv3hVIWJVfmbXIbeAnk2~NnJTtn5pIxTG6cOQHLLKUpNP-BGEum1i3F0juABXHA1MAKYlOOGaZdihdIihjg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal