Ectopic expression of fibroblast growth factor receptor 3 (FGFR3) associated with t(4;14) has been implicated in the pathogenesis of human multiple myeloma. Some t(4;14) patients have activating mutations of FGFR3, of which a minority are K650E (thanatophoric dysplasia type II [TDII]). To investigate the role of autophosphorylated tyrosine residues in FGFR3 signal transduction and transformation, we characterized a series of FGFR3 TDII mutants with single or multiple Y→F substitutions. Phenylalanine substitution of Y760, essential for phospholipase Cγ (PLCγ) binding and activation, significantly attenuated FGFR3 TDII–mediated PLCγ activation, as well as transformation in Ba/F3 cells and a murine bone marrow transplant leukemia model. In contrast, single substitution of Y577, Y724, or Y770 had minimal to moderate effects on TDII-dependent transformation. Substitution of all 4 non–activation loop tyrosine residues significantly attenuated, but did not abolish, TDII transforming activity. Similar observations were obtained in the context of a constitutively activated fusion TEL-FGFR3 associated with t(4;12)(p16;p13) peripheral T-cell lymphomas. Moreover, 2 independent EμSR-FGFR3 TDII transgenic mouse lines developed a pro-B-cell lymphoma, and PLCγ was highly activated in primary lymphoma cells as assessed by tyrosine phosphorylation. These data indicate that engagement of multiple signaling pathways, including PLCγ-dependent and PLCγ-independent pathways, is required for full hematopoietic transformation by constitutively activated FGFR3 mutants.
Introduction
Multiple myeloma affects terminally differentiated plasma B cells and is among the most common hematologic malignancies in patients older than 65 years. Recent molecular and cytogenetic advances have shown that recurrent translocations involving 14q32 into the immunoglobulin heavy (IgH) chain switch region are frequent in human multiple myeloma cells.1,2 The translocations usually result in dysregulated expression of several heterogeneous partners including cyclin D1,3 c-maf,4 and fibroblast growth factor receptor 3 (FGFR3).5 FGFR3 is 1 of 4 receptor-tyrosine kinases that responds to fibroblast growth factor (FGF) and negatively regulates bone formation in mammals. The murine knock out of FGFR3 results in long bone overgrowth and other skeletal abnormalities.6,7 The t(4;14) translocation involving FGFR3 has been identified in approximately 15% of multiple myeloma patients and 30% of cell lines.5,8,9 In some cases, the translocated FGFR3 gene contains an activating mutation K650E that, when present in the germ line, causes thanatophoric dysplasia type II (TDII).10 FGFR3 is composed of an extracellular ligand-binding domain, a transmembrane domain, and a split cytoplasmic tyrosine kinase domain.11 FGFR3 is activated by oligomerization induced by ligand binding. The consequent transautophosphorylation at tyrosine residues in the cytoplasmic domains is required for stimulation of the intrinsic catalytic activity and activation of downstream signaling pathways.12,13
Growing evidence has suggested a pathogenic role of FGFR3 in multiple myeloma disease progression. Expression of FGFR3 wild-type or activated FGFR3 TDII mutant transforms murine B9 myeloma cells to interleukin-6 (IL-6)–independent growth, with elevated phosphorylation of signal transducer and activator of transcription 3 (STAT3) and expression of the survival factor Bcl-XL.14 NIH3T3 cells transformed by the activated form of FGFR3 are tumorigenic when injected into nude mice.15 Moreover, in a murine bone marrow transplantation (BMT) model, mice that received transplants of bone marrow cells transduced by retroviral vectors carrying wild-type Fgfr3 or FGFR3 TDII mutant developed lethal pro–B or pre–B-cell lymphomas, respectively.16 Interestingly, in humans, activating mutations of FGFR3 do not occur concurrently in the same myeloma cells with the activating mutations of K-ras and N-ras, which are present in approximately 40% of multiple myeloma patients. Thus, FGFR3 may share the signaling pathways with ras activating mutations and play a similar role in multiple myeloma progression.15
Activation of receptor tyrosine kinases normally results in autophosphorylation at multiple tyrosine residues that provide docking sites for signaling protein factors through their respective Src homology 2 (SH2) phosphotyrosine binding domains. In FGFR1, 7 tyrosine residues have been mapped as autophosphorylation sites.17 There are 5 corresponding residues conserved in FGFR3 that are required for kinase activity, including Y647 and Y648 in the activation loop12 as well as the non–activation loop residues Y577, Y724, and Y760.13 Autophosphorylation site Y760 of FGFR3 mediates binding of phospholipase Cγ (PLCγ), which is the only cellular SH2 domain–containing target of FGFR3 that is well characterized so far.18 The potential SH2 domain–containing partners for the other FGFR3 non–activation loop tyrosine residues remain unknown. All of the postulated autophosphorylation sites of tyrosine residues in FGFR3, as well as a C-terminal Y770 that is conserved in all 4 FGFR family members, have been examined in detail by systematic mutational analysis.13 Single or multiple mutations of distinct tyrosine residues were introduced into FGFR3 cytoplasmic domain derivatives, which contained an N-terminal myristylation signal for plasma-membrane localization and a point mutation K650E for constitutive kinase activation. Multiple signaling components, including mitogen-activated protein kinases (MAPKs), STAT1, STAT3, STAT5, PLCγ, phosphatidylinositol 3–kinase (PI3K), and protein tyrosine phosphatase Shp2, are activated by the activated membrane-targeted FGFR3 derivative in a number of attached mammalian cell lines.13,19 Substitution of all non–activation loop tyrosine residues abolished the constitutively activated kinase activity of this FGFR3 construct conferred by the K650E mutation. However, “add-back” of the Y724 tyrosine residue restored the ability of this construct to phosphorylate and activate PI3K, MAPK, STAT1, and STAT3, indicating a critical role of Y724 in the activation of multiple signaling pathways, in the context of this activated plasma-membrane–targeted FGFR3 derivative.13
In this report, we investigated the roles of specific tyrosine autophosphorylation sites in FGFR3-dependent signaling and transformation in hematopoietic cells in vitro and in vivo. We constructed a series of tyrosine-to-phenylalanine (Y→F) mutants at all 6 conserved tyrosine residues in the constitutively activated FGFR3 constructs including FGFR3 TDII (K650E) mutant and TEL-FGFR3 fusion tyrosine kinase. TEL-FGFR3 is associated with t(4;12)(p16;p13) peripheral T-cell lymphoma.20 The gene rearrangement results in expression of a chimeric protein with the N-terminal domain of the transcription factor TEL (ETV6) fused to the FGFR3 C-terminal segment of entire intracellular tyrosine kinase domains. TEL N-terminal pointed domain (PNT) mediates selfassociation that might result in constitutive activation of FGFR3 tyrosine kinase domain by mimicking ligand-induced oligomerization. Our results demonstrate an important role of Y760-mediated PLCγ activation in both FGFR3 TDII– and TEL-FGFR3–induced transformation in hematopoietic cells. The full transforming activity of these leukemogenic FGFR3 mutants requires multiple signaling pathways including PLCγ-dependent and -independent pathways.
Materials and methods
DNA constructs and site-directed mutagenesis
Retroviral vectors MSCV-neoEB and MSCV2.2-IRESGFP were converted to Gateway destination vectors using Gateway Vector Conversion kit (Invitrogen life technologies, Carlsbad, CA). Full-length FGFR3 wild-type and TDII (K650E) cDNAs were individually subcloned into retroviral MSCV-Gateway-neoEB and MSCV2.2-Gateway-IRESGFP. Single or multiple mutations of Y577F, Y760F, Y724F, or Y770F were introduced into FGFR3 TDII using QuikChange-XL site-directed mutagenesis kit (Stratagene, La Jolla, CA). FGFR3 TDII 3F (Y577F/Y760F/Y770F) and FF4F (Y647F/Y648F, 4F) were generated by fragment exchange from pKH143 and pKH146,13 respectively, to the retroviral plasmids containing FGFR3 TDII using XhoI and PvuI sites. TEL-FGFR3 was constructed in the Gateway entry vector pDONR201 using the unique BsrBI site in FGFR3 near the break point. Mutations were generated by site-directed mutagenesis and fragment exchange as described.
Cell cultures
Ba/F3 cells were cultured in RPMI 1640 medium with 10% fetal bovine serum (FBS) and 1.0 ng/mL interleukin-3 (IL-3) (R & D Systems, Minneapolis, MN). 293T cells were cultured in Dulbecco modified Eagle medium (DMEM) with 10% FBS. Retroviral stocks were generated and viral titers were determined as described previously.21 For murine BMT experiments, the viral titers of all constructs were normalized to 5 × 105 infectious units/mL. Stable Ba/F3 cell lines expressing distinct FGFR3 variants were generated, and IL-3–independent proliferation assay and cell viability assay were performed as described.22,23 Cells transduced by full-length wild-type FGFR3 or TDII mutants were treated with acidic FGF (aFGF, 1.0 nM) and heparin (30 μg/mL) during the assay.
Immunoblotting analysis
To assay for the phosphorylation of various proteins, Ba/F3 cells were treated with serum starvation in plain RPMI 1640 media for 4 hours prior to lysis. Activation of PLCγ and MAPK was induced by aFGF (1.0 nM) with heparin (30 μg/mL) for 24 hours and 5 minutes, respectively. Applied antibodies include anti-PI3K (p85) antiserum and anti–phosphotyrosine 4G10 (Upstate Biotechnology, Lake Placid, NY); antibodies against FGFR3, STAT5b, Bcl-XL, and phospho-PI3K p85 (Tyr-508) (Santa Cruz Biotechnology, Santa Cruz, CA); phospho-FGFR, phospho-STAT5 (Tyr-694), STAT3, phospho-STAT3 (Tyr-705), PLCγ, phospho-PLCγ (Tyr-783), MAPK p42/p44, and phospho-MAPK p42/p44 (Cell Signaling, Beverly, MA).
Mice
Murine BMT assays were performed as described previously.24 Animals were carefully monitored under the auspices of institutionally approved protocols for humane care of animals. Donor bone marrow cells were transduced with retroviral supernatant carrying MSCV2.2-Gateway-IRESGFP-FGFR3 constructs. Cells (5 × 105/0.5 mL) were injected into the lateral tail veins of lethally irradiated (2 × 450 cGy) BALB/c recipient mice. The diseased FGFR3 TDII BMT mice were examined each day, and killed at first signs of morbidity including scruffy coat, lethargy, weight loss, tachypnea, splenomegaly palpable beyond the midline, or hind limb paresis. White blood count (WBC) and weights of organs including spleen and liver were recorded when an individual diseased mouse was killed.
The plasmid pEμSR containing the immunoglobulin-mu enhancer (Eμ) SRα promoter, and poly A sequences was converted to a Gateway destination vector, and the Gateway subcloning cassette was inserted 3′ of the EμSR cassette. FGFR3 TDII cDNA was inserted into the vector by Gateway reaction, and prokaryotic plasmid sequences were removed from the resulting construct using NotI. The gel-purified construct was microinjected into the pronucleus of an FVB strain murine oocyte that was implanted into the oviduct of a pseudopregnant mouse at the Transgenic mouse facility, Brigham and Women's Hospital. At 2 weeks of age, genomic DNA was isolated from tail clippings and the positive founder mice were identified by Southern blot analysis.
Histopathology and flow cytometric immunophenotyping
Histopathologic analyses were performed as described previously.25 Prior to flow cytometric analysis, cell samples of single-cell suspensions were washed in the staining buffer (phosphate-buffered saline [PBS] with 0.1% NaN3 and 0.1% bovine serum albumin) and stained for 20 minutes on ice with combinations of labeled monoclonal antibodies including allophycocyanin-conjugated anti-B220, phycoerythrin-conjugated anti-CD19, phycoerythrin-conjugated anti–c-kit, biotinylated anti-CD43, biotinylated anti-CD25, and biotinylated anti–BP-1 (all antibodies from BD Biosciences, San Diego, CA). Binding of biotinylated primary antibodies was detected by subsequent staining with allophycocyanin-conjugated streptavidin (Caltag, Burlingame, CA). After washing, the cells were resuspended in staining buffer containing 0.5 μg/mL 7-amino-actinomycin D (BD Biosciences) to allow discrimination of nonviable cells, and flow cytometric analysis was done on a FACSCalibur cytometer (BD Biosciences). At least 10 000 events were acquired, and the data were analyzed using CellQuest software (Version 3.3, BD Biosciences, San Jose, CA). The results are presented as dot plots of viable cells selected on the basis of scatter and 7-amino-actinomycin D staining.
Results
Y760F mutation abolishes PLCγ activation and attenuates transformation of hematopoietic cells by FGFR3 TDII
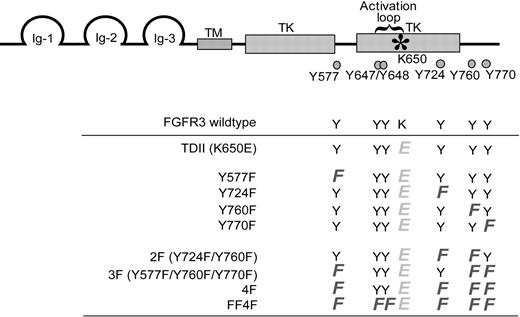
Activation of FGFR3 leads to the phosphorylation and activation of multiple signaling components, which may be critical in mediating FGFR3-dependent transformation. The FGFR3 TDII mutant is constitutively activated, which can be further activated in the presence of ligand.26 To determine the role of specific tyrosine residues in regulation of FGFR3-dependent transformation, we generated and analyzed a series of mutants with single or multiple substitutions of the non–activation loop tyrosine residues including Y577, Y724, Y760, and Y770, as well as the activation loop residues Y647/Y648, in the context of FGFR3 TDII (K650E) mutant (Figure 1).
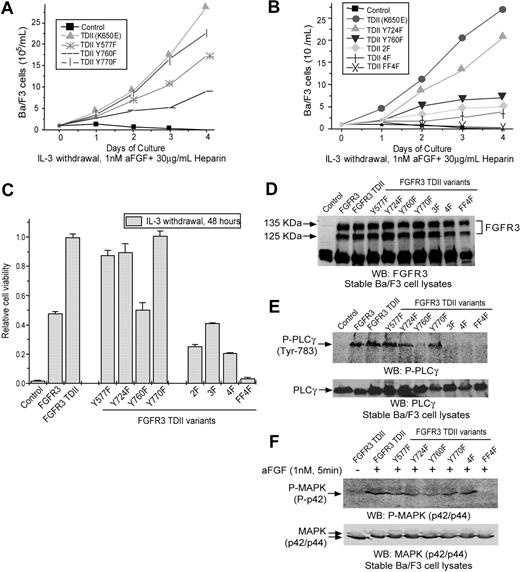
Y760, when phosphorylated, mediates PLCγ binding and activation.18 We first tested the effect of a phenylalanine substitution at Y760 on the FGFR3 TDII–dependent transformation of hematopoietic cells. FGFR3 wild type, TDII, and TDII Y760F constructs were subcloned individually into a retroviral vector with a neomycin resistance gene and stably expressed in murine Ba/F3 hematopoietic cells following retroviral transduction. The resultant stable cell lines were assayed for the ability to grow in the absence of IL-3 induced by FGFR3 variants. In the presence of ligand aFGF, both FGFR3 wild-type and constitutively activated FGFR3 TDII conferred factor-independent growth to Ba/F3 cells, whereas the control Ba/F3 cells transduced with empty vector underwent apoptosis in the absence of IL-3 because Ba/F3 cells do not express FGFRs or respond to FGF27 (Figure 2A). FGFR3 TDII was a much more potent oncoprotein to confer factor-independent growth to Ba/F3 cells compared with wild-type FGFR3. In contrast, Ba/F3 cells that were stably transduced with the FGFR3 TDII Y760F mutant had a significantly slower proliferative rate compared with cells expressing FGFR3 TDII (Figure 2A). Similar results were obtained using a cell viability–based assay in which Y760F mutation significantly decreased FGFR3 TDII transforming activity (P < .001, Figure 2C and Table 1). Immunoblotting results demonstrated that the stable cell lines expressed a comparable amount of FGFR3 protein, indicating that the difference in the transforming activity was not due to altered protein level (Figure 2D). Moreover, substitution of Y760 in FGFR3 TDII abolished the TDII-dependent PLCγ phosphorylation and activation (Figure 2E), suggesting that PLCγ is a critical effector of phosphorylation of Y760.
Cell viability comparison among TDII Y > F mutants
Cell viability comparison . | P . |
|---|---|
| TDII | |
| Y577F | .006 |
| Y724F | .04 |
| Y760F | < .001 |
| Y770F | .7 |
| Y760F | |
| 2F | .001 |
| 3F | .08 |
| 4F | .001 |
Cell viability comparison . | P . |
|---|---|
| TDII | |
| Y577F | .006 |
| Y724F | .04 |
| Y760F | < .001 |
| Y770F | .7 |
| Y760F | |
| 2F | .001 |
| 3F | .08 |
| 4F | .001 |
Schematic diagram of the locations of conserved tyrosine residues in FGFR3 and mutant constructs designed for this study. Full-length FGFR3 contains an N-terminal extracellular ligand-binding domain, a transmembrane domain (TM), and a split cytoplasmic tyrosine kinase (TK) domain. The activating mutation K650E in the activation loop is indicated by an asterisk, and 6 conserved tyrosine residues are marked by. The bottom panel shows the spectrum of FGFR3 mutants generated. All the mutants contain the activating mutation K650E except FGFR3 wild type. Single or multiple substitutions of the non–activation loop tyrosine residues Y577, Y724, Y760, and Y770 were introduced into the FGFR3 TDII (K650E). The FF4F mutant contains the mutations at the 2 activation loop tyrosine residues (Y647/Y648). All the numbering of mutations is as for native human FGFR3.
Schematic diagram of the locations of conserved tyrosine residues in FGFR3 and mutant constructs designed for this study. Full-length FGFR3 contains an N-terminal extracellular ligand-binding domain, a transmembrane domain (TM), and a split cytoplasmic tyrosine kinase (TK) domain. The activating mutation K650E in the activation loop is indicated by an asterisk, and 6 conserved tyrosine residues are marked by. The bottom panel shows the spectrum of FGFR3 mutants generated. All the mutants contain the activating mutation K650E except FGFR3 wild type. Single or multiple substitutions of the non–activation loop tyrosine residues Y577, Y724, Y760, and Y770 were introduced into the FGFR3 TDII (K650E). The FF4F mutant contains the mutations at the 2 activation loop tyrosine residues (Y647/Y648). All the numbering of mutations is as for native human FGFR3.
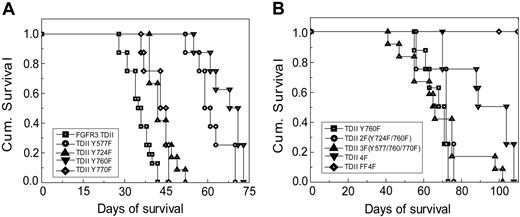
When tested in a murine BMT assay, mutation at Y760 also resulted in a statistically significant prolongation in disease latency (Figure 3A; Tables 2 and 3). Mice receiving bone marrow cells transduced by activated FGFR3 TDII induced a rapidly fatal pre–B-cell lymphoma disease, characterized by splenomegaly, lymphadenopathy, a variable leukocytosis, and a prominent marrow involvement by immature lymphoid cells (Figure 3A; Tables 2 and 3). Immunophenotypic analysis by flow cytometry indicated that these cells were B220+, CD19+, CD25+, and CD43+, but BP-1- and c-Kit- (Figure 4), consistent with a pre–B-cell phenotype. In contrast, although mice that received transplants of TDII Y760F died of a similar pre–B-cell lymphoid disease, they exhibited a significantly prolonged disease latency (median survival = 69.5 days; P < .001) (Figure 3A; Table 2). These data demonstrate that Y760 is required for FGFR3 TDII full transforming activity in vivo, and suggest a critical role for PLCγ signaling pathway in FGFR3 TDII–mediated transformation.
Disease latency comparisons among animals that received transplants of distinct TDII Y > F mutant by comparison with TDII and TDII Y760F
. | Median latency, d . | Comparison with TDII, P . | Comparison with TDII Y760F, P . |
|---|---|---|---|
| TDII | 35.5 | — | < .001 |
| TDII Y577F | 60 | < .001 | .04 |
| TDII Y724F | 42 | .001 | .001 |
| TDII Y760F | 69.5 | .001 | — |
| TDII Y770F | 44 | .001 | .001 |
| TDII 2F | 72 | — | .16 |
| TDII 3F | 65.5 | — | .35 |
| TDII 4F | 96.5 | — | .004 |
. | Median latency, d . | Comparison with TDII, P . | Comparison with TDII Y760F, P . |
|---|---|---|---|
| TDII | 35.5 | — | < .001 |
| TDII Y577F | 60 | < .001 | .04 |
| TDII Y724F | 42 | .001 | .001 |
| TDII Y760F | 69.5 | .001 | — |
| TDII Y770F | 44 | .001 | .001 |
| TDII 2F | 72 | — | .16 |
| TDII 3F | 65.5 | — | .35 |
| TDII 4F | 96.5 | — | .004 |
— indicates not applicable.
Analyses of mice transplanted with distinct FGFR3 (K650E) TDII variants
. | No. diseased/no. transplanted . | Latency, d/median . | Spleen weight, mg/median . | WBC, 106/mL/median . |
|---|---|---|---|---|
| FGFR3 TDII | 16/16 | 28-42/35.5 | 381-864/517.5 | 6.1-28.5/15 |
| TDII Y577F | 8/8 | 52-70/60 | 165-711/340 | 2-13.5/7 |
| TDII Y724F | 12/12 | 39-52/42 | 318-682/396 | 5-22.5/10.25 |
| TDII Y760F | 8/8 | 55-73/69.5 | 230-714/304 | 5.5-18/11.25 |
| TDII Y770F | 8/8 | 36-46/44 | 175-534/301.5 | 0.82-15.13/3.54 |
| TDII 2F | 8/8 | 56-76/72 | 326-667/506.5 | 1.5-9/2.25 |
| TDII 3F | 12/12 | 41-102/65.6 | 89.3-423.2/319 | 2-67/7.5 |
| TDII 4F | 8/8 | 70-108/96.5 | 181-512/300 | 2.5-19.4/12.7 |
| TDII FF4F | 0/12 | NA/ > 180 | 71-97.2/90 | 1.8-7.8/3.6 |
. | No. diseased/no. transplanted . | Latency, d/median . | Spleen weight, mg/median . | WBC, 106/mL/median . |
|---|---|---|---|---|
| FGFR3 TDII | 16/16 | 28-42/35.5 | 381-864/517.5 | 6.1-28.5/15 |
| TDII Y577F | 8/8 | 52-70/60 | 165-711/340 | 2-13.5/7 |
| TDII Y724F | 12/12 | 39-52/42 | 318-682/396 | 5-22.5/10.25 |
| TDII Y760F | 8/8 | 55-73/69.5 | 230-714/304 | 5.5-18/11.25 |
| TDII Y770F | 8/8 | 36-46/44 | 175-534/301.5 | 0.82-15.13/3.54 |
| TDII 2F | 8/8 | 56-76/72 | 326-667/506.5 | 1.5-9/2.25 |
| TDII 3F | 12/12 | 41-102/65.6 | 89.3-423.2/319 | 2-67/7.5 |
| TDII 4F | 8/8 | 70-108/96.5 | 181-512/300 | 2.5-19.4/12.7 |
| TDII FF4F | 0/12 | NA/ > 180 | 71-97.2/90 | 1.8-7.8/3.6 |
NA indicates not applicable.
The Y577F mutation has a modest effect on FGFR3 TDII–mediated transformation, whereas Y724 and Y770F have minimal effects
We next tested whether phenylalanine substitution at the other non–activation loop tyrosine residues including Y577, Y724, and Y770 could also attenuate FGFR3 TDII transforming activity in hematopoietic cells. Ba/F3 cell lines that stably expressed distinct FGFR3 TDII mutants were generated and tested. Y577F mutation resulted in a modest decrease in the IL-3–independent growth of TDII-transduced Ba/F3 cells, compared with cells expressing FGFR3 TDII in a cell population–based assay, but not as significant a decrease as for the Y760F mutation (Figure 2A). Y577F also resulted in a modest decrease in the proliferative rate of Ba/F3 cells in the cell viability assay (P = .006, Figure 2C and Table 1). Consistent with this observation, TDII Y577F mutant caused a similar pre–B-cell lymphoma in mice in the BMT assay, but with a significant prolongation in disease latency compared with TDII mice (median = 60 days; P < .001), similar to the latency of mice receiving bone marrow cells expressing TDII Y760F (P = .04) (Figure 3A; Tables 2 and 3).
In contrast, although Y724F mutation in TDII resulted in a modest decrease in IL-3–independent outgrowth of stably transduced Ba/F3 cells in a cell population–based assay (Figure 2B), it caused only a borderline significant decrease in the proliferative rate of cells in the cell viability assay (P = .04, Figure 2C and Table 1). Similarly, there was no effect of Y770F mutation on FGFR3 TDII–mediated IL-3–independent proliferation and survival of Ba/F3 cells in either cell assay (Figure 2A and 2C, respectively; Table 1). Consistent with these observations, both Y724F and Y770F mutants induced similar pre–B-lymphoid disease in the murine BMT assay (Figure 4). Although a statistically significant difference in disease latency was observed between Y724F or Y770F and FGFR3 TDII mice (median survival = 42 or 44 days, P = .001 or .001, respectively), Y724F and Y770F mutants had less effect on disease latency compared with Y760F (P < .001 and P = .001, respectively; Figure 3A; Tables 2 and 3). Taken together, these observations indicate that the Y577F mutation has a modest effect on FGFR3 TDII transforming activity, whereas the Y724F or Y770F mutations have minimal effect.
Effects of single or multiple substitutions at tyrosine residues on FGFR3 TDII activity in Ba/F3 cells. Ba/F3 cells transduced with an empty retroviral vector were included as a negative control. (A) IL-3–independent growth of Ba/F3 cell lines stably expressing distinct FGFR3 TDII single mutants. Cells were cultured with aFGF and heparin in the absence of IL-3 and counted daily. (B) Effects of multiple mutations at tyrosine residues on FGFR3 TDII–dependent IL-3–independent growth of Ba/F3 cells. (C) Effects of diverse tyrosine mutations on FGFR3 TDII transforming activity in a cell viability assay. The relative cell viability was normalized to the viability of cells stably expressing FGFR3 TDII mutant control. Data presented are mean ± standard error (n = 3). (D) Expression of distinct FGFR3 TDII variants in stably transduced Ba/F3 cells. FGFR3 TDII proteins were detected with antibody recognizing FGFR3 C-terminal tail. Visualization of double bands in each lane is due to the 2 alternative translational start sites in FGFR3. WB indicates Western blot. (E) Mutation Y760F abolishes FGFR3 TDII–dependent phosphorylation and activation of PLCγ. PLCγ specifically phosphorylated at activating tyrosine residue (Y783) and expression of total PLCγ were detected by immunoblotting. (F) Non–activation loop tyrosine residues are dispensable for FGFR3 TDII–dependent phosphorylation and activation of MAPK. Cells were treated with 1 nM aFGF and 30μg/mL heparin for 5 minutes prior to lysis. Phospho-MAPK and total MAPK protein were examined. FGFR3 TDII stable cells without ligand treatment were included as a control.
Effects of single or multiple substitutions at tyrosine residues on FGFR3 TDII activity in Ba/F3 cells. Ba/F3 cells transduced with an empty retroviral vector were included as a negative control. (A) IL-3–independent growth of Ba/F3 cell lines stably expressing distinct FGFR3 TDII single mutants. Cells were cultured with aFGF and heparin in the absence of IL-3 and counted daily. (B) Effects of multiple mutations at tyrosine residues on FGFR3 TDII–dependent IL-3–independent growth of Ba/F3 cells. (C) Effects of diverse tyrosine mutations on FGFR3 TDII transforming activity in a cell viability assay. The relative cell viability was normalized to the viability of cells stably expressing FGFR3 TDII mutant control. Data presented are mean ± standard error (n = 3). (D) Expression of distinct FGFR3 TDII variants in stably transduced Ba/F3 cells. FGFR3 TDII proteins were detected with antibody recognizing FGFR3 C-terminal tail. Visualization of double bands in each lane is due to the 2 alternative translational start sites in FGFR3. WB indicates Western blot. (E) Mutation Y760F abolishes FGFR3 TDII–dependent phosphorylation and activation of PLCγ. PLCγ specifically phosphorylated at activating tyrosine residue (Y783) and expression of total PLCγ were detected by immunoblotting. (F) Non–activation loop tyrosine residues are dispensable for FGFR3 TDII–dependent phosphorylation and activation of MAPK. Cells were treated with 1 nM aFGF and 30μg/mL heparin for 5 minutes prior to lysis. Phospho-MAPK and total MAPK protein were examined. FGFR3 TDII stable cells without ligand treatment were included as a control.
Effect of substitution of multiple non–activation loop tyrosine residues on FGFR3 TDII transforming activity
We next tested the effect of mutation at other non–activation loop tyrosine residues in addition to Y760F on FGFR3 TDII transforming activity. FGFR3 TDII variants with multiple Y→F mutations including 2F (Y724F/Y760F), 3F (Y577F/Y760F/Y770F), and 4F (Y577F/Y724F/Y760F/Y770F) were generated and stably transduced into Ba/F3 cells. The 2F (Y724/Y760) mutant resulted in further decrease in the induction of IL-3–independent growth of Ba/F3 cells compared with cells transduced by FGFR3 TDII Y760F in both cell population– and viability-based assays (Figure 2B and 2C, respectively; Table 1). In contrast, the 3F (Y577F/Y760F/Y770F) mutant did not show further effect on TDII transforming activity compared with Y760F in Ba/F3 cells (P = .08; Figure 2C and Table 1). Surprisingly, in the murine BMT assay, neither of the mutants attenuated the transforming activity of FGFR3 TDII Y760F mutant. Mice receiving bone marrow cells expressing 2F or 3F mutants predominantly developed pre–B-cell lymphoma with median latency of 72 or 65.5 days, respectively, comparable with a 69.5-day median of mice that received transplants of the Y760F mutant (P = .16 or .35, respectively; Figure 3B; Tables 2 and 3). However, substitution of all 4 non–activation loop tyrosine residues including Y577, Y724, Y760, and Y770 significantly attenuated the FGFR3 TDII–induced transformation both in vitro in Ba/F3 cells (Figure 2B-C; Table 1) and in vivo in the murine BMT assay (Figures 3B,4; Tables 2 and 3). The 4F mutant also induced a pre–B-cell lymphoma in mice with a further prolonged disease latency of 96.5 days, compared with 69.5 days of TDII Y760F mice (P = .004).
Taken together, these data indicate that Y760 is required for full transforming activity of FGFR3 TDII through activation of the PLCγ signaling pathway. Mutation of the other non–activation loop tyrosine residues (Y577, Y724, or Y770) did not affect Y760-mediated activating tyrosine phosphorylation of PLCγ by FGFR3 TDII (Figure 2E). Moreover, substitution of any or all of the 4 non–activation tyrosine residues had no effect on FGFR3 TDII–mediated MAPK activation (Figure 2F). Thus, the autophosphorylated Y577 and Y724 might contribute to the full transforming activity of FGFR3 TDII by providing docking sites for potential downstream SH2 domain–containing signaling components, which mediate signal transduction pathways not involving PLCγ or MAPK. The conserved Y770 is not an autophosphorylation site13 and probably not required for transforming activity in the context of the FGFR3 TDII mutant.
Kaplan-Meier survival plot of mice receiving bone marrow cells transduced by distinct FGFR3 TDII constructs. Mice that received transplants of various FGFR3 TDII constructs (except FF4F) succumbed to a fatal pre–B-cell lymphoma. None of 12 mice that received transplants of FF4F developed any disease by 180 days after transplantation when the experiment was terminated.
Kaplan-Meier survival plot of mice receiving bone marrow cells transduced by distinct FGFR3 TDII constructs. Mice that received transplants of various FGFR3 TDII constructs (except FF4F) succumbed to a fatal pre–B-cell lymphoma. None of 12 mice that received transplants of FF4F developed any disease by 180 days after transplantation when the experiment was terminated.
Tyrosine kinase activity is required for FGFR3 TDII transforming activity
Further substitution of the 2 activation loop tyrosine residues (Y647/Y648) abolished transforming activity of FGFR3 TDII, despite the presence of the activating mutation K650E. The activation loop tyrosine residues are required for kinase activity of FGFR3 or FGFR3 constitutively activated mutants.12 Ba/F3 cells stably transduced by the kinase-inactive FF4F (Y647F/Y648F, 4F) mutant underwent apoptosis in the absence of IL-3 (Figure 2B-C; Table 1). Mice that received transplants of FF4F were healthy and had no evidence of disease by gross examination, or by histopathologic and flow cytometric analysis when killed approximately 180 days after transplantation (Figures 3 and 4; Tables 2 and 3). These data indicate that, as expected, tyrosine kinase activity is required for FGFR3 TDII–dependent transformation in hematopoietic cells.
Effect of single or multiple tyrosine substitutions on TEL-FGFR3 fusion tyrosine kinase
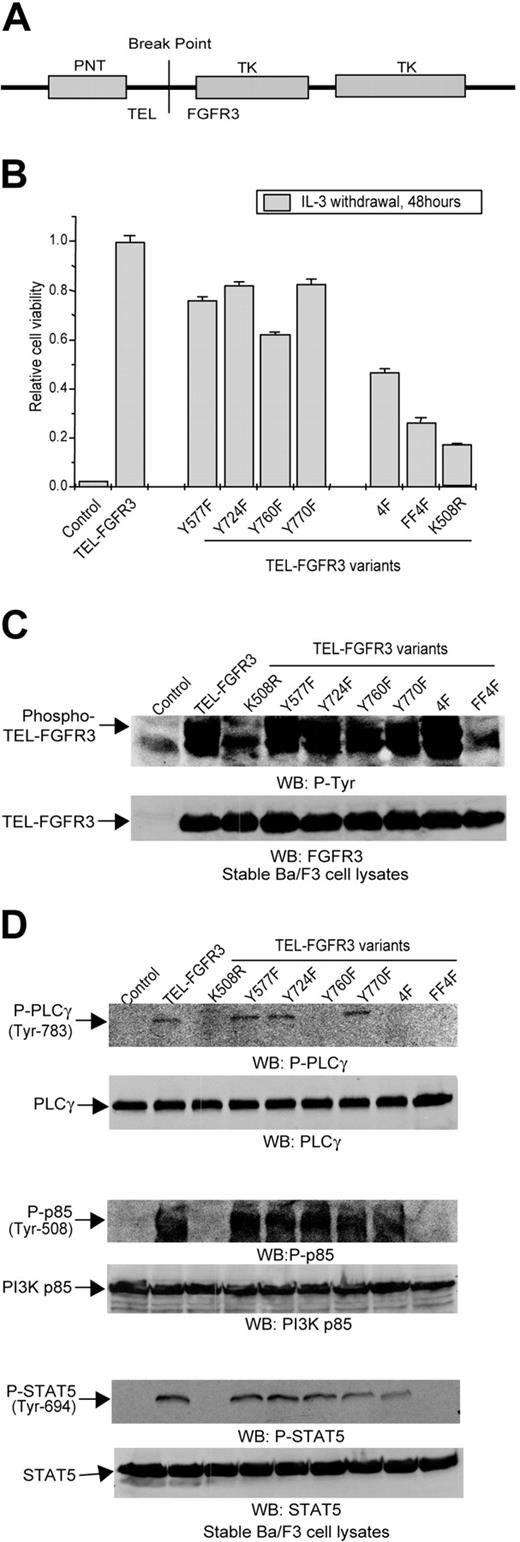
TEL-FGFR3 fusion is expressed as a consequence of t(4;12)(p16; p13) peripheral T-cell lymphoma (Figure 5A). TEL-FGFR3 is constitutively activated independent of ligand and transforms Ba/F3 cells to factor-independent outgrowth (Figure 5B; Table 4). To examine the regulatory roles of autophosphorylated tyrosine residues in the context of TEL-FGFR3, mutants with single or multiple Y→F substitutions, including Y577F, Y724F, Y760, Y770F, 4F (Y577F/Y724F/Y760F/Y770F), and FF4F (Y647F/Y648F,4F), were generated and stably transduced into Ba/F3 cells. Data similar to that were observed with these mutants in the context of full-length FGFR3 TDII. The Y760F mutation significantly impaired TEL-FGFR3 transforming activity in Ba/F3 cells (P < .001). Individual Y577F, Y724F, and Y770F mutations resulted in a modest decrease in TEL-FGFR3–dependent transformation of cells. Substitution of all 4 non–activation loop tyrosine residues (4F) further attenuated TEL-FGFR3 transforming activity (Figure 5B; Table 4). These differences could not be attributed to different levels of protein expression of TEL-FGFR3 variants (Figure 5C).
Cell viability comparisons among TEL-FGFR3 Y > F mutants
. | Comparison with TEL-FGFR3, P . | Comparison with Y760F, P . |
|---|---|---|
| TEL-FGFR3 | — | < .001 |
| Y577F | < .001 | < .001 |
| Y724F | < .001 | < .001 |
| Y760F | < .001 | — |
| Y770F | .001 | < .001 |
| 4F | — | < .001 |
| FF4F | — | < .001 |
. | Comparison with TEL-FGFR3, P . | Comparison with Y760F, P . |
|---|---|---|
| TEL-FGFR3 | — | < .001 |
| Y577F | < .001 | < .001 |
| Y724F | < .001 | < .001 |
| Y760F | < .001 | — |
| Y770F | .001 | < .001 |
| 4F | — | < .001 |
| FF4F | — | < .001 |
—indicates not applicable.
Constitutively activated TEL-FGFR3 was hyperautophosphorylated (Figure 5C). Interestingly, diverse single or multiple substitutions at the non–activation loop tyrosine residues did not affect the TEL-FGFR3 autokinase activity, indicating that none of these tyrosine residues is involved in kinase activation in the context of TEL-FGFR3 (Figure 5C). In contrast, substitution of the activation loop Y647/Y648 residues abolished TEL-FGFR3 kinase activity, similar to the FGFR3 kinase-inactivating mutation K508R28 (Figure 5C). The resulting kinase-inactive mutants FF4F and K508R showed minimal transforming activity in Ba/F3 cells (Figure 5B; Table 4). These data demonstrate that the transforming activity of TEL-FGFR3 depends on its kinase activity.
TEL-FGFR3 activated diverse downstream signaling pathways including PLCγ, PI3K, and STAT5 in Ba/F3 cells (Figure 5D). In contrast, the kinase-inactive FF4F and K508R mutants were not capable of activating any of these downstream signaling components (Figure 5D). Consistent with previous observations, TEL-FGFR3 mutants containing Y760F substitution, including Y760F and 4F, failed to phosphorylate and activate PLCγ (Figure 5D). However, substitution of any or all of the non–activation loop tyrosine residues had no effect on TEL-FGFR3–mediated phosphorylation and activation of PI3K and STAT5 (Figure 5D). These data indicate that an engagement of multiple signaling pathways is required for TEL-FGFR3–dependent transformation in Ba/F3 cells, including Y760-mediated activation of PLCγ, as well as other pathways that are independent of the non–activation loop tyrosine residues.
FGFR3 TDII mutants induce pre–B-cell lymphoma in BMT mice. (A) Sections of tissues from a representative FGFR3 TDII mouse show effacement of normal splenic and lymph node architecture, as well as replacement of normal hematopoietic cells within the bone marrow by an atypical population of intermediate to large lymphoid cells. Focal infiltration by these lymphoid cells was also frequently observed in the liver in both a sinusoidal and perivascular distribution. Magnifications are as indicated (hematoxylin and eosin [H&E]). The samples were analyzed by using Nikon Eclipse E400 microscope with Nikon 10 ×/0.25, 40 ×/1.30, or 60 ×/1.40 objective lenses (Nikon, Melville, NY). The pictures were taken with an RT Color Spot camera and analyzed with acquisition software Spot Version 4.0.6. (Diagnostic Instruments, Sterling Heights, MI) and Adobe Photoshop 6.0 (Adobe, San Jose, CA). (B) Spleen sections of mice that received transplants of FGFR3 TDII Y724F, Y760F, and 4F mutants show similar effacement of splenic architecture by atypical lymphoid cells as observed in the FGFR3 TDII mice, whereas FF4F mice did not develop disease (shown at a lower magnification that demonstrates normal splenic architecture). (C) Flow cytometry analysis confirms an immunophenotype of pre–B-cell lymphoma in mice that received transplants of FGFR3 TDII, identified as B220+, CD19+, CD25+, CD43+, but BP-1- and c-KIT-. (D) Distinct FGFR3 TDII variants induce a similar immature B-cell lymphoid disease in mice. Flow cytometry demonstrates a predominant population of pre–B cells stained by B220 and CD19 in bone marrow cells. The numbers of percentage of double-stained cells in the inside quadrant of the dot plots are indicated.
FGFR3 TDII mutants induce pre–B-cell lymphoma in BMT mice. (A) Sections of tissues from a representative FGFR3 TDII mouse show effacement of normal splenic and lymph node architecture, as well as replacement of normal hematopoietic cells within the bone marrow by an atypical population of intermediate to large lymphoid cells. Focal infiltration by these lymphoid cells was also frequently observed in the liver in both a sinusoidal and perivascular distribution. Magnifications are as indicated (hematoxylin and eosin [H&E]). The samples were analyzed by using Nikon Eclipse E400 microscope with Nikon 10 ×/0.25, 40 ×/1.30, or 60 ×/1.40 objective lenses (Nikon, Melville, NY). The pictures were taken with an RT Color Spot camera and analyzed with acquisition software Spot Version 4.0.6. (Diagnostic Instruments, Sterling Heights, MI) and Adobe Photoshop 6.0 (Adobe, San Jose, CA). (B) Spleen sections of mice that received transplants of FGFR3 TDII Y724F, Y760F, and 4F mutants show similar effacement of splenic architecture by atypical lymphoid cells as observed in the FGFR3 TDII mice, whereas FF4F mice did not develop disease (shown at a lower magnification that demonstrates normal splenic architecture). (C) Flow cytometry analysis confirms an immunophenotype of pre–B-cell lymphoma in mice that received transplants of FGFR3 TDII, identified as B220+, CD19+, CD25+, CD43+, but BP-1- and c-KIT-. (D) Distinct FGFR3 TDII variants induce a similar immature B-cell lymphoid disease in mice. Flow cytometry demonstrates a predominant population of pre–B cells stained by B220 and CD19 in bone marrow cells. The numbers of percentage of double-stained cells in the inside quadrant of the dot plots are indicated.
Activation of PLCγ in an FGFR3 TDII transgenic murine model
Next we tested the oncogenic activity of the FGFR3 TDII mutant in a transgenic mouse model. The immunoglobulin-mu enhancer Eμ was used to direct FGFR3 TDII expression to the lymphoid compartment. Of 10 positive founders, 2 developed a rapid lethal pro-B-cell lymphoma with splenomegaly, lymphadenopathy, and extensive infiltration of bone marrow by neoplastic lymphoid cells within 6 weeks after birth (Figure 6). The remaining 8 founders were followed-up up to more than one year, and none of the founders or F1 offspring developed any disease. This phenotypic difference in disease penetrance might be due to an integration site effect of the transgene that resulted in insufficient expression of FGFR3 TDII in these founders that did not develop disease.
Flow cytometric analysis of bone marrow single cell suspensions confirmed the pro-B-cell lymphoma phenotype as B220+, CD43+, CD25+, CD19+, and c-Kit+, but BP-1- (Figure 6E). FGFR3 TDII expression and activation as assessed by tyrosine autophosphorylation were confirmed by immunoblotting in the tumor tissue samples from the 2 diseased founders (Figure 7A). Hyperphosphorylation and activation of PLCγ were detected, along with elevated STAT3 phosphorylation and antiapoptotic factor Bcl-XL expression in the FGFR3 TDII–expressing tumors (Figure 7B-D). These data suggest that multiple signaling pathways including PLCγ are also engaged in FGFR3 TDII–dependent hematopoietic transformation in primary lymphoid cells.
Discussion
Characterization of the signaling properties of FGFR3 in hematopoietic transformation provides an understanding of the pathways that are important in the pathogenesis of multiple myeloma, as well as insights into potential therapeutic strategies in clinical treatments. Using systematic mutational analysis, we studied the importance of individual tyrosine residues in FGFR3-mediated signaling and transformation in hematopoietic cells. The results demonstrate that Y760 is required for the full transforming activity of constitutively activated FGFR3 mutants; multiple downstream signaling pathways, including Y760-mediated PLCγ-dependent and -independent pathways, are required for full hematopoietic transformation by activated FGFR3 mutants.
Effects of single or multiple Y→F substitutions on TEL-FGFR3 activity. Ba/F3 cells transduced with empty retroviral vector were included as a control. (A) Schematic diagram of TEL-FGFR3 fusion tyrosine kinase that contains an N-terminal TEL PNT dimerization domain and a cytoplasmic FGFR3 intracellular tyrosine kinase (TK) domain. (B) Effects of diverse tyrosine mutations on TEL-FGFR3 transforming activity in Ba/F3 cells in cell viability assay. The relative cell viability was normalized to the viability of cells stably expressing TEL-FGFR3 control. Data presented are mean ± standard error (n = 3). (C) Autophosphorylation of distinct TEL-FGFR3 variants assessed by specific phosphotyrosine antibody. Expression of distinct TEL-FGFR3 mutants was examined. (D) Tyrosine phosphorylation of PLCγ, PI3K, and STAT5 by TEL-FGFR3 variants. Mutation Y760F abolishes TEL-FGFR3–dependent phosphorylation and activation of PLCγ.
Effects of single or multiple Y→F substitutions on TEL-FGFR3 activity. Ba/F3 cells transduced with empty retroviral vector were included as a control. (A) Schematic diagram of TEL-FGFR3 fusion tyrosine kinase that contains an N-terminal TEL PNT dimerization domain and a cytoplasmic FGFR3 intracellular tyrosine kinase (TK) domain. (B) Effects of diverse tyrosine mutations on TEL-FGFR3 transforming activity in Ba/F3 cells in cell viability assay. The relative cell viability was normalized to the viability of cells stably expressing TEL-FGFR3 control. Data presented are mean ± standard error (n = 3). (C) Autophosphorylation of distinct TEL-FGFR3 variants assessed by specific phosphotyrosine antibody. Expression of distinct TEL-FGFR3 mutants was examined. (D) Tyrosine phosphorylation of PLCγ, PI3K, and STAT5 by TEL-FGFR3 variants. Mutation Y760F abolishes TEL-FGFR3–dependent phosphorylation and activation of PLCγ.
Phenylalanine substitution of Y760 abolished PLCγ tyrosine phosphorylation and activation by FGFR3 TDII or TEL-FGFR3, and significantly attenuated the ability of FGFR3 to transform Ba/F3 cells to survive and proliferate in the absence of IL-3. Moreover, in a murine BMT assay, the Y760F mutation impairs FGFR3 TDII–induced development of a pre–B-lymphoid disease. These findings are consistent with a critical role of Y760, as well as Y760-mediated PLCγ signaling pathway in the full transforming activity of constitutively activated FGFR3 mutants. Mutation of the Y760 corresponding tyrosine residue Y766 in FGFR1 results in impaired phosphatidylinositol (PI) hydrolysis and receptor internalization.29 This suggests a possible mechanism by which PLCγ may contribute to FGFR3 transformation signaling.
In contrast, substitution of another non–activation loop autophosphorylated tyrosine residue, Y577, results in a more modest decrease in FGFR3 TDII or TEL-FGFR3 transforming activity in vitro and in vivo. Additionally, the Y577F mutant, in the context of both FGFR3 TDII and TEL-FGFR3, phosphorylates and activates PLCγ to comparable levels as the control kinases. These data suggest that Y577 also plays a positive regulatory role in FGFR3-mediated transformation, and that certain PLCγ-independent pathway(s) may signal through Y577 and contribute to the transformation by activated FGFR3 mutants. While the Y724F single mutation shows only minimal effects on FGFR3 TDII transforming activity in vitro and in vivo, addition of the Y724F mutation to TDII Y760F mutant further attenuates the decreased factor-independent proliferation of Ba/F3 cells (Figure 2C; Table 1). Moreover, further phenylalanine substitution at Y724 in the context of the TDII 3F mutant (Y577F/Y760F/Y770F) substantially decreases transforming activity of TDII 3F in the murine BMT assay. These results suggest that the postulated autophosphorylation site Y724 probably is also required for full transforming activity in the context of FGFR3 TDII.
These findings contrast with the observations in the context of plasma-membrane–targeted FGFR3 TDII truncation constructs,13 which suggest that Y577F has no effect on transforming activity of the FGFR3 TDII derivatives, whereas substitution of Y724 results in abolishment of both TDII-dependent transformation and activation of downstream pathways. This difference might be due to the structural properties and activation mechanisms of different FGFR3 constructs in question. The kinase activity of truncated FGFR3 TDII derivatives depends on the K650E activating mutation instead of receptor dimerization, and thus these mutants do not respond to FGF ligand. In contrast, constitutively activated full-length FGFR3 TDII is a transmembrane receptor tyrosine kinase that can be further activated in the presence of FGF ligand,26 whereas TEL-FGFR3 is located in cytosol but similarly activated by dimerization induced by the TEL PNT domain,30 which mimics ligand-dependent oligomerization and activation of FGFR3.
Y770F mutation was suggested to positively regulate PI3K activation and transformation in the context of the plasma-membrane–targeted truncated form of FGFR3 TDII in certain attached mammalian cell lines.13 However, our mutational analysis revealed that mutation Y770F has minimal effect on the hematopoietic transformation by FGFR3 TDII or TEL-FGFR3. Perhaps because FGFR3 TDII or TEL-FGFR3 is maximally activated in hematopoietic cells by ligand- or TEL PNT motif–induced oligomerization, respectively, substitution at Y770 in these contexts does not result in further enhancement of transforming activity.
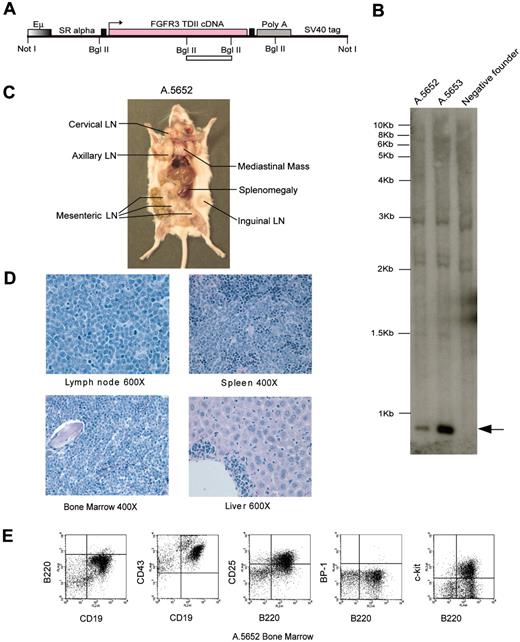
EμSR-FGFR3 TDII transgenic mice develop a fatal pro–B-cell lymphoma. (A) Schematic diagram of EμSR-FGFR3 TDII transgenic construct. FGFR3 TDII cDNA was inserted following the 3′ of EμSR promoter/enhancer using a Gateway subcloning cassette. The 2 filled boxes flanking the FGFR3 TDII construct are Gateway recombination sequences. The open bar indicates the target fragment for Southern blot analysis. (B) Southern blot identification of 2 positive founders with EμSRFGFR3 TDII transgenic construct. Genomic tail DNA was digested with BglII, and the fragment containing FGFR3 TDII cDNA sequence was detected (indicated by an arrow). An FGFR3 TDII transgene-negative FVB founder tail DNA is shown as a control. (C) Gross analysis of affected EμSR-FGFR3 TDII transgenic founder A.5652 shows splenomegaly and massive lymphadenopathy in various lymph node (LN) groups as indicated. (D) Sections of tissues from mouse A.5652 demonstrate extensive infiltration and effacement by immature lymphoid cells in lymph node, spleen, and bone marrow and focally in the liver. Magnifications are as indicated (H&E). (E) Flow cytometry analysis of lymph nodes in mouse A.5652 illustrates an immunophenotype with a high percentage of pro–B lymphoid cells, identified as B220+, CD19+, CD25+, CD43+, and c-KIT+ but BP-1-. Images were captured as described for Figure 4.
EμSR-FGFR3 TDII transgenic mice develop a fatal pro–B-cell lymphoma. (A) Schematic diagram of EμSR-FGFR3 TDII transgenic construct. FGFR3 TDII cDNA was inserted following the 3′ of EμSR promoter/enhancer using a Gateway subcloning cassette. The 2 filled boxes flanking the FGFR3 TDII construct are Gateway recombination sequences. The open bar indicates the target fragment for Southern blot analysis. (B) Southern blot identification of 2 positive founders with EμSRFGFR3 TDII transgenic construct. Genomic tail DNA was digested with BglII, and the fragment containing FGFR3 TDII cDNA sequence was detected (indicated by an arrow). An FGFR3 TDII transgene-negative FVB founder tail DNA is shown as a control. (C) Gross analysis of affected EμSR-FGFR3 TDII transgenic founder A.5652 shows splenomegaly and massive lymphadenopathy in various lymph node (LN) groups as indicated. (D) Sections of tissues from mouse A.5652 demonstrate extensive infiltration and effacement by immature lymphoid cells in lymph node, spleen, and bone marrow and focally in the liver. Magnifications are as indicated (H&E). (E) Flow cytometry analysis of lymph nodes in mouse A.5652 illustrates an immunophenotype with a high percentage of pro–B lymphoid cells, identified as B220+, CD19+, CD25+, CD43+, and c-KIT+ but BP-1-. Images were captured as described for Figure 4.
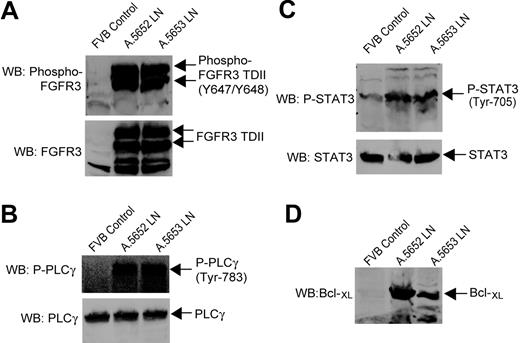
Activation of PLCγ and STAT3 in tumors of EμSR-FGFR3 TDII transgenic mice. Spleen samples from a healthy normal FVB mouse were included as a control. (A) FGFR3 TDII is expressed and constitutively activated in the tumor tissues of EμSR-FGFR3 TDII transgenic mice A.5652 and A.5653. Expression of FGFR3 TDII was detected by immunoblotting in lymph node cell lysates. The activating tyrosine-phosphorylated FGFR3 TDII was detected by an antibody specifically recognizing the 2 phosphorylated activation loop tyrosine residues in FGFRs. (B) PLCγ is hyperphosphorylated and activated in the tumor tissues by FGFR3 TDII in transgenic mice. (C-D) Elevated tyrosine phosphorylation of STAT3 and expression of Bcl-XL in tumor tissues of FGFR3 TDII transgenic mice.
Activation of PLCγ and STAT3 in tumors of EμSR-FGFR3 TDII transgenic mice. Spleen samples from a healthy normal FVB mouse were included as a control. (A) FGFR3 TDII is expressed and constitutively activated in the tumor tissues of EμSR-FGFR3 TDII transgenic mice A.5652 and A.5653. Expression of FGFR3 TDII was detected by immunoblotting in lymph node cell lysates. The activating tyrosine-phosphorylated FGFR3 TDII was detected by an antibody specifically recognizing the 2 phosphorylated activation loop tyrosine residues in FGFRs. (B) PLCγ is hyperphosphorylated and activated in the tumor tissues by FGFR3 TDII in transgenic mice. (C-D) Elevated tyrosine phosphorylation of STAT3 and expression of Bcl-XL in tumor tissues of FGFR3 TDII transgenic mice.
Surprisingly, a combination of mutations at Y724 with Y760, or Y577 and Y770 with Y760 fails to significantly further attenuate TDII Y760F–mediated transformation in mice, although such multiple substitutions result in some decrease in TDII Y760F transforming activity in Ba/F3 cells. However, substitution of all 4 non–activation loop tyrosine residues significantly attenuated the TDII Y760F–induced transformation in vivo and in vitro. These data suggest that a minimal engagement of multiple pathways, which apparently include the Y760-mediated PLCγ signaling pathway, signal through non–activation loop tyrosine residues in TDII-dependent transformation. Given that the 4F mutant in the contexts of both FGFR3 TDII and TEL-FGFR3 still retains basal transforming activity, certain pathway(s) independent of all 4 non–activation loop tyrosine residues may be also involved in full transformation by the activated FGFR3 mutants. Consistent with this possibility, FGFR3 TDII mutants with substitution of any or all the non–activation loop tyrosine residues still activate MAPK (Figure 2F). Similarly, all of the non–activation loop tyrosine residues in FGFR1 are dispensable for FGFR1-dependent activation of MAPK and consequent mitogenesis in L6 myoblasts, as well as neurite outgrowth stimulated by FGFR1. These observations indicate that the signaling required for these cellular processes is independent of those tyrosine residues.17
One potential explanation for the progressive decrements in hematopoietic transformation by FGFR3 TDII or TEL-FGFR3 variants is that there might be progressive disruption of protein tyrosine kinase activity due to the Y→F substitutions. However, we observed comparable autokinase activities of TEL-FGFR3 mutants with multiple substitutions as wild-type TEL-FGFR3 (Figure 5C). Thus, substitution of non–activation loop tyrosine residues in the constitutively activated FGFR3 mutants does not grossly alter the kinase activity. Consistent with this concept, the TEL-FGFR3 4F mutant is still able to activate STAT5 and PI3K (Figure 5D). Similarly, the FGFR3 TDII 4F mutant activates MAPK to a comparable level as the control FGFR3 TDII (Figure 2F).
Together, the data of mutational analysis suggest that the full transforming activity of FGFR3 requires, at a minimum, an engagement of signaling pathways besides PLCγ. Indeed, activation of STAT1 and increased expression of p21(WAF1/CIP1) is observed in cartilage cells from the thanatophoric dysplasia type II (TDII) fetus, but not in those from the healthy fetus.31 The Ras/Raf/MAPK pathway is required for cell proliferation stimulated by FGFR3 in L6 cells.32 In particular, the focus formation of FGFR3 TDII–transformed NIH3T3 cells is inhibited by treatment of PD98059 or LY294002, specific inhibitors of mitogen-induced extracellular kinase (MEK) and PI3K, respectively.33 Consistent with these observations, we observed that FGFR3 TDII activates PLCγ and MAPK in Ba/F3 cells. Furthermore, expression of FGFR3 TDII resulted in hyperphosphorylation and activation of PLCγ as well as elevated activation of STAT3 and expression of antiapoptotic Bcl-XL, in a transgenic mouse model of FGFR3 TDII–induced pro-B-cell lymphoma presented herein. This is consistent with the recent report that STAT3 activation has been detected in 48% of a series of multiple myeloma cases investigated, and a high level of Bcl-XL expression was identified in 89% of these cases.34 Activation of STAT3 has been demonstrated to confer resistance to apoptosis in human U266 multiple myeloma cells.35 Thus, STAT3 may promote disease progression in the FGFR3 TDII transgenic mice by protecting tumor cells from apoptosis, probably through stimulation of Bcl-XL expression. In the context of TEL-FGFR3, the fusion tyrosine kinase activates PLCγ, PI3K, and STAT5 in Ba/F3 cells. Y724 was suggested as a candidate site for recruitment and activation of PI3K because this tyrosine residue is located in a YMXM motif representing a consensus binding site for PI3K p85 subunit.13 However, substitution of Y724 or other non–activation loop tyrosine residues does not alter tyrosine phosphorylation levels of PI3K p85 or STAT5 by TEL-FGFR3, indicating that the activation of these signaling components is independent of these tyrosine residues in the context of TEL-FGFR3. A recent report36 demonstrates that substitution of Y718 in murine FGFR3 TDII, which is corresponding to Y724 in human FGFR3, abolished activation of Janus kinase 1 (JAK1)/STAT1 pathway by TDII, suggesting that Y724 might be required for JAK1 recruitment and activation. Identification of the pathway signaling through non–activation loop Y577, Y724, and Y770 will be of interest.
In summary, these studies demonstrate that activated FGFR3 mutants induce hematopoietic transformation through an engagement of multiple signaling pathways including PLCγ-dependent and -independent pathways. Activating mutations of FGFR3 such as kinase domain mutation K650E, as well as extracellular domain mutations R248C, S249C, and G370C, have also been identified in human bladder and cervical carcinomas.37 Thus, these findings may also have therapeutic implications with regard to various hematopoietic and solid tumors associated with dysregulation of FGFR3.
Prepublished online as Blood First Edition Paper, March 22, 2005; DOI 10.1182/blood-2004-09-3686.
Supported in part by National Institutes of Health (NIH) grants DK50654 and CA66996, and the Leukemia and Lymphoma Society. J.C. is a Fellow of the Leukemia and Lymphoma Society, and D.G.G. is an Investigator of the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We gratefully acknowledge administrative assistance from Alexis Bywater and valuable discussion with members of the Gilliland lab.

![Figure 4. FGFR3 TDII mutants induce pre–B-cell lymphoma in BMT mice. (A) Sections of tissues from a representative FGFR3 TDII mouse show effacement of normal splenic and lymph node architecture, as well as replacement of normal hematopoietic cells within the bone marrow by an atypical population of intermediate to large lymphoid cells. Focal infiltration by these lymphoid cells was also frequently observed in the liver in both a sinusoidal and perivascular distribution. Magnifications are as indicated (hematoxylin and eosin [H&E]). The samples were analyzed by using Nikon Eclipse E400 microscope with Nikon 10 ×/0.25, 40 ×/1.30, or 60 ×/1.40 objective lenses (Nikon, Melville, NY). The pictures were taken with an RT Color Spot camera and analyzed with acquisition software Spot Version 4.0.6. (Diagnostic Instruments, Sterling Heights, MI) and Adobe Photoshop 6.0 (Adobe, San Jose, CA). (B) Spleen sections of mice that received transplants of FGFR3 TDII Y724F, Y760F, and 4F mutants show similar effacement of splenic architecture by atypical lymphoid cells as observed in the FGFR3 TDII mice, whereas FF4F mice did not develop disease (shown at a lower magnification that demonstrates normal splenic architecture). (C) Flow cytometry analysis confirms an immunophenotype of pre–B-cell lymphoma in mice that received transplants of FGFR3 TDII, identified as B220+, CD19+, CD25+, CD43+, but BP-1- and c-KIT-. (D) Distinct FGFR3 TDII variants induce a similar immature B-cell lymphoid disease in mice. Flow cytometry demonstrates a predominant population of pre–B cells stained by B220 and CD19 in bone marrow cells. The numbers of percentage of double-stained cells in the inside quadrant of the dot plots are indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/1/10.1182_blood-2004-09-3686/4/m_zh80130580740004.jpeg?Expires=1769129256&Signature=MpJdfAVi39ObVUEuI~35fR5DnwI79g~rJgfa-uckg0TyrEibF3ZV5J3wc89yES2rxdJQX07iA5zT6MupTRGAaBeQ8nUboY834-hP~Ffoz8tiALHB9uz4uU8kzS-tpTmgr4M7zDAugSFgn1ChbvAZDPH~MieZ8JN6cYm6j8-JVy5OQNeZ8RNqWO8o~S2kli7FPp-jwlFLwI767yIFqw3Av5sEspQBafo208JmrrzfuLL9OUQjv7CDRHkMhuJuP31okEmJBceWF0u0qFpqB4Z8ZjUcj0-FVirhjk3DICOd11UWXpVucFYcOiEzkmq~nBPtli0XAKpngV4B7NV4iEj6Xg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal