Pregnant women often develop anemia concomitant with the increase in serum erythropoietin levels, which are actually lower than those of nonpregnant anemic women due to the possible suppressive effect of endogenous estradiol on erythropoietin induction. The anemia, derived from hemodilution, does not act as a drive for erythropoietin induction, but iron deficiency, often observed during pregnancy, might. In order to demonstrate this, we investigated the effects of iron deficiency on estradiol-induced suppression of erythropoietin induction in rats. Single doses of estradiol suppressed hypoxia-, cobalt-, and bleeding-stimulated elevation of plasma erythropoietin levels and renal erythropoietin mRNA expression. Repeated administration of estradiol at 0.1 and 1 mg/kg for 2 months induced a slight anemic trend without elevation of plasma erythropoietin. Feeding an iron-deficient diet for 2 months induced plasma erythropoietin elevation without obvious anemia, but the simultaneous repeated administration of estradiol suppressed it and reversed the iron deficiency. Plasma erythropoietin levels had distinct negative correlations with plasma iron, plasma ferritin, and iron concentrations in the organs, but not with plasma hemoglobin level. These results suggest that iron deficiency would significantly stimulate erythropoietin induction during pregnancy, although estradiol might suppress it through iron restoration.
Introduction
Red blood cells (RBCs) circulating in peripheral blood are maintained at a fixed level on the basis of an equilibrium between production and destruction. One of the important factors involved in the regulation of RBC production is erythropoietin (Epo), an erythroid-specific glycoprotein hormone produced mainly by the kidney in adults.1 Renal Epo production is up-regulated by anemia, hypoxia, transition metals such as cobalt (Co) or nickel, and iron chelators.2 Plasma Epo levels, reflecting the amount of renal production, are useful in clinical diagnosis of renal disease–related anemia, including renal tubular dysfunction3-5 as well as chronic renal failure,6,7 in which plasma Epo levels are not elevated in response to the anemia. Chronic diseases without renal injury, such as malignancy or inflammation, can also produce anemia due to the functional suppression of Epo induction in the kidney.8,9
Pregnancy is also known to induce anemia concomitant with suppression of Epo production. Pregnant women often show elevation of plasma Epo,10,11 but the levels are in fact relatively low compared with those of nonpregnant women with the same degree of anemia.12,13 Since the body hormonal environment changes drastically during pregnancy, some of the relevant hormones have been suggested to attenuate Epo production. Especially, 17β-estradiol (E2), the plasma level of which gradually increases along with the progress of pregnancy and reaches as much as 30 ng/mL at term, around 100 times that of nonpregnant women,14 has been well studied, with an inhibitory effect on Epo production reported in animal experiments.15-18
However, the anemia observed in pregnancy is not derived from E2-induced insufficient Epo production, since actual RBC generation increases; the increase in plasma volume surpasses that in RBC mass (about 150% and 120% of those in a nonpregnant woman, respectively), resulting in “dilutional” or “physiologic” anemia.19 The expansion of RBC volume is obviously derived from increased Epo production, even though it is somewhat suppressed by the large amount of endogenously secreted E2 or diluted by the increase of plasma volume. But it is not yet clear what trigger stimulates Epo production during pregnancy. The anemia, which would be an important stimulant for Epo production in nonpregnant women, does not act as a significant drive, since pregnant women do not show the close correlation between serum Epo levels and hemoglobin (Hb) levels seen in patients with nonrenal anemia.20,21 These facts suggest that other factors would act as more significant triggers for Epo production in pregnancy.
The increase in maternal RBC mass and the growth of the fetus during pregnancy require increased amounts of iron,22,23 leading to a rapid decline in maternal iron stores.24,25 Of interest, iron has been suggested to play an important role in Epo production. Iron supplementation inhibits Epo production, whereas iron depletion by iron chelators, such as desferrixamine, stimulates Epo production both in vitro and in vivo,26-29 although desferrixamine inversely inhibits hypoxia-induced Epo production.30 In fact, there have been some reports suggesting a relationship between hematinic status and increased serum Epo levels in pregnant women.31,32 Therefore, iron depletion, not “dilutional anemia,” might be a key factor explaining why the increase in Epo production and the resultant RBC expansion are observed in pregnancy despite the suppressive effect of E2 on Epo production observed in animal experiments.
In order to demonstrate this, we performed 2 series of experiments using female rats. First, we checked and described in detail the effects within 24 hours of various doses of E2 on Epo induction enhanced by stimulants. Second, we investigated the hematologic effects of a large amount of E2 on rats fed iron-deficient chows for 2 months, thereby mimicking the situation in pregnant women, to elucidate the role of iron deficiency in erythropoiesis during pregnancy. Based on these results, we made a comprehensive analysis of the major determinants of maternal erythropoiesis during pregnancy.
Materials and methods
Animals and treatment
We used female Wistar rats aged 5 weeks, which were semi-immature, because 3-week-old rats, often used as immature animals to observe the effect of a small dose of E2, showed much higher baseline plasma Epo than did those aged 5 weeks or older.33 In addition, the doses of E2 used in this study were high enough to allow us to ignore endogenously secreted E2. The rats were not ovariectomized, to avoid confounding effects on Epo induction by blood loss or inflammation due to the surgery.9 We performed 2 kinds of experiments using these rats: one-shot injection of E2 at 1 μg/kg to 1 mg/kg along with exposure to stimulants of Epo induction to observe outcomes within 24 hours and repeated administration at 0.1 and 1 mg/kg for 2 months with an iron-deficient diet. The rats were fed CLEA Rodent Diet CE-2 (CLEA Japan, Tokyo, Japan) for 24-hour experiments; this diet is made primarily from soybeans and therefore might have some confounding effects on the suppression of Epo induction by E2 due to the presence of soy phytoestrogen.34 Low-Iron Purified Diet and Basal Diet 5755 for the control group (TestDiet, Richmond, IN) (the iron contents are 10-20 ppm and 60 ppm, respectively), which are casein based, were used in the 2-month experiments to observe the effects of iron deficiency. The estrogenic activities in the CLEA Diet and the Basal Diet measured using a human ovarian carcinoma cell line, BG1, which was stably transformed with an estrogen-responsive element fused luciferase reporter gene, were 3.1 and 1.2, respectively. However, in rats fed the Basal Diet, the suppressive effect of E2 at 100 μg/kg on hypoxia-stimulated Epo induction was almost the same as that of rats fed the CLEA Diet (30% and 40% decreases, respectively), indicating that phytoestrogen in the diet did not significantly affect the suppression of Epo induction by E2.
For the 24-hour experiments, we adopted 3 kinds of stimuli for Epo induction. The first was hypobaric hypoxia: rats were put in an airtight vacuum cage in which the air pressure was maintained at 0.65 atm with airflow at 100 mL/min. The second was Co injection at 15 mg/kg subcutaneously.35 The third was bleeding: 2 mL peripheral blood was bled from the heart, and the same amount of saline was injected intraperitoneally under ether anesthesia, resulting in about 90 g/L (9.0 g/dL) peripheral Hb (the normal level is about 130 g/L [13.0 g/dL]). We adopted these conditions because they increased plasma Epo levels approximately 7 to 10 times from baseline, which were the maximum magnitudes of enhancement without lethal effects. Immediately before exposure to the stimuli, rats were injected subcutaneously with E2 (Sigma, St Louis, MO) suspended in propylene glycol. Control rats were injected with the same amount of vehicle. The rats were killed by taking heparinized peripheral blood from the heart under ether anesthesia at 24 hours after treatment to measure Epo levels in peripheral blood, or at 6 hours to take total RNA from the kidneys.
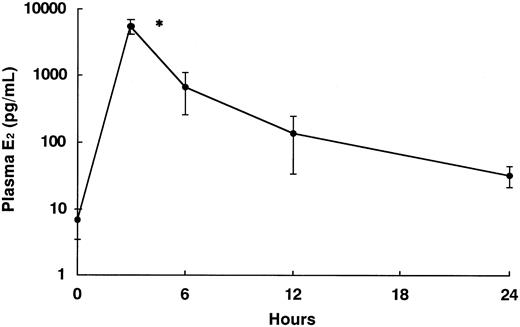
In the 2-month experiments, rats were injected with E2 at 0.1 or 1 mg/kg subcutaneously, and the controls were injected with the same amount of vehicle 3 times a week for 2 months. After that, rats were killed by taking heparinized peripheral blood from the heart as well as the liver and kidneys under ether anesthesia. After one-shot injection of E2 at 1 mg/kg, the plasma E2 level increased rapidly up to about 4 to 8 ng/mL at 3 hours, which was more than 800 times higher than baseline, then decreased gradually, still maintaining about 5 times the baseline level at 24 hours (Figure 1). Therefore, injection with E2 at 1 mg/kg 3 times a week for 2 months should be sufficient to maintain plasma E2 levels that mimic the maternal hormonal environment. These experiments were carried out under the control of the Animal Research Committee in accordance with the Guidelines on Animal Experiments of Jichi Medical School and the Animal Protection and Management Law (no. 105).
Analysis of peripheral blood
Peripheral RBC count was determined by a Bürker-Türk hemacytometer (ERMA, Tokyo, Japan) after dilution of the whole blood with Hayem reagent solution. The concentration of Hb in whole blood was measured by the cyan methemoglobin method.36 Hematocrit (Hct) was measured by centrifugation of aspirated blood using a microdispenser. Mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) were calculated from these results.37 Plasma samples were prepared by centrifugation of the heparinized whole blood and stocked at –80°C before analysis. The levels of iron, total iron-binding capacity (TIBC), ferritin, Epo, and E2 in the plasma samples were measured by a colorimetric method, competitive protein binding assay, enzyme immunoassay, radioimmunoassay,38 and radioimmunoassay, respectively.
Measurement of iron concentrations in organs
After removal of one of the hepatic lobes and the right kidney, the peripheral blood remaining in the organs was washed out via the hilus by phosphate-buffered saline using syringes. The tissue homogenates were then prepared with phosphate-buffered saline using a Potter-type homogenizer and digested with an acid mixture (HNO3/HClO4/H2SO4, 8:2:1, vol/vol and HNO3/HClO4, 5:1, vol/vol) on a hot plate at 130°C to 200°C. After resuspension of the residues in milli-Q water, the iron concentrations were analyzed by flameless atomic absorption spectrometry, using a Z-9000 Polarized Zeeman Atomic Absorption Spectrophotometer (Hitachi, Tokyo, Japan).
Ribonuclease protection assay
We prepared DNA templates for making riboprobes by ligating SP6 RNA polymerase promoter (Ambion, Austin, TX) into a 365-bp fragment of the rat Epo gene (GenBank accession no. M12930)4 and a 285-bp fragment of the rat β-actin gene (Promega, Madison WI), and then amplifying them by polymerase chain reaction (PCR) using a commercially available kit (Ambion). The Epo and β-actin riboprobes labeled by α32P–cytidine triphosphate (CTP) (NEN, Boston, MA) were transcribed from those DNA templates. Total RNA was purified from 100 mg renal cortex tissue using TRIzol reagent (Invitrogen, Carlsbad, CA). Total RNA (40 μg) was hybridized with excess amounts of Epo and β-actin riboprobes, then digested by RNase A and RNase T1. The protected RNAs were electrophoresed on 5% polyacrylamide gel and visualized with BAS 2000 (FUJI-FILM, Tokyo, Japan).
Plasma E2 levels in rats at 0 (no treatment), 3, 6, 12, and 24 hours after injection of E2 at 1 mg/kg. Each group included 5 rats. *Significant difference (P < .05) compared with the nontreatment group. Data are presented as mean values of plasma E2 levels with error bars indicating standard deviation (SD).
Plasma E2 levels in rats at 0 (no treatment), 3, 6, 12, and 24 hours after injection of E2 at 1 mg/kg. Each group included 5 rats. *Significant difference (P < .05) compared with the nontreatment group. Data are presented as mean values of plasma E2 levels with error bars indicating standard deviation (SD).
Measurement of plasma volume
We measured plasma volumes of the rats by the Evans blue dye dilution technique.39 In brief, rats were injected with approximately 1 mg/kg body weight of Evans blue (Wako, Osaka, Japan) diluted in saline into the heart under ether anesthesia, and after 10 minutes peripheral blood was taken from the heart by the method described in “Animals and treatment.” The whole blood samples were used for Hct measurement, and plasma samples prepared by centrifugation of the whole blood were used for measurement of the optical density at 620 nm with a blank of plasma from an untreated rat. The plasma volume was calculated from the optical density ratio of the used dye solution and plasma sample, adjusted by the value of the optical density at 740 nm. The RBC volume was further calculated from the values of the plasma volume and Hct.
Kinetic analysis of iron absorption from the intestine
Following overnight fasting for more than 12 hours, rats were administered approximately 18 kBq 59Fe (FeCl3 in 0.5 N hydrochloric acid; Amersham Biosciences, Buckinghamshire, United Kingdom) with 1 mL of 100 μg/mL iron nitrate by gavage using an esophageal cannula. After 6 hours, the rats were killed by taking heparinized peripheral blood from the heart under ether anesthesia, followed by removing the liver and the intestine, which was further slit longitudinally to wash the contents off with phosphate-buffered saline and divided into 3 portions: the duodenum (proximal 10% of total length), jejunum (following 40%), and ileum (remaining 50%).40 The radioactivity of the whole liver, intestines, and 1 mL blood as well as the whole 59Fe solution before administration were counted for 10 minutes using an Auto Well Gamma System ARC-2000 (Aloka, Tokyo, Japan), and the relative 59Fe uptakes in the organs were calculated. The relative count remaining in the cannula after administration was less than 0.1%.
Statistical analysis
The significance of differences between groups of rats was examined by one-way or 2-way analysis of variance (ANOVA), followed by comparison between the control groups and others using the Bonferroni multiple comparison procedure or Student t test. We determined the necessary number of rats in each group according to the variation in plasma Epo values.
Results
Suppression of Epo induction by E2
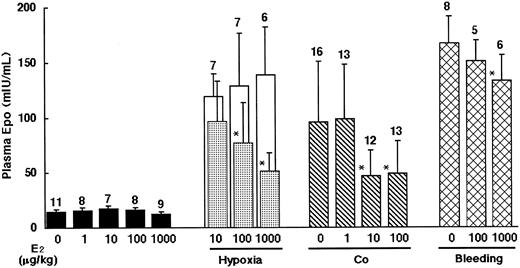
First, we observed the plasma Epo levels of the rats 24 hours after exposure to 3 kinds of stimulants for Epo induction: hypoxia, Co injection, or bleeding, with or without administration of various doses of E2 (Figure 2). E2 did not have any significant effects on basal plasma Epo levels, but it suppressed Epo induction stimulated by hypoxia dose dependently: the magnitudes of suppression were 20%, 40%, and 60% at 10 μg/kg, 100 μg/kg, and 1 mg/kg of E2, respectively. For Epo induction by Co, E2 showed a stronger suppressive effect, producing a 50% decrease at 10 μg/kg. Co concentration in the kidneys after administration of Co and E2 at 10 μg/kg did not differ from that after Co and vehicle administration, indicating that the suppression of Epo induction by E2 was not derived from the change of Co distribution (data not shown). In contrast, only 20% suppression of bleeding-stimulated Epo induction was observed at the highest dose of E2, 1 mg/kg. These results suggest that E2 would have a suppressive effect on Epo induction, although there is a difference in the effective doses among the stimulants.
Suppressive effects of E2 on Epo induction stimulated by hypoxia, Co, and bleeding for 24 hours in rats. ▪ indicate without stimulation; □ and ▦, hypoxia (vehicle- and E2-injected rats, respectively, confined to one hypoxic cage simultaneously); ▧, co-injection; and ▩, bleeding. Data are presented as mean values of plasma Epo levels with SD bars. The numbers of animals are shown on the tops of the bars. *Significant difference (P < .05) compared with each vehicle-injected group.
Suppressive effects of E2 on Epo induction stimulated by hypoxia, Co, and bleeding for 24 hours in rats. ▪ indicate without stimulation; □ and ▦, hypoxia (vehicle- and E2-injected rats, respectively, confined to one hypoxic cage simultaneously); ▧, co-injection; and ▩, bleeding. Data are presented as mean values of plasma Epo levels with SD bars. The numbers of animals are shown on the tops of the bars. *Significant difference (P < .05) compared with each vehicle-injected group.
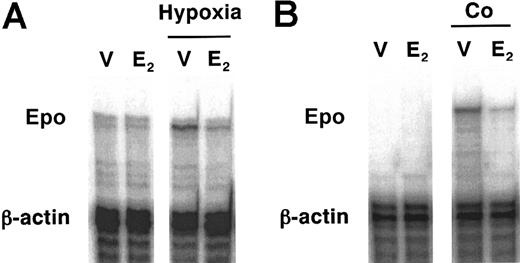
We further looked into the effect of E2 on Epo mRNA induction stimulated by hypoxia or Co injection in the kidneys of rats by the ribonuclease protection assay. Renal Epo mRNA expression was induced by 6-hour exposure to hypoxia, and E2 suppressed it at 1 mg/kg, which decreased the plasma Epo level effectively as well (Figure 3A). E2 at 100 μg/kg also suppressed Epo mRNA expression in the kidneys 6 hours after Co injection (Figure 3B). These results suggest that the suppression of Epo by E2 is induced at the mRNA level.
Hematologic effects of E2 along with iron deficiency
We next performed experiments in which we repeatedly administered high doses of E2 to rats fed the Low-Iron Purified Diet (Basal Diet for the controls) to create pregnancy-like hormonal and iron conditions and observed the actual hematologic effects. The E2-exposed group fed the Basal Diet showed slight but significant dose-dependent decreases in RBC, Hct, and Hb in a normocytic and normochromic manner without a biologically significant increase of plasma Epo level (Table 1). This result suggests that the suppression of Epo induction by E2 observed in the 6- and 24-hour experiments might induce the anemic trend. However, the involvement of hemodilution has to be taken into consideration since E2 has a profound ability to increase plasma volume.41 Therefore, we measured the volumes of circulating plasma and RBCs by the Evans blue dye dilution technique in the different animal groups (Table 2). A relative increase in plasma volumes compared with RBC volumes caused by E2 was observed; plasma volumes adjusted by body weights in the rats administered E2 were about 1.4 times those in the vehicle-treated rats, while there was no significant difference in RBC volumes between them. This suggests that E2-induced hemodilution, in addition to Epo suppression, would contribute to the progress of anemia, which is very similar to actual human pregnancy. The levels of plasma iron and TIBC were significantly elevated, whereas the ferritin level, an indicator of iron storage,42 was slightly decreased by E2 exposure dose-dependently, indicating that iron metabolism would be activated by E2, including transferrin induction or mobilization of the stored iron.
Hematologic effects of E2 in iron-deficient rats
. | Vehicle . | E2, 0.1 mg/kg . | E2, 1 mg/kg . |
|---|---|---|---|
| RBC, 104/mm3 | |||
| Basal Diet | 589.1 ± 60.5 | 514.5 ± 65.0* | 513.3 ± 65.5* |
| Low-Iron Diet | 955.6 ± 89.9† | 813.9 ± 70.5*† | 680.9 ± 49.5*† |
| Hematocrit, % | |||
| Basal Diet | 46.9 ± 1.9 | 44.1 ± 1.6* | 40.8 ± 1.3* |
| Low-Iron Diet | 46.0 ± 1.9 | 46.0 ± 2.0 | 42.0 ± 2.1* |
| Hemoglobin, g/dL | |||
| Basal Diet | 14.9 ± 0.6 | 14.1 ± 0.5* | 13.1 ± 0.6* |
| Low-Iron Diet | 13.6 ± 0.9† | 14.0 ± 0.8 | 12.9 ± 0.5* |
| MCV, μm3 | |||
| Basal Diet | 80.2 ± 7.3 | 86.8 ± 10.0 | 80.5 ± 10.2 |
| Low-Iron Diet | 48.6 ± 5.4† | 57.0 ± 6.3*† | 61.9 ± 4.0*† |
| MCH, pg | |||
| Basal Diet | 25.5 ± 2.5 | 27.7 ± 2.9 | 25.9 ± 3.1 |
| Low-Iron Diet | 14.4 ± 1.9† | 17.3 ± 2.2*† | 19.0 ± 1.6*† |
| MCHC, % | |||
| Basal Diet | 31.8 ± 0.6 | 31.9 ± 0.6 | 32.2 ± 0.8 |
| Low-Iron Diet | 29.6 ± 1.3† | 30.4 ± 1.1† | 30.7 ± 1.5† |
| Plasma iron, μg/dL | |||
| Basal Diet | 322.3 ± 37.2 | 348.9 ± 71.3 | 423.8 ± 63.8* |
| Low-Iron Diet | 54.0 ± 10.9† | 83.2 ± 51.7† | 198.6 ± 64.1*† |
| TIBC, μg/dL | |||
| Basal Diet | 527.3 ± 28.9 | 577.4 ± 42.0* | 667.8 ± 43.1* |
| Low-Iron Diet | 718.7 ± 31.0† | 751.8 ± 33.0† | 757.0 ± 50.1*† |
| Ferritin, ng/mL | |||
| Basal Diet | 97.6 ± 43.7 | 96.2 ± 39.1 | 82.1 ± 22.9 |
| Low-Iron Diet | 16.4 ± 8.2† | 17.5 ± 20.1† | 36.3 ± 29.1† |
| Plasma Epo, mIU/mL | |||
| Basal Diet | 15.0 ± 2.5 | 19.3 ± 4.8 | 21.2 ± 5.1 |
| Low-Iron Diet | 45.5 ± 13.9† | 51.3 ± 17.1† | 19.7 ± 7.0* |
. | Vehicle . | E2, 0.1 mg/kg . | E2, 1 mg/kg . |
|---|---|---|---|
| RBC, 104/mm3 | |||
| Basal Diet | 589.1 ± 60.5 | 514.5 ± 65.0* | 513.3 ± 65.5* |
| Low-Iron Diet | 955.6 ± 89.9† | 813.9 ± 70.5*† | 680.9 ± 49.5*† |
| Hematocrit, % | |||
| Basal Diet | 46.9 ± 1.9 | 44.1 ± 1.6* | 40.8 ± 1.3* |
| Low-Iron Diet | 46.0 ± 1.9 | 46.0 ± 2.0 | 42.0 ± 2.1* |
| Hemoglobin, g/dL | |||
| Basal Diet | 14.9 ± 0.6 | 14.1 ± 0.5* | 13.1 ± 0.6* |
| Low-Iron Diet | 13.6 ± 0.9† | 14.0 ± 0.8 | 12.9 ± 0.5* |
| MCV, μm3 | |||
| Basal Diet | 80.2 ± 7.3 | 86.8 ± 10.0 | 80.5 ± 10.2 |
| Low-Iron Diet | 48.6 ± 5.4† | 57.0 ± 6.3*† | 61.9 ± 4.0*† |
| MCH, pg | |||
| Basal Diet | 25.5 ± 2.5 | 27.7 ± 2.9 | 25.9 ± 3.1 |
| Low-Iron Diet | 14.4 ± 1.9† | 17.3 ± 2.2*† | 19.0 ± 1.6*† |
| MCHC, % | |||
| Basal Diet | 31.8 ± 0.6 | 31.9 ± 0.6 | 32.2 ± 0.8 |
| Low-Iron Diet | 29.6 ± 1.3† | 30.4 ± 1.1† | 30.7 ± 1.5† |
| Plasma iron, μg/dL | |||
| Basal Diet | 322.3 ± 37.2 | 348.9 ± 71.3 | 423.8 ± 63.8* |
| Low-Iron Diet | 54.0 ± 10.9† | 83.2 ± 51.7† | 198.6 ± 64.1*† |
| TIBC, μg/dL | |||
| Basal Diet | 527.3 ± 28.9 | 577.4 ± 42.0* | 667.8 ± 43.1* |
| Low-Iron Diet | 718.7 ± 31.0† | 751.8 ± 33.0† | 757.0 ± 50.1*† |
| Ferritin, ng/mL | |||
| Basal Diet | 97.6 ± 43.7 | 96.2 ± 39.1 | 82.1 ± 22.9 |
| Low-Iron Diet | 16.4 ± 8.2† | 17.5 ± 20.1† | 36.3 ± 29.1† |
| Plasma Epo, mIU/mL | |||
| Basal Diet | 15.0 ± 2.5 | 19.3 ± 4.8 | 21.2 ± 5.1 |
| Low-Iron Diet | 45.5 ± 13.9† | 51.3 ± 17.1† | 19.7 ± 7.0* |
The rats were administered E2 at the indicated doses 3 times a week for 2 months and fed the Basal Diet 5755 (n = 8) or the Low-Iron Purified Diet (n = 9) from TestDiet. Data are presented as mean ± SD.
P < .05 (compared with vehicle group).
P < .05 (compared with Basal Diet group).
Plasma and RBC volumes in rats
. | Vehicle . | E2, 1 mg/kg . |
|---|---|---|
| Body weight, g | ||
| Basal Diet | 189.8 ± 3.8 | 162.0 ± 5.4* |
| Low-Iron Diet | 174.8 ± 8.9† | 157.2 ± 7.9* |
| Hematocrit, % | ||
| Basal Diet | 45.4 ± 3.4 | 40.7 ± 2.0* |
| Low-Iron Diet | 43.5 ± 2.7 | 41.8 ± 5.0 |
| Plasma volume, mL | ||
| Basal Diet | 8.1 ± 1.2 | 9.4 ± 2.1 |
| Low-Iron Diet | 7.7 ± 1.1 | 9.7 ± 3.0 |
| Plasma volume/body weight, mL/kg | ||
| Basal Diet | 42.8 ± 6.2 | 57.9 ± 13.3* |
| Low-Iron Diet | 44.3 ± 7.3 | 61.7 ± 17.4 |
| RBC volume, mL | ||
| Basal Diet | 6.7 ± 0.7 | 6.5 ± 2.0 |
| Low-Iron Diet | 5.9 ± 0.8 | 6.9 ± 1.8 |
| RBC volume/body weight, mL/kg | ||
| Basal Diet | 35.5 ± 3.8 | 40.4 ± 12.5 |
| Low-Iron Diet | 34.0 ± 4.6 | 43.9 ± 10.4 |
. | Vehicle . | E2, 1 mg/kg . |
|---|---|---|
| Body weight, g | ||
| Basal Diet | 189.8 ± 3.8 | 162.0 ± 5.4* |
| Low-Iron Diet | 174.8 ± 8.9† | 157.2 ± 7.9* |
| Hematocrit, % | ||
| Basal Diet | 45.4 ± 3.4 | 40.7 ± 2.0* |
| Low-Iron Diet | 43.5 ± 2.7 | 41.8 ± 5.0 |
| Plasma volume, mL | ||
| Basal Diet | 8.1 ± 1.2 | 9.4 ± 2.1 |
| Low-Iron Diet | 7.7 ± 1.1 | 9.7 ± 3.0 |
| Plasma volume/body weight, mL/kg | ||
| Basal Diet | 42.8 ± 6.2 | 57.9 ± 13.3* |
| Low-Iron Diet | 44.3 ± 7.3 | 61.7 ± 17.4 |
| RBC volume, mL | ||
| Basal Diet | 6.7 ± 0.7 | 6.5 ± 2.0 |
| Low-Iron Diet | 5.9 ± 0.8 | 6.9 ± 1.8 |
| RBC volume/body weight, mL/kg | ||
| Basal Diet | 35.5 ± 3.8 | 40.4 ± 12.5 |
| Low-Iron Diet | 34.0 ± 4.6 | 43.9 ± 10.4 |
The rats were administered E2 at 1 mg/kg 3 times a week for 2 months and fed the Basal Diet 5755 (vehicle group, N = 5; E2, N = 7) or the Low-Iron Purified Diet (vehicle group, n = 6; E2 group, n = 5) from TestDiet. Data are presented as mean ± SD.
P < .05 (compared with vehicle group).
P < .05 (compared with Basal Diet group).
Vehicle-administered control rats fed the low-iron diet showed only a small decrease of Hb and no change in Hct, with intensive decreases in plasma iron and ferritin levels along with TIBC elevation compared with the rats fed the Basal Diet, indicating that they developed distinct iron deficiency without anemia. The increase of RBC count could be ascribed to microcytosis due to the iron deficiency, evidenced by the reductions in MCV, MCH, and MCHC values. Despite almost-normal levels of Hct and Hb, however, the plasma Epo level was significantly elevated in the iron-deficient rats, indicating that Epo could be induced by the shortage of iron stores alone even in the absence of anemia; in other words, iron itself would be an important regulator for Epo induction.
Suppressive effects of E2 on Epo mRNA expression in the kidneys. Rats were exposed to hypoxia (A) or injected with Co (B) for 6 hours demonstrated by ribonuclease protection assay. (A) V indicates vehicle; E, E2 at 1 mg/kg. (B) V indicates vehicle; E, E2 at 100 μg/kg. These are representative data from 3 independent experiments.
Suppressive effects of E2 on Epo mRNA expression in the kidneys. Rats were exposed to hypoxia (A) or injected with Co (B) for 6 hours demonstrated by ribonuclease protection assay. (A) V indicates vehicle; E, E2 at 1 mg/kg. (B) V indicates vehicle; E, E2 at 100 μg/kg. These are representative data from 3 independent experiments.
In contrast, E2 administration, especially at 1 mg/kg, increased plasma iron and ferritin levels in the iron-deficient rats, indicating that E2 would increase the efficiency of iron absorption in the iron-deficient animals (Table 1). The decline of RBC count and the increases in MCV and MCH values were dose dependent, meaning that the iron deficiency was improved by E2. The elevation of TIBC values in both iron-deficient and non–iron-deficient rats suggests that E2 could increase transferrin induction regardless of the amount of iron stores. Of interest, the elevated plasma Epo in the iron-deficient rats was abruptly reduced by 1 mg/kg E2, which was in an inverse manner to ferritin, suggesting again that iron deficiency would be a significant factor to induce Epo.
Unlike the situation in the non–iron-deficient rats, the Hct and Hb values in the iron-deficient rats did not show any decreases even after the administration of E2 at 0.1 mg/kg, suggesting that the drive to induce Epo caused by iron deficiency would overcome the E2 effect to suppress Epo induction, resulting in sustained erythropoiesis. In contrast, 1 mg/kg E2 induced decreases in Hct and Hb without elevation of plasma Epo in the iron-deficient rats; this was essentially the same hematologic status as that of the non–iron-deficient rats with E2 administration. This result indicates that 1 mg/kg E2 suppressed erythropoiesis even in the iron-deficient rats by restoring iron.
Since these hematologic examination results suggest that E2 would enhance iron absorption from the intestine, we confirmed it by observing short-time kinetics of orally administered iron using 59Fe in different groups of animals (Table 3). The 59Fe fractions of the duodenum, jejunum, and ileum 6 hours after administration were increased significantly by E2 in the non–iron-deficient rats. On the other hand, that of the duodenum decreased while those of peripheral blood and the liver increased in the vehicle-treated iron-deficient rats, meaning that there was a quick passage of iron through the intestine into the blood circulation due to the strong ability of iron deficiency to enhance iron absorption. Furthermore, those of blood and the liver in the E2-administered rats with iron deficiency showed great increases, as much as approximately 5 times that of the controls. These results indicate that E2 stimulates iron absorption from the intestine and accumulation in the liver, and it is extremely enhanced by iron deficiency, supporting the result shown in Table 1.
Weights and relative fractions of 59Fe in the organs and peripheral blood of rats at 6 hours after oral administration of 59Fe by gavage
. | Vehicle . | E2 1 mg/kg . |
|---|---|---|
| Weight, g | ||
| Duodenum | ||
| Basal Diet | 0.51 ± 0.05 | 0.56 ± 0.07 |
| Low-Iron Diet | 0.48 ± 0.04 | 0.56 ± 0.05* |
| Jejunum | ||
| Basal Diet | 1.38 ± 0.08 | 1.38 ± 0.15 |
| Low-Iron Diet | 1.24 ± 0.06† | 1.39 ± 0.14* |
| Ileum | ||
| Basal Diet | 1.33 ± 0.18 | 1.33 ± 0.17 |
| Low-Iron Diet | 1.26 ± 0.13 | 1.36 ± 0.13 |
| Liver | ||
| Basal Diet | 4.3 ± 0.4 | 5.5 ± 0.4* |
| Low-Iron Diet | 3.8 ± 0.4 | 5.1 ± 0.3* |
| 59Fe fraction, % | ||
| Duodenum | ||
| Basal Diet | 6.8 ± 1.5 | 10.9 ± 3.4* |
| Low-Iron Diet | 2.3 ± 0.9† | 3.9 ± 0.7*† |
| Jejunum | ||
| Basal Diet | 6.2 ± 1.1 | 10.8 ± 2.1* |
| Low-Iron Diet | 5.2 ± 2.0 | 6.7 ± 2.3† |
| Ileum | ||
| Basal Diet | 0.9 ± 0.2 | 1.8 ± 0.6* |
| Low-Iron Diet | 1.6 ± 0.3† | 2.3 ± 0.6* |
| Blood, 1 mL | ||
| Basal Diet | 0.3 ± 0.2 | 0.3 ± 0.1 |
| Low-Iron Diet | 0.8 ± 0.2† | 1.4 ± 0.3*† |
| Liver | ||
| Basal Diet | 5.4 ± 1.5 | 7.3 ± 4.5 |
| Low-Iron Diet | 8.5 ± 1.4 | 29.6 ± 13.6*† |
. | Vehicle . | E2 1 mg/kg . |
|---|---|---|
| Weight, g | ||
| Duodenum | ||
| Basal Diet | 0.51 ± 0.05 | 0.56 ± 0.07 |
| Low-Iron Diet | 0.48 ± 0.04 | 0.56 ± 0.05* |
| Jejunum | ||
| Basal Diet | 1.38 ± 0.08 | 1.38 ± 0.15 |
| Low-Iron Diet | 1.24 ± 0.06† | 1.39 ± 0.14* |
| Ileum | ||
| Basal Diet | 1.33 ± 0.18 | 1.33 ± 0.17 |
| Low-Iron Diet | 1.26 ± 0.13 | 1.36 ± 0.13 |
| Liver | ||
| Basal Diet | 4.3 ± 0.4 | 5.5 ± 0.4* |
| Low-Iron Diet | 3.8 ± 0.4 | 5.1 ± 0.3* |
| 59Fe fraction, % | ||
| Duodenum | ||
| Basal Diet | 6.8 ± 1.5 | 10.9 ± 3.4* |
| Low-Iron Diet | 2.3 ± 0.9† | 3.9 ± 0.7*† |
| Jejunum | ||
| Basal Diet | 6.2 ± 1.1 | 10.8 ± 2.1* |
| Low-Iron Diet | 5.2 ± 2.0 | 6.7 ± 2.3† |
| Ileum | ||
| Basal Diet | 0.9 ± 0.2 | 1.8 ± 0.6* |
| Low-Iron Diet | 1.6 ± 0.3† | 2.3 ± 0.6* |
| Blood, 1 mL | ||
| Basal Diet | 0.3 ± 0.2 | 0.3 ± 0.1 |
| Low-Iron Diet | 0.8 ± 0.2† | 1.4 ± 0.3*† |
| Liver | ||
| Basal Diet | 5.4 ± 1.5 | 7.3 ± 4.5 |
| Low-Iron Diet | 8.5 ± 1.4 | 29.6 ± 13.6*† |
The rats were administered E2 at 1 mg/kg 3 times a week for 2 months and fed the Basal Diet 5755 (vehicle group, n = 6; E2 group, n = 5) or the Low-Iron Purified Diet (vehicle group, n = 6; E2 group, n = 6) from TestDiet. Data are presented as mean ± SD.
P < .05 (compared with vehicle group).
P < .05 (compared with Basal Diet group).
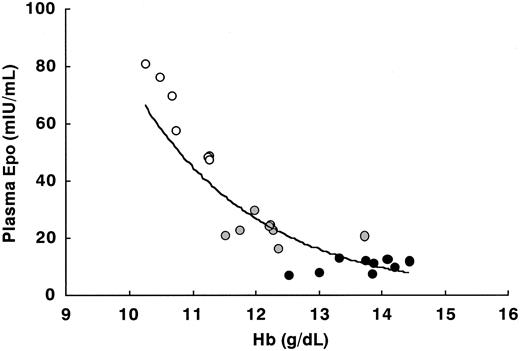
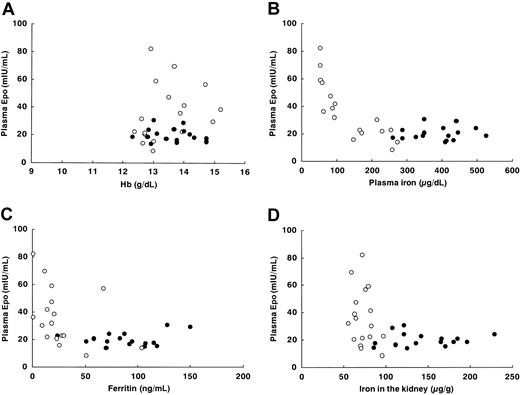
In order to further demonstrate the significance of iron deficiency as an inducer of Epo, we made scatter diagrams of the relationship between plasma Epo and other relevant factors, where the data for E2-administered rats in Table 1 (n = 33) were used for comparison with human pregnancy. While there was a clear inverse relationship between Hb and plasma Epo in rats with acute anemia induced by bleeding without E2 administration (Figure 4), there was no significant association in E2-administered rats, including iron-deficient rats (Figure 5A). This result is consistent with data on pregnant women.20,21 In contrast, the scatter diagrams of plasma Epo and plasma iron or ferritin showed distinct negative correlations, making “inverse J curves” (Figure 5B-C). These diagrams indicate that in pregnancy iron storage is much more important for Epo induction than is the degree of anemia.
Relationships between plasma Epo and iron concentrations in organs
We measured iron concentrations in the liver and the kidney to further confirm the actual effects of iron accumulated in these organs on Epo induction (Table 4). While the iron concentration in the liver, one of the iron-storage organs, was not changed significantly by E2 exposure in the non–iron-deficient rats, the total accumulated amount of iron increased when the increase in the liver weight was taken into account. The hepatic iron concentration, which showed a severe decrease in the rats fed the Low-Iron Diet, was restored dose dependently by E2 administration. These results verify the effect of E2 in increasing iron stores. It is noteworthy that the pattern of hepatic iron concentrations was almost perfectly consistent with plasma ferritin levels (Table 1). The iron concentration in the kidney was increased by E2 exposure, especially at 1 mg/kg, in the non–iron-deficient rats, while the effect of E2 in correcting the iron deficiency induced by feeding the Low-Iron Diet was not so obvious as that observed for the hepatic iron concentration (Table 4). The scatter diagram of renal iron and plasma Epo in the rats given E2 showed the characteristic “L curve”: excess induction of Epo was observed only in the limited, lower range of renal iron concentrations (Figure 5D). The same correlation was observed between hepatic iron and plasma Epo (data not shown). These results indicate that the actual levels of iron accumulated in the body would play a significant role in the induction of Epo.
Weights of the livers and the kidneys and iron concentrations in rats
. | Vehicle . | E2, 0.1 mg/kg . | E2, 1 mg/kg . |
|---|---|---|---|
| Weight, g | |||
| Liver | |||
| Basal Diet | 5.8 ± 0.7 | 6.5 ± 0.7 | 7.3 ± 0.9* |
| Low-Iron Diet | 4.9 ± 0.6† | 5.6 ± 0.6*† | 7.2 ± 0.8* |
| Kidney | |||
| Basal Diet | 0.58 ± 0.03 | 0.58 ± 0.07 | 0.65 ± 0.06* |
| Low-Iron Diet | 0.60 ± 0.04 | 0.61 ± 0.05 | 0.67 ± 0.05* |
| Iron concentration, μg/g | |||
| Liver | |||
| Basal Diet | 330.3 ± 71.6 | 336.5 ± 90.1 | 310.6 ± 74.2 |
| Low-Iron Diet | 58.9 ± 18.6† | 65.2 ± 13.3† | 106.5 ± 51.7*† |
| Kidney | |||
| Basal Diet | 114.5 ± 28.0 | 125.4 ± 32.9 | 165.3 ± 40.1* |
| Low-Iron Diet | 63.4 ± 15.3† | 70.9 ± 9.0† | 75.1 ± 15.0† |
. | Vehicle . | E2, 0.1 mg/kg . | E2, 1 mg/kg . |
|---|---|---|---|
| Weight, g | |||
| Liver | |||
| Basal Diet | 5.8 ± 0.7 | 6.5 ± 0.7 | 7.3 ± 0.9* |
| Low-Iron Diet | 4.9 ± 0.6† | 5.6 ± 0.6*† | 7.2 ± 0.8* |
| Kidney | |||
| Basal Diet | 0.58 ± 0.03 | 0.58 ± 0.07 | 0.65 ± 0.06* |
| Low-Iron Diet | 0.60 ± 0.04 | 0.61 ± 0.05 | 0.67 ± 0.05* |
| Iron concentration, μg/g | |||
| Liver | |||
| Basal Diet | 330.3 ± 71.6 | 336.5 ± 90.1 | 310.6 ± 74.2 |
| Low-Iron Diet | 58.9 ± 18.6† | 65.2 ± 13.3† | 106.5 ± 51.7*† |
| Kidney | |||
| Basal Diet | 114.5 ± 28.0 | 125.4 ± 32.9 | 165.3 ± 40.1* |
| Low-Iron Diet | 63.4 ± 15.3† | 70.9 ± 9.0† | 75.1 ± 15.0† |
The rats were administered E2 at the indicated doses 3 times a week for 2 months and fed the Basal Diet 5755 (n = 8 for all groups) or the Low-Iron Purified Diet (vehicle and 0.1 mg/kg E2 groups, n = 9; 1 mg/kg E2 group, n = 8) from TestDiet. Samples were taken from the same rats as in Table 1. Data are presented by mean ± SD.
P < .05 (compared with vehicle group).
P < .05 (compared with Basal Diet group).
Discussion
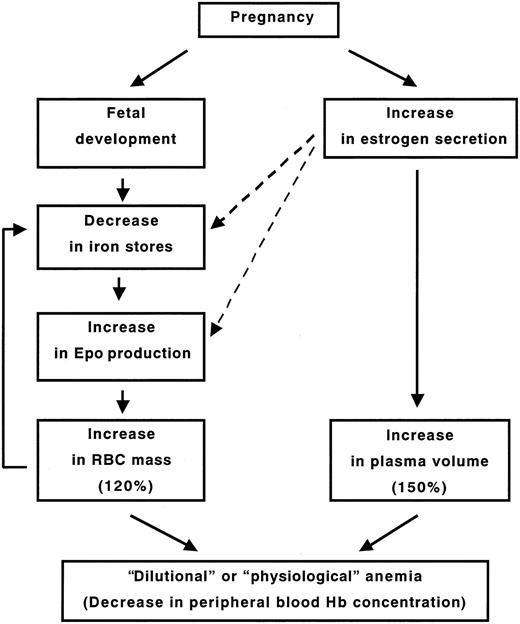
In this study, we demonstrated that E2 could suppress Epo induction at 24 hours by 3 well-known stimulants, hypoxia, Co, and bleeding, and that repeated administration of large amounts of E2 for 2 months actually induced a slight but significant decrease in Hb levels without elevation of plasma Epo in rats, although it was accompanied by hemodilution. However, the same experiments using iron-deficient rats revealed that Epo could be efficiently induced by iron deficiency, not by the degree of anemia, but the Epo induction was suppressed by E2 indirectly through reversal of the iron deficiency. These results provide mechanistic insights into the hematologic status during pregnancy, as illustrated in Figure 6. In the process of pregnancy, fetal development and maternal RBC expansion require much iron mobilization from the maternal iron stores, leading to iron deficiency that induces Epo and consequent RBC production, creating a progressing cycle that continues until delivery. However, these trends are kept in check by secretion of a large amount of endogenous E2 during pregnancy, which suppresses Epo production and limits iron deficiency. At the same time, plasma volume increases even more than the increase in RBC mass by the E2 effect, resulting in “dilutional anemia.”
We used experimental animal models with E2 exposure and iron deficiency to mimic human pregnancy, but the differences between the models and actual pregnancy must be taken into account. First, there is no fetal erythropoiesis in the animal models, which would require a large amount of iron in pregnant women, resulting in the accumulation of excess iron in the rat bodies due to the E2-induced acceleration of iron absorption. Second, possible modifications of Epo induction by other hormones induced from the placenta are lacking in the models. For example, serum levels of human chorionic somatotropin were reported to correlate with plasma Epo in pregnant women.43 However, the scatter diagram of the relationship between plasma Epo and Hb in the rats (Figure 5A) showed the same pattern as that of human pregnant women,20,21 indicating that the animal models we used were sufficiently similar to actual erythropoiesis in pregnancy.
The relationship between plasma Epo and Hb in peripheral blood in female rats at 8 weeks with various degrees of bleeding. • indicates no bleeding (n = 9); ⬡, 1-mL bleeding (n = 8); and ○, 2-mL bleeding (n = 7). The regression equation and correlation coefficient of the regression line are y = 13 255e-0.5172x and 0.871, respectively.
The relationship between plasma Epo and Hb in peripheral blood in female rats at 8 weeks with various degrees of bleeding. • indicates no bleeding (n = 9); ⬡, 1-mL bleeding (n = 8); and ○, 2-mL bleeding (n = 7). The regression equation and correlation coefficient of the regression line are y = 13 255e-0.5172x and 0.871, respectively.
The relationships between plasma Epo and the relevant factors in rats. Hb in peripheral blood (A), plasma iron (B), plasma ferritin (C), and iron concentration in the kidney (D). The rats were injected with E2 at 1 mg/kg 3 times a week for 2 months and fed the Basal Diet 5755 (solid circles, n = 16) or the Low-Iron Purified Diet (open circles, n = 17).
The relationships between plasma Epo and the relevant factors in rats. Hb in peripheral blood (A), plasma iron (B), plasma ferritin (C), and iron concentration in the kidney (D). The rats were injected with E2 at 1 mg/kg 3 times a week for 2 months and fed the Basal Diet 5755 (solid circles, n = 16) or the Low-Iron Purified Diet (open circles, n = 17).
It is quite intriguing that plasma Epo levels and all of the indicators of iron stores, including plasma iron and ferritin as well as the iron concentrations in the liver and the kidney, showed the characteristic inverse J curve or L curve (Figure 5). This suggests that there would be a critical threshold level of iron concentration, especially in the kidney, to induce extra Epo, since renal iron is thought to affect the regulation of Epo induction at the kidney in situ. According to the scatter diagram of plasma Epo and renal iron, the threshold of renal iron must be approximately 70 to 80 μg/g (Figure 5D); plasma Epo remained low above the threshold but started increasing below the threshold as if a specific sensor switch were turned on. Of interest, there have been some recent reports to suggest that iron itself would be significantly involved in the oxygen-sensing mechanism. The induction of the Epo gene is mediated by hypoxia-inducible factor-1 (HIF-1), which binds to a hypoxia-response element (HRE) in the 3′ enhancer of the gene.2 HIF consists of 2 subunits, HIFα and HIFβ; the former has a highly conservative residue, proline 564, which is hydroxylated to bind with von Hippel-Lindau protein (pVHL) in the presence of oxygen and iron, leading to the degradation of HIFα by a ubiquitin ligase.44,45 Therefore, Epo induction in the kidney might be “switched on” when the renal iron is depleted to a low level that would be insufficient to hydroxylate the proline residue of HIFα.
The hypothetical scheme of the mechanism underlying “dilutional anemia.” Solid arrows indicate direct action, and dashed arrows indicate inverse action.
The hypothetical scheme of the mechanism underlying “dilutional anemia.” Solid arrows indicate direct action, and dashed arrows indicate inverse action.
We have shown that E2 has some significant effects on iron metabolism. It was surprising that iron absorption through the intestine was synergistically enhanced to as much as approximately 30% by iron deficiency and E2, resulting in an increase of iron accumulation in the organs, although the ability of E2 alone to increase iron absorption has been reported before.46,47 Some kinds of iron transporters, such as divalent metal transporter 1 (DMT-1)48 and hepcidin,49 might be involved in this effect. In addition, plasma TIBC levels were increased in the rats by the repeated administration of a large amount of E2, although they did not show any significant changes 24 hours after a one-shot injection of E2 (data not shown), suggesting that E2 would stimulate transferrin induction. This observation would be supported by previous reports that serum TIBC levels are elevated in pregnant women50,51 and that E2 increases the rate of transferrin gene expression in human breast cancer cells in vitro.52 These changes in iron metabolism induced by E2 would be physiologically reasonable for pregnant women, whose iron stores tend to be insufficient due to active mobilization to the growing fetus and the expanding RBC mass.
It is as yet unknown how E2 would suppress Epo induction at 24 hours stimulated by hypoxia, Co, and bleeding. The iron concentrations in the liver and the kidney did not significantly change 24 hours after administration of E2 (data not shown), suggesting that suppression of the Epo induction by E2 might be mediated in a different way from long-term administration, in which the attenuation of iron deficiency might work indirectly. However, the possibility cannot be excluded that E2 might directly alter intracellular iron distribution,53 affecting the oxygen-sensing system that uses iron. In addition to iron, other kinds of intracellular transition metals might be mobilized by E2 to affect Epo induction, since some of them, such as zinc, are known to suppress Epo gene induction.54 Other than that, some previous reports suggest that the effect of E2 on Epo gene regulation acts through steroid hormone receptors; there are tandem repeats of steroid hormone receptor response element half-sites in the Epo promoter and enhancer regions,55 and the testicular receptor 2 (TR2) orphan receptor, which shares structural homology with members of the steroid/thyroid hormone receptor superfamily, suppresses Epo gene expression.56 Another recent report demonstrated that E2 attenuated Epo expression by interfering with hypoxic increases in HIF-1α protein through an estrogen receptor–dependent mechanism.57 However, the results indicating possible involvement of steroid hormone receptors or HIF-1α were all obtained from in vitro experiments, and we actually observed that E2 did not inhibit Epo induction in vitro using Epo-producing Hep3B cells derived from human hepatoma cells (data not shown). Further studies will be necessary to elucidate the mechanism of the suppressive effect of E2 on Epo induction.
In conclusion, E2 suppresses Epo induction and induces a resultant slight anemic trend along with hemodilution when administered in a large amount over a long period of time. Iron deficiency is a significant stimulant of Epo induction, which is suppressed by E2 through an iron-restoring effect. These findings from animal experiments suggest that in human pregnancy, the concomitant iron deficiency would stimulate extra Epo induction, leading to the production of RBCs, although the endogenously secreted large amount of E2 might partly suppress it. The expanded RBC mass, however, is surpassed by the increase in plasma volume induced by E2, resulting in “dilutional anemia.” These findings are relevant to pregnant women with anemia as well as to an understanding of the physiology of pregnancy.
Prepublished online as Blood First Edition Paper, March 22, 2005; DOI 10.1182/blood-2004-06-2350.
Supported by research grants from CREST-JST (F.K.), and a Grant-in-Aid for Encouragement of Young Scientists (A) (no. 12770179) from the Ministry of Education, Science, and Culture of Japan (H.H).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms Hiroko Ohtsuka for technical assistance in the measurement of estrogenic activity in the diets.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal