Abstract
Hemophagocytic syndrome (HPS) is characterized by an uncontrolled and poorly understood activation of T-helper 1 (Th-1) lymphocytes and macrophages. We studied 20 patients with HPS secondary to infections, autoimmune disease, lymphoma, or cancer and observed that the concentrations of serum interleukin 18 (IL-18), a strong inducer of Th-1 responses, interferon γ (IFN-γ) production, and stimulation of macrophages and natural killer (NK) cells were highly increased in HPS but not in control patients. In contrast, concentrations of its natural inhibitor, the IL-18 binding protein (IL-18BP), were only moderately elevated, resulting in a high level of biologically active free IL-18 in HPS (4.6-fold increase compared with controls; P < .001). Free IL-18 but not IL-12 concentrations significantly correlated with clinical status and the biologic markers of HPS such as anemia (P < .001), hypertriglyceridemia, and hyperferritinemia (P < .01) and also with markers of Th-1 lymphocyte or macrophage activation, such as elevated concentrations of IFN-γ and soluble IL-2 and tumor necrosis factor α (TNF-α) receptor concentrations. Despite high IL-18 elevation, in vitro NK-cell cytotoxicity was severely impaired in HPS patients, in part due to NK-cell lymphopenia that was observed in a majority of patients but also secondary to an intrinsic NK-cell functional deficiency. We concluded that a severe IL-18/IL-18BP imbalance results in Th-1 lymphocyte and macrophage activation, which escapes control by NK-cell cytotoxicity and may allow for secondary HPS in patients with underlying diseases.
Introduction
Hemophagocytic syndrome (HPS) is a rare and severe disease in which abnormal activation and proliferation of well-differentiated macrophages/histiocytes with an increased phagocytic activity are present.1 The primary clinical and biochemical features of HPS include nonremitting high fever, hepatosplenomegaly, cytopenia, hypertriglyceridemia, and hyperferritinemia. The hallmarks of this diagnosis are usually found in the bone marrow with the presence of numerous well-differentiated macrophages phagocytosing hematopoietic cells.1,2 Despite improved diagnosis and treatment of HPS, its prognosis remains severe with 50% mortality.2 HPS can be primary as an inherited disorder such as hereditary lymphohistiocytosis or Chédiak-Higashi or Griscelli syndromes.3,4 But the disease is most commonly secondary to infections usually due to intracellular organisms and particularly viruses of the herpes family, malignancy but notably non-Hodgkin lymphoma, as well as inflammatory/autoimmune diseases such as systemic juvenile rheumatoid arthritis and adult-onset Still disease.5-9
The pathogenesis of HPS remains poorly understood; however, uncontrolled macrophage and T-helper 1 (Th-1) lymphocyte activation appear to be crucial mechanisms of the syndrome.2-4 Excess production of cytokines mainly involved in Th-1 lymphocyte and macrophage activation, such as interferon γ (IFN-γ), soluble interleukin 2 receptor (sIL-2R), tumor necrosis factor α (TNF-α), IL-1, or IL-6, has been consistently reported.10-12 These cytokines may mediate an autoamplification loop of lymphocyte and macrophage activation as well as the hematologic and metabolic manifestations of HPS, such as cytopenia due to IFN-γ and TNF-α, hemophagocytosis for IFN-γ, and hypertriglyceridemia for TNF-α.10-12 During the past 5 years a deficiency of natural killer (NK)–cell cytotoxicity has been identified as part of the mechanism of primary HPS, since genetic defects affecting proteins of the granule cytotoxic secretory pathway have been identified in these patients.13,14
IL-18 is a proinflammatory cytokine belonging to the IL-1 family; IL-18 is present constitutively in monocytes/macrophages, antigen-presenting cells, and epithelial cells of healthy humans and mice as an inactive precursor.15,16 Biologically active IL-18 results from the cleavage of the precursor by caspase-1, an intracellular cysteine protease that cleaves the IL-1β precursor into an active cytokine. Although IL-18 was discovered for its ability to induce IFN-γ production in a mouse model of endotoxemia, IL-18 is more than an IFN-γ inducer. IL-18 acts in synergy with IL-12 to sustain the Th-1 immune response, induces chemokines and cell-adhesion molecules, stimulates inflammatory cytokine secretion such as IL-1 and TNF-α, and enhances NK-cell cytotoxicity through up-regulation of Fas ligand and perforin pathways.15-18 IL-18 has also been shown to be involved in the pathogenesis of several Th-1 immune diseases, graft-versus-host disease, rheumatoid arthritis, Crohn disease, and multiple sclerosis.19-22
Although soluble receptors for IL-18 exist, they are of low affinity for the ligand; in contrast, a natural secreted inhibitor, IL-18 binding protein (IL-18BP), was discovered. IL-18BP has high-affinity binding for IL-18 and neutralizes the biologic activity of mature IL-18.23 Since IL-18 is an important cytokine in both macrophage and Th-1 immune activation, two important pathogenic mechanisms in HPS, we asked whether IL-18 was involved in secondary HPS.
Patients, materials, and methods
Patients and controls
Patients hospitalized in the internal medicine division of Hospital Conception, Marseille, between 2000 and 2004 were included in this prospective study upon fulfilling the revised criteria of the International Histiocyte Society for the diagnosis of HPS.2 These criteria consist of fever higher than 38.5°C for at least the previous 72 hours; monocytopenia, bicytopenia, or tricytopenia (hemoglobin level < 110 g/L [11 g/dL] and/or platelet count < 100 000/mm3 and/or polymorphonuclear cells [PMNs] < 1 × 109/L [1000/mm3]); elevated lactic acid dehydrogenase (LDH) level greater than 500 IU/L (normal values, 125-240 IU/L); ferritin level greater than 1000 μg/L (normal, 10-120 μg/L); and triglyceride level greater than 2 mM (normal, 0.6-1.7 mM). In addition, the presence of hemophagocytosis in bone marrow, spleen, or lymph nodes was required. Two control groups were also studied: healthy volunteers (healthy control group); and patients hospitalized in the internal medicine division for viral, bacterial, or parasite infections, malignant hemopathy, or cancer, but without criteria of HPS (disease control group). Peripheral-blood samples were obtained after informed personal consent.
Cytokines and soluble receptor measurement
Serum IL-18 (MBL, Nagoya, Japan), IFN-γ (Beckman Coulter, France), IL-12 p70 (high-sensitivity assay; R&D Systems, Abingdon, United Kingdom), sIL-2Rα (R&D Systems), and soluble TNF-α receptor type 1 and type 2 receptors (sTNFR1, sTNFR2; R&D Systems) were measured using commercial ELISA following the manufacturer's instructions. The limit of detection was 12.5 pg/mL for IL-18, 0.1 IU/mL for IFN-γ, 0.1 pg/mL for IL-12 p70, 10 pg/mL for sIL-2Rα, 15 pg/mL for sTNFR1, and 12 pg/mL for sTNFR2. IL-18BP was measured using a previously described sandwich ELISA and free IL-18 resulted from calculation, as previously reported.24
PBMC preparation
Peripheral-blood mononuclear cells (PBMCs) from patients and controls were freshly isolated from heparinized venous blood on Ficoll-hypaque density gradient and were then used for RNA preparation.
Determination of steady-state IL-18 mRNA concentrations in PBMCs by real-time reverse transcriptase–polymerase chain reaction (RT-PCR)
Total RNA was extracted with the high pure RNA isolation kit (Roche, Meylan, France) following the manufacturer's instructions. The purity of the extraction and RNA quantification was determined by spectrophotometry (λ= 260/280 nm). Complementary DNA was generated from 0.5 to 4 μg of total RNA using Super Script II and oligo-(dT)15, according to the manufacturer's protocol (Gibco-Invitrogen, France).
Real-time PCR was performed with 5 μL of reverse transcription products (2-50 ng of total RNA), 25 μL/well of Brillant Sybr Green QPCR master mix (Stratagene, Amsterdam, The Netherlands), with 500 nM primers. Each sample was performed in duplicate. For each PCR reaction, the following sequential procedures were performed on spectrofluorometric thermal cycler (MX 4000; Stratagene): one cycle (10 minutes at 95°C) corresponding to denaturation of cDNA and activation of Taq polymerase (hot start DNA polymerase), followed by 40 cycles including denaturation at 95°C for 30 seconds, annealing at 55°C for 60 seconds, and elongation at 72°C for 30 seconds. Primer sequences were designed by Stratagene: sense 5′-GCC TCT ATT TGA AGA TAT GAC TGA-3′, antisense 5′-GAG ATA GT ACA GCC ATA CCT CTA-3′ for IL-18; sense 5′-GGG TCA GAA GGA TTC CTA TG-3′, antisense 5′-GGT CTC AAA CAT GAT CTG GG-3′ for β-actin. The purity of each PCR product was controlled by systematic study of the melting curve.
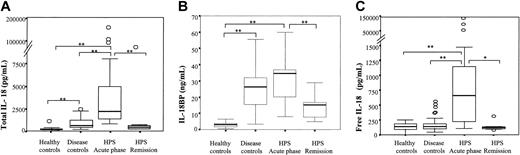
Increased IL-18 concentrations in the serum of HPS patients. (A) Total IL-18 and (B) IL-18BP concentrations were measured and (C) free IL-18 concentrations were calculated in HPS patients during the acute phase or after remission of the disease as well as in control groups composed of healthy volunteers (healthy controls) or patients suffering from infection, malignant hemopathy, or cancer without HPS (disease controls). Box plot representation, horizontal line within boxes represents median. ○ indicates outside values. *P < .01, **P ≤ .001 compared with respective controls.
Increased IL-18 concentrations in the serum of HPS patients. (A) Total IL-18 and (B) IL-18BP concentrations were measured and (C) free IL-18 concentrations were calculated in HPS patients during the acute phase or after remission of the disease as well as in control groups composed of healthy volunteers (healthy controls) or patients suffering from infection, malignant hemopathy, or cancer without HPS (disease controls). Box plot representation, horizontal line within boxes represents median. ○ indicates outside values. *P < .01, **P ≤ .001 compared with respective controls.
To standardize real-time PCR and to perform relative quantification of each cytokine gene expression, a series of dilutions of the calibration sample was included in each assay. The calibration sample was prepared from THP-1 cells for IL-18 and β-actin. The cycle threshold values were determined for each sample and RNA concentrations of each cytokine were calculated from calibration curve (MX 4000; Stratagene). To correct variation in amount of initial material between samples, cytokine RNA levels were normalized to β-actin set as one.
Lymphocyte subpopulation determination
Immunophenotypic analysis of circulating lymphocytes was determined in whole blood by fluorescence-activated cell sorter (FACS) analysis (XL Coultronics; Beckman Coulter) using labeled monoclonal antibodies: anti-CD2–phycoerythrin (PE), anti-CD19–fluorescein isothiocyanate (FITC), anti-CD3–phycocyanin 5, anti-CD4–PE, anti-CD8–PE Texas Red, anti-CD45–FITC, anti-CD16–FITC, anti-CD56–PE, and respective immunoglobulin G (IgG) isotype controls (Beckman Coulter). Normal values obtained in a cohort of healthy subjects are CD3 (71% ± 9%, 1050-2800/mm3), CD4 (45% ± 9%, 700-1800/mm3), CD8 (27% ± 5%, 405-1080/mm3), CD19 (13% ± 5%, 200-520/mm3), and CD3–CD16+CD56+ (5%-15%, 200-400/mm3).
NK-cell cytotoxicity analysis
Natural NK activity was measured using a 51Cr-releasing assay in which PBMCs from the patients or a healthy control were incubated with 104 × 51Cr-labeled K562 target cells for 4 hours at 37°C at a 50:1, 25:1, or 12.5:1 effector-target (E/T) cell ratio. Results were expressed as lytic units (LUs)/107 effector cells, LUs corresponding to the number of effector cells required to obtain 20% lysis.25
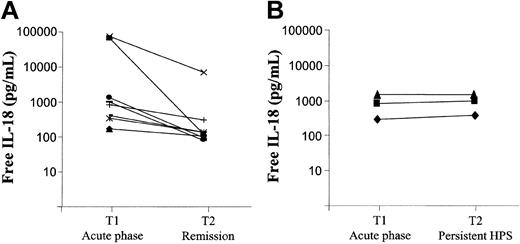
IL-18 concentrations follow-up in patients with HPS. Free IL-18 concentrations were calculated from IL-18 and IL-18BP measured concentrations in 12 patients with HPS at 2 different times. Patients were divided into 2 different subgroups depending on clinico-biologic evolution (A, remission n = 9; B, persistent n = 3).
IL-18 concentrations follow-up in patients with HPS. Free IL-18 concentrations were calculated from IL-18 and IL-18BP measured concentrations in 12 patients with HPS at 2 different times. Patients were divided into 2 different subgroups depending on clinico-biologic evolution (A, remission n = 9; B, persistent n = 3).
For antibody-dependent cell cytotoxicity (ADCC), target cells consist of 51Cr-labeled P815 cells sensitized with rabbit IgG antimouse lymphocytes (Biovalley, SpA, France). The assay was performed under the same conditions as those used for natural NK activity and results were expressed as LUs/107 effector cells.
Statistical analysis
Analysis was performed using the SPSS Base statistical package version 11.5 (SPSS, Chicago, IL). The values were expressed as the median. Differences between 2 groups were determined by the Mann-Whitney U test corrected by the Bonferroni method. The correlation coefficient (R) was obtained by using the Spearman rank correlation test. Box plot representation was used in Figures 1, 3, 5, and 7B-C. Briefly, the horizontal line in the box marks the median of the samples, whereas the hinges of each box represent the 25th and 75th percentiles (thereby including 50% of the data within a box). The vertical line extending above and below each box indicates the range of values that fall within 1.5 standard deviation of the hinges. Values between 1.5 and 3 standard deviations outside the hinges are considered outside values.
Results
Characteristics of patients and controls
Twenty patients (9 females and 11 males aged 17 to 86 years; median, 57 years) who exhibited 21 separate HPS episodes as defined by the modified International Histiocyte Society criteria (patient no. 18 had 2 episodes of HPS) were included in this study between the years 2000 and 2004. In 14 of these patients, infection was established or highly suspected as the cause of HPS (viral, 8; bacterial, 5; parasitic, 1), 3 patients were diagnosed with non-Hodgkin lymphoma, 1 patient presented with metastatic prostate cancer, and no underlying cause was identified in 2 patients (Table 1). At the time of diagnosis, the markers of HPS (in median values) in the 20 patients were hemoglobin level 88 g/L, platelet count 100 × 109/L (73 000/mm3), PMNs 2800/mm3, LDH level 552 IU/L, triglyceridemia 3.26 mM, and ferritinemia 2807 μg/L.
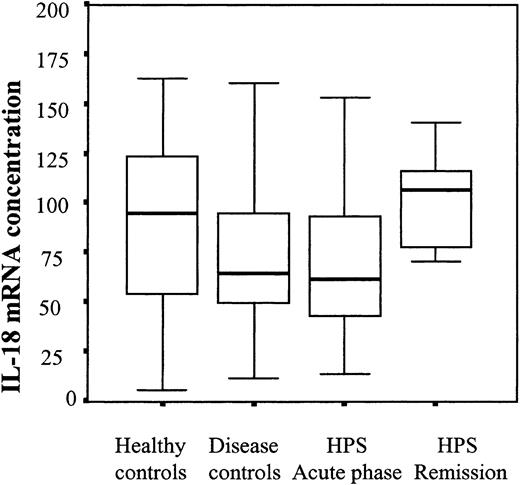
IL-18 mRNA concentrations in patients' PBMCs. Steady-state IL-18 mRNA concentrations were measured using RT-PCR in freshly isolated PBMCs from HPS patients and controls. Box plot representation, horizontal line within boxes represents median.
IL-18 mRNA concentrations in patients' PBMCs. Steady-state IL-18 mRNA concentrations were measured using RT-PCR in freshly isolated PBMCs from HPS patients and controls. Box plot representation, horizontal line within boxes represents median.
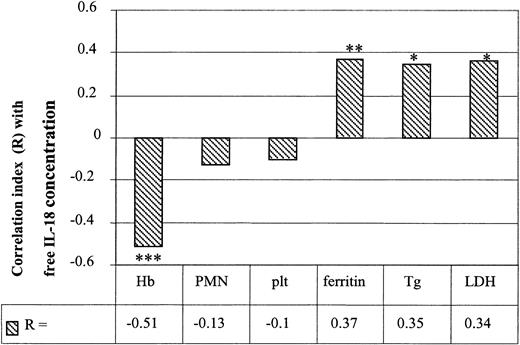
Correlations between free IL-18 concentrations and biologic markers in HPS patients. Correlations were studied between initial serum-free IL-18 concentrations and the various biologic markers of HPS such as hemoglobin (Hb), polymorphonuclear cells (PMNs), platelets (plt), ferritinemia, triglyceridemia (Tg), and LDH (*P < .05, **P < .01, ***P < .001; n = 21).
Correlations between free IL-18 concentrations and biologic markers in HPS patients. Correlations were studied between initial serum-free IL-18 concentrations and the various biologic markers of HPS such as hemoglobin (Hb), polymorphonuclear cells (PMNs), platelets (plt), ferritinemia, triglyceridemia (Tg), and LDH (*P < .05, **P < .01, ***P < .001; n = 21).
Patient characteristics
. | . | . | Medical history . | . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | Sex . | Age, y . | Hemopathy . | Neoplasia . | Others . | Immunosuppression . | Cause of HPS . | Evolution . | |||
| 1 | F | 48 | No | No | No | CS | Parasite infection | Favorable | |||
| 2 | M | 15 | No | No | No | No | Viral infection | Favorable | |||
| 3 | F | 86 | No | Colon | Dermatomyositis | CS | Bacterial infection | Death | |||
| 4 | M | 42 | No | No | No | No | Viral infection | Favorable | |||
| 5 | F | 55 | No | No | Still disease | CS and CP | Bacterial infection | Favorable | |||
| 6 | M | 39 | No | No | No | Chronic ethylic intoxication | Infection (K pneumoniae) | Favorable | |||
| 7 | M | 59 | No | No | No | No | Viral infection | Favorable | |||
| 8 | M | 81 | No | No | No | No | Viral infection (HSV) | Favorable | |||
| 9 | F | 83 | No | No | No | No | Non-Hodgkin lymphoma | Death | |||
| 10 | M | 83 | No | Prostate | No | No | Metastatic neoplasia | Death | |||
| 11 | M | 58 | No | No | No | Chronic ethylic intoxication | Viral infection | Favorable | |||
| 12 | F | 77 | No | No | No | No | Non-Hodgkin lymphoma | Death | |||
| 13 | M | 62 | No | No | No | No | Viral infection (VZV) | Favorable | |||
| 14 | M | 62 | No | No | No | No | Non-Hodgkin lymphoma | Death | |||
| 15 | M | 50 | CLL | No | No | No | Bacterial infection | Favorable | |||
| 16 | F | 37 | No | No | AIHA | No | Viral infection | Favorable | |||
| 17 | F | 62 | No | No | No | No | Viral infection | Favorable | |||
| 18 | M | 61 | No | No | No | No | Nonidentified | Favorable | |||
| 18 bis | No | No | No | No | Nonidentified | Favorable | |||||
| 19 | F | 44 | No | No | Chronic hepatitis C | No | Nonidentified | Death | |||
| 20 | F | 41 | No | No | SLE/RA/APS | CS and splenectomy | Bacterial infection | Favorable | |||
. | . | . | Medical history . | . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | Sex . | Age, y . | Hemopathy . | Neoplasia . | Others . | Immunosuppression . | Cause of HPS . | Evolution . | |||
| 1 | F | 48 | No | No | No | CS | Parasite infection | Favorable | |||
| 2 | M | 15 | No | No | No | No | Viral infection | Favorable | |||
| 3 | F | 86 | No | Colon | Dermatomyositis | CS | Bacterial infection | Death | |||
| 4 | M | 42 | No | No | No | No | Viral infection | Favorable | |||
| 5 | F | 55 | No | No | Still disease | CS and CP | Bacterial infection | Favorable | |||
| 6 | M | 39 | No | No | No | Chronic ethylic intoxication | Infection (K pneumoniae) | Favorable | |||
| 7 | M | 59 | No | No | No | No | Viral infection | Favorable | |||
| 8 | M | 81 | No | No | No | No | Viral infection (HSV) | Favorable | |||
| 9 | F | 83 | No | No | No | No | Non-Hodgkin lymphoma | Death | |||
| 10 | M | 83 | No | Prostate | No | No | Metastatic neoplasia | Death | |||
| 11 | M | 58 | No | No | No | Chronic ethylic intoxication | Viral infection | Favorable | |||
| 12 | F | 77 | No | No | No | No | Non-Hodgkin lymphoma | Death | |||
| 13 | M | 62 | No | No | No | No | Viral infection (VZV) | Favorable | |||
| 14 | M | 62 | No | No | No | No | Non-Hodgkin lymphoma | Death | |||
| 15 | M | 50 | CLL | No | No | No | Bacterial infection | Favorable | |||
| 16 | F | 37 | No | No | AIHA | No | Viral infection | Favorable | |||
| 17 | F | 62 | No | No | No | No | Viral infection | Favorable | |||
| 18 | M | 61 | No | No | No | No | Nonidentified | Favorable | |||
| 18 bis | No | No | No | No | Nonidentified | Favorable | |||||
| 19 | F | 44 | No | No | Chronic hepatitis C | No | Nonidentified | Death | |||
| 20 | F | 41 | No | No | SLE/RA/APS | CS and splenectomy | Bacterial infection | Favorable | |||
F indicates female; CS, corticosteroids; M, male; CP, cyclophosphamide; K pneumoniae, Klebsiella pneumoniae; HSV, herpes simplex virus; VZV, varicella zoster virus; CLL, chronic lymphoid leukemia; AIHA, autoimmune hemolytic anemia; SLE, systemic lupus erythematosus; RA, rheumatism arthritis; and APS, antiphospholipid syndrome.
The 2 control groups were composed of 27 non–HPS disease control patients (11 females and 16 males aged 22 to 91 years; median, 59 years) including 15 patients with infection, 7 patients with chronic lymphocytic leukemia or non-Hodgkin lymphoma, and 5 patients with other neoplasia but without symptoms of HPS. There were 46 healthy volunteers (29 females and 17 males aged 22 to 60 years; median, 30 years).
Increased serum IL-18 concentrations in HPS patients during the active phase of the disease
Total serum IL-18 concentrations were significantly higher in HPS patients than in healthy controls (median, 2170 pg/mL vs 163 pg/mL; P < .001; Figure 1A) or in disease controls (2170 pg/mL vs 618 pg/mL; P < .001; Figure 1A). The concentrations of IL-18BP, the natural IL-18 antagonist, were also significantly higher in HPS patients than in healthy controls (34.5 ng/mL vs 3 ng/mL; P < .001; Figure 1B) but were not significantly different from disease control patients (34.5 ng/mL vs 26.3 ng/mL; P = not significant; Figure 1B). Overall, when calculated, the concentrations of free IL-18 were significantly higher in the HPS patient group during the active phase of their diseases (despite their etiology) than in controls (659 pg/mL vs 141 pg/mL in healthy controls; 659 pg/mL vs 139 pg/mL in non–HPS disease controls; P < .001; Figure 1C). Noteworthy was the fact that free IL-18 concentrations were not significantly different in the 2 control groups.
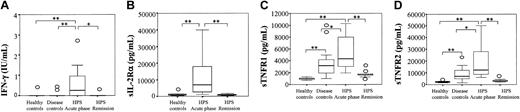
Concentrations of various cytokines and soluble receptors. Concentrations of IFN-γ (A), sIL-2Rα (B), sTNFR1 (C), and sTNFR2 (D) were measured in the serum of control individuals and HPS patients during the acute phase or after remission of the disease. Box plot representation, horizontal line within boxes represents median. ○ indicates outside values (*P < .05, **P ≤ .001 compared with respective controls).
Concentrations of various cytokines and soluble receptors. Concentrations of IFN-γ (A), sIL-2Rα (B), sTNFR1 (C), and sTNFR2 (D) were measured in the serum of control individuals and HPS patients during the acute phase or after remission of the disease. Box plot representation, horizontal line within boxes represents median. ○ indicates outside values (*P < .05, **P ≤ .001 compared with respective controls).
Serum samples were obtained for 12 of 21 HPS patients at different times in the course of their disease, 9 of 15 who had a favorable outcome and 3 of 6 who died. Total IL-18 and free IL-18 concentrations significantly decreased during remission of the disease and returned to lower values in several patients (free IL-18 concentrations during remission 122 pg/mL vs 659 pg/mL during the acute phase; P < .01; Figure 1A,C and Figure 2A-B). On the other hand, in patients with persistent severe HPS who died, total (not shown) and free IL-18 concentrations remained elevated (Figure 2A-B).
IL-18 mRNA concentrations in PBMCs from HPS patients are not elevated
To determine whether circulating PBMCs were activated to produce IL-18, we studied steady-state IL-18 mRNA concentrations in freshly isolated PBMCs from HPS patients or control groups, using real-time RT-PCR. As shown in Figure 3, IL-18 mRNA concentrations were not different in the various groups studied.
IL-18 concentrations correlate with biologic markers of HPS
Correlations were studied between free IL-18 concentrations and various known markers of HPS. A negative significant correlation was observed between IL-18 and hemoglobin concentrations (R =–0.51; P < .001; Figure 4) but not with PMNs or platelet numbers (R =–0.13 and R =–0.10, respectively). A positive correlation was observed between IL-18 concentrations and ferritinemia (R = 0.37; P = .007), triglyceridemia (R = 0.35; P < .05), and LDH levels (R = 0.34; P < .05; Figure 4).
IL-18 concentrations correlate with immune markers of Th-1 lymphocyte or macrophage activation
Serum concentrations of Th-1 lymphocyte and macrophage products such as IFN-γ, sIL-2Rα, IL-12, or sTNFR1 and sTNFR2 were studied in HPS patients compared with control groups. Concentrations of IFN-γ (0.25 IU/mL in HPS vs undetectable levels in disease controls; P < .001; Figure 5A), sIL-2Rα (6924 pg/mL in HPS vs 629 pg/mL in healthy patients; P < .001; Figure 5B), sTNFR1 (4334 pg/mL in HPS vs 3184 pg/mL in disease controls; P < .05; Figure 5C), and sTNFR2 (12 154 pg/mL in HPS vs 7159 pg/mL in disease controls; P < .05; Figure 5D) but not IL-12 (0.1 pg/mL in HPS vs undetectable levels in healthy controls; data not shown) were found significantly elevated in HPS patients compared with control groups. Of importance, these elevated levels returned to lower values in patients in which HPS remitted (Figure 5A-D). A positive correlation was observed between free IL-18 and IFN-γ (R = 0.34; P < .01; Figure 6), sIL-2Rα (R = 0.45; P < .001), sTNFR1 (R = 0.36; P < .01), and sTNFR2 (R = 0.45; P < .001) respective concentrations but not with IL-12 (R = 0.23; P = .14; Figure 6).
Correlations between various Th-1 cytokines, soluble receptors, and free IL-18 concentrations in HPS patients. Correlations were studied in HPS patients between calculated free IL-18 initial concentrations and the measured concentrations of other cytokines (*P < .01, **P < .001).
Correlations between various Th-1 cytokines, soluble receptors, and free IL-18 concentrations in HPS patients. Correlations were studied in HPS patients between calculated free IL-18 initial concentrations and the measured concentrations of other cytokines (*P < .01, **P < .001).
Decreased NK-cell numbers correlate with IL-18 concentrations in HPS patients
When analyzed in HPS patients, the absolute number of total circulating lymphocytes was within the normal range in 10 patients (median, 1525/mm3) and moderately decreased in 10 patients (median, 835 mm3). A detailed lymphocyte subset count was available for 15 of 20 patients and was found abnormal in each. A global lymphopenia affecting T, B, and NK cells was found in 1 of the 15 available patients. In 7 of this group, there was a low CD4 count (< 500/mm3) and in 6, decreased CD8 numbers (< 300/mm3). In 8 of the 15 patients, a low number of CD19+ cells were present (< 95/mm3), whereas CD8 lymphocytosis (> 1100/mm3) was present in 1 patient. The most frequent finding was a significant decrease in the NK-cell count (CD3–CD56+CD16+ < 150/mm3), which was observed in 14 of the 15 patients available for this study (Table 2). In addition, a significant negative correlation was found between free IL-18 concentrations and NK-cell count (R =–0.73; P < .001; Figure 7A).
Patients' lymphocyte subpopulation counts
Patient . | Lymphocytes, ACN . | CD4, % . | CD4, ACN . | CD8, % . | CD8, ACN . | CD19, % . | CD19, ACN . | NK, % . | NK, ACN . | Comments . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1650 | 48 | 792 | 34 | 561 | 6 | 99 | 5 | 82 | NK lymphopenia |
| 3 | 2780 | 32 | 884 | 48 | 1332 | 4 | 103 | 4 | 108 | NK lymphopenia, TCD8 lymphocytosis |
| 4 | 940 | 54 | 508 | 10 | 94 | 20 | 187 | 10 | 98 | TCD8, NK lymphopenia |
| 5 | 1480 | 47 | 696 | 41 | 607 | 6 | 89 | 5 | 74 | B and NK lymphopenia |
| 7 | 910 | 58 | 525 | 22 | 198 | 6 | 55 | 11 | 100 | TCD8, B and NK lymphopenia |
| 10 | 1190 | 57 | 676 | 28 | 332 | 8 | 95 | 6 | 71 | NK lymphopenia |
| 11 | 530 | 23 | 122 | 38 | 202 | 10 | 53 | 19 | 101 | Lymphopenia |
| 12 | 1020 | 35 | 355 | 8 | 81 | 34 | 340 | 9 | 92 | T and NK lymphopenia |
| 13 | 950 | 38 | 358 | 34 | 325 | 12 | 118 | 11 | 103 | TCD4 and NK lymphopenia |
| 14 | 910 | 29 | 263 | 43 | 390 | 10 | 91 | 5 | 45 | TCD4, B and NK lymphopenia |
| 15 | 5840 | 5 | 292 | 2 | 117 | 93 | 5427 | 1 | 58 | T and NK lymphopenia, CLL |
| 16 | 660 | 30 | 198 | 54 | 357 | 12 | 79 | 4 | 26 | TCD4, B and NK lymphopenia |
| 18 | 1040 | 31 | 321 | 35 | 363 | 6 | 62 | 17 | 176 | TCD4 and B lymphopenia |
| 19 | 1000 | 54 | 539 | 28 | 280 | 8 | 80 | 8 | 80 | TCD8 and B and NK lymphopenia |
| 20 | 1400 | 57 | 801 | 40 | 558 | 0 | 1 | 1 | 14 | B and NK lymphopenia |
Patient . | Lymphocytes, ACN . | CD4, % . | CD4, ACN . | CD8, % . | CD8, ACN . | CD19, % . | CD19, ACN . | NK, % . | NK, ACN . | Comments . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1650 | 48 | 792 | 34 | 561 | 6 | 99 | 5 | 82 | NK lymphopenia |
| 3 | 2780 | 32 | 884 | 48 | 1332 | 4 | 103 | 4 | 108 | NK lymphopenia, TCD8 lymphocytosis |
| 4 | 940 | 54 | 508 | 10 | 94 | 20 | 187 | 10 | 98 | TCD8, NK lymphopenia |
| 5 | 1480 | 47 | 696 | 41 | 607 | 6 | 89 | 5 | 74 | B and NK lymphopenia |
| 7 | 910 | 58 | 525 | 22 | 198 | 6 | 55 | 11 | 100 | TCD8, B and NK lymphopenia |
| 10 | 1190 | 57 | 676 | 28 | 332 | 8 | 95 | 6 | 71 | NK lymphopenia |
| 11 | 530 | 23 | 122 | 38 | 202 | 10 | 53 | 19 | 101 | Lymphopenia |
| 12 | 1020 | 35 | 355 | 8 | 81 | 34 | 340 | 9 | 92 | T and NK lymphopenia |
| 13 | 950 | 38 | 358 | 34 | 325 | 12 | 118 | 11 | 103 | TCD4 and NK lymphopenia |
| 14 | 910 | 29 | 263 | 43 | 390 | 10 | 91 | 5 | 45 | TCD4, B and NK lymphopenia |
| 15 | 5840 | 5 | 292 | 2 | 117 | 93 | 5427 | 1 | 58 | T and NK lymphopenia, CLL |
| 16 | 660 | 30 | 198 | 54 | 357 | 12 | 79 | 4 | 26 | TCD4, B and NK lymphopenia |
| 18 | 1040 | 31 | 321 | 35 | 363 | 6 | 62 | 17 | 176 | TCD4 and B lymphopenia |
| 19 | 1000 | 54 | 539 | 28 | 280 | 8 | 80 | 8 | 80 | TCD8 and B and NK lymphopenia |
| 20 | 1400 | 57 | 801 | 40 | 558 | 0 | 1 | 1 | 14 | B and NK lymphopenia |
ACN indicates absolute cell number/mm3; and CLL, chronic lymphoid leukemia.
Impaired NK-cell cytotoxicity in HPS patients
In vitro NK-cell cytotoxicity was determined in the peripheral blood of 7 of the 20 HPS patients during the acute phase of their disease and compared with NK-cell cytotoxicity in cells from both control groups. Six of these HPS patients had moderate to severe in vivo NK lymphopenia (15 to 150/mm3) but normal NK cell percentage (4%-11%) in 5 of the 7. With the exception of one patient, both NK-cell natural cytotoxicity (median, 7 vs 61 LUs; P < .01; Figure 7B) and ADCC (11 vs 57 LUs; P = .001; Figure 7C) were low and significantly decreased in HPS patients compared with healthy controls. Among these 6 patients, 2 had a reduced percentage of NK cells, thus a NK cell lymphopenia within the assay, possibly explaining the reduced cytotoxicity. On the other hand, 4 of the 6 exhibited severely decreased NK cytotoxicity despite normal percentage of NK cells (4%-11%), suggesting a functional deficiency. In addition, both functions of cytotoxicity were lower in HPS compared with disease control patients but did not reach significance since cytotoxicity was also decreased in the non–HPS disease control group (Figure 7B-C).
Discussion
HPS can be due to various genetic defects affecting the cytotoxic functions of immune cells or secondary to specific predisposing conditions such as infection by intracellular organisms, lymphoma, metastatic cancer, or autoimmune inflammatory diseases.1-9 In the present study we observed an unusually high level of circulating free IL-18 in patients with active HPS compared with patients with similar predisposing clinical disease but without HPS. In addition, we observed a paradoxic decrease of NK-cell numbers and cytotoxic functions in cells from patients with secondary HPS.
HPS is characterized by an uncontrolled macrophage and Th-1 lymphocyte stimulation with a prominent cytokine response, consisting in elevated levels of circulating IFN-γ, sIL-2Rα, TNF-α, sTNFRs, and IL-6.10-12 The study of HPS in animal models has also shown a major role of IFN-γ in its pathogenesis.26 Here we studied both IL-18, a potent IFN-γ–inducing and proinflammatory cytokine involved in immune defense against intracellular infections and cancers, and IL-18BP, its natural inhibitor. We observed that although serum levels of IL-18BP were increased in patients with HPS compared with healthy controls, its levels were not significantly different from those of disease controls. However, when calculated for free and bound IL-18,24 we observed that in HPS, and not disease controls, the free IL-18 was distinctly elevated. Thus, despite the high levels of IL-18BP in HPS, these concentrations are insufficient to bind most of the IL-18. As a result, it is likely that the free IL-18 reflects an imbalance favoring a pro–Th-1 and inflammatory state. Of considerable importance were the findings that free IL-18 was elevated in each HPS patient despite different underlying diseases and moreover correlated with clinical status, anemia, hypertriglyceridemia, and hyperferritinemia. In addition, a significant correlation was observed between concentrations of free IL-18 and those of other cytokines involved in macrophage or Th-1 lymphocyte activation such as sTNFR, IFN-γ, and also sIL-2Rα, which has recently been added to the HPS diagnosis criteria.27 Of note, however, serum IL-12 was not elevated and did not correlate with any parameter in agreement with previous report.28,29
Correlations between CD3–CD56+CD16+-cell numbers corresponding to NK cells and free IL-18 calculated concentrations in HPS patients during the acute phase of the disease. Correlations were studied between NK-cell numbers and free IL-18 concentrations (A), NK natural cytotoxicity (B), and antibody-dependent cell cytotoxicity (ADCC; C) in HPS and control patients. In vitro NK cytotoxicity and ADCC were studied in HPS patients (n = 7) and compared with 2 control groups (healthy controls, n = 30; and disease controls, n = 21). Box plot representation, horizontal line within boxes represents median (*P < .01, **P = .001 compared with healthy controls). ○ indicates outside values.
Correlations between CD3–CD56+CD16+-cell numbers corresponding to NK cells and free IL-18 calculated concentrations in HPS patients during the acute phase of the disease. Correlations were studied between NK-cell numbers and free IL-18 concentrations (A), NK natural cytotoxicity (B), and antibody-dependent cell cytotoxicity (ADCC; C) in HPS and control patients. In vitro NK cytotoxicity and ADCC were studied in HPS patients (n = 7) and compared with 2 control groups (healthy controls, n = 30; and disease controls, n = 21). Box plot representation, horizontal line within boxes represents median (*P < .01, **P = .001 compared with healthy controls). ○ indicates outside values.
In order to identify the cellular source of IL-18 we studied steady-state levels of IL-18 mRNA concentrations in patients' PBMCs using RT-PCR and found no significant difference between HPS and control patients. There are 2 possibilities to explain this finding: first, tissue and bone marrow macrophages may be the cellular source of IL-18,28,30 or second, elevated serum IL-18 may represent caspase-1–mediated cleavage and release of the IL-18 precursor, which is present in PBMCs.31 The present findings are consistent with 2 previous reports of marked increases in IL-18 production in patients with primary HPS, although in the latter studies free IL-18 levels remain uncalculated.28,32 Furthermore, in mice deficient in perforin, an HPS-like phenotype has been reported in which IFN-γ has been proposed to play a pathologic role.26
Several findings suggest that elevated levels of IL-18 and IFN-γ may not be the sole triggers of HPS. Elevated concentrations of IL-18 have been found in other diseases such as sepsis, malignant hematologic disorders, or autoimmune diseases but in the absence of HPS.15,16,20,21,33 Nevertheless, a large excess of IL-18 is necessary to induce HPS. To date, however, concentrations of IL-18 in the order of nanograms such as those found in HPS have only been reported in one autoimmune/inflammatory disease, adult-onset Still disease and its pediatric equivalent systemic juvenile rheumatoid arthritis.34,35 This disease of unknown origin shares several features in common with HPS, notably fever and hyperferritinemia, but not the characteristic and important neutrophilic leukocytosis.36 Adult-onset Still disease can evolve into HPS and becomes apparent with the development of cytopenia.8,9 The role of IL-18 and IFN-γ may account for the cytopenia; indeed cytopenia is characteristic of IFN-γ therapy in cancer patients.
IL-18 is known to participate in host defense against infection and cancer through stimulation of NK and CD8 cytotoxicity as well as IFN-γ and TNF-α secretion and Th-1 lymphocyte and macrophage activations.15-18 The latter mechanisms obviously appear to be fully active in HPS. On the contrary, much evidence has demonstrated an abnormal NK cytotoxic response in primary HPS, and genetic defects of proteins involved in granule-cytotoxic secretory cell-death pathway such as perforin, LYST, and RAB27 have been associated with hereditary lymphohistiocytosis, Chédiak-Higashi syndrome, and Griscelli syndrome, respectively.37-40 In addition, virally infected perforin knockout (KO) mice, in which NK and CD8 cytotoxicity are abolished, appears to be a highly relevant animal model of HPS.26 NK cytotoxicity, however, has been poorly studied in humans with secondary HPS. Therefore, in the present study, NK-cell numbers and functions were investigated in our patients.
We observed a severe decrease in both natural NK cytotoxicity as well as ADCC in 6 of the 7 patients tested. The mechanisms of this marked deficiency are likely to be acquired, since most of the patients were older than 30 years. In vivo, NK-cell lymphopenia is likely to be one of these mechanisms and indeed 15 of the 20 available patients presented with a moderate to severe reduction in NK-cell numbers. This is in agreement with a report by Grom et al41 in patients with HPS complicating juvenile rheumatoid arthritis, in which 7 of the 7 patients exhibited NK functional deficiency and 4 of these manifested a severe NK lymphopenia. An intriguing finding in the present study is the inverse correlation between NK-cell number and IL-18 concentrations, suggesting that IL-18 may participate in NK lymphopenia. A similar observation has been made recently in several autoimmune diseases and IL-18 may contribute to activation-induced NK-cell apoptosis.42 Some of our patients, as well as some of Grom et al's patients,41 exhibited a severe deficiency of in vitro cytotoxicity, despite normal NK-cell number in the experimental assay, suggesting that other mechanisms may induce NK-cell dysfunction. Variations in NK cytotoxic receptor expression or impaired interactions between these receptors and their ligands on target cells may likely impair NK functions. Indeed, both mechanisms have been described in acute myeloid leukemia and in cytomegalovirus infection and may explain tumor-cell or virus escape from the immune system.43,44
Regardless of the mechanisms, reduced NK-cell and probably CD8 cytotoxicity, despite high IL-18 levels, may impair the effector arm of host defenses. More importantly, the NK-dependent dysregulation of immune responses may result in uncontrolled Th-1 lymphocyte and macrophage activation, leading to HPS.14,45 High levels of IL-18 appear to play a central role in this syndrome being at the center of a vicious circle of Th-1, CD8, and macrophage activation leading to large excess IFN-γ production. In addition, IL-18 may induce NK-cell apoptosis, maintaining and increasing the fatal cytotoxicity impairment. Despite treatment with antimicrobial drugs, intravenous immunoglobulins, steroids, etoposide, cyclosporine, and more recently anti–TNF-α agents, HPS prognosis remains severe and underscores the need for new therapeutic strategy. In the recently reported virally infected perforin KO mice, anti–IFN-γ but not anti–IL-18 antibodies demonstrated a beneficial effect on animal survey.26 The residual concentrations of IL-18, however, were not reported in this study. Moreover, blocking IFN-γ in humans might not be an easy solution, since HPS is often secondary to infection by intracellular organisms. Although important in immune defenses against intracellular organisms, IL-18 appears to play a less crucial role than IFN-γ. The results of the present study, especially the relatively low IL-18BP concentrations resulting in IL-18/IL-18BP imbalance, may support the use of IL-18BP in addition to antimicrobial drugs in the treatment of severe cases of HPS.
Prepublished online as Blood First Edition Paper, July 14, 2005; DOI 10.1182/blood-2005-05-1980.
Supported by the Université de la Méditerranée; the Assistance Publique-Hôpitaux de Marseille; Serono International, SpA, Geneva, Switzerland; National Institutes of Health (NIH) Grants AI-15614 (C.A.D.) and HL-68743 (C.A.D.); and the Colorado Cancer Center.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank S. Gayet, E. Bernit, and N. Galatis for clinical help and M. Barbier, J. Bideau, M.-L. Bonhoure, and D. Taxis for technical assistance.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal