Abstract
Our recent results on autocrine nerve growth factor (NGF) synthesis in B lymphocytes, which directly regulates the expression and release of calcitonin gene-related peptide (CGRP), a neuropeptide known to down-regulate immune response, led us to propose an anti-inflammatory action of NGF. In the present work, we investigated whether the endogenous synthesis of NGF can regulate the expression of CGRP in other antigen-presenting cells, such as monocytes, and whether this may have a functional effect. Our data indicate that human monocytes synthesize basal levels of NGF and CGRP and that, following lipopolysaccharide (LPS) stimulation, NGF and CGRP expression are both up-regulated. When endogenous NGF is neutralized, the up-regulation of CGRP expression induced by LPS is inhibited. The expression of membrane molecules involved in T-cell activation such as human leukocyte antigen-DR (HLA-DR) and CD86 is affected by endogenous NGF, and similar effects were obtained using a CGRP1 receptor antagonist. In addition, NGF deprivation in LPS-treated monocytes significantly decreases interleukin 10 (IL-10) synthesis. Our findings indicate that endogenous NGF synthesis has a functional role and may represent a physiologic mechanism to down-regulate major histocompatibility complex (MHC) class II and CD86 expression and alter the development of immune responses.
Introduction
The intensity and duration of an inflammatory process depend on the local balance between pro- and anti-inflammatory factors. Substances of neuronal origin have been the subject of considerable research because they exert specific effects on immune cells and can regulate inflammation.1,2
In vivo studies of inflammatory diseases have shown that the synthesis of nerve growth factor (NGF) is up-regulated in the cerebrospinal fluid of patients with multiple sclerosis (MS)3 and in the sera of patients with systemic lupus erythematosus (SLE).4 A growing number of studies on inflammatory diseases have demonstrated that the inflammatory state is characterized by up-regulation of NGF synthesis.5,6 Numerous cytokines such as interleukin 1 β (IL-1β), tumor necrosis factor α (TNF-α), and IL-6 can induce NGF production in fibroblasts, endothelial cells, and glial cells.7-9 In addition, immune cells involved in innate and acquired immunity show a basal expression of NGF, whose synthesis is enhanced after stimulation with specific antigens and cytokines.10-15 The immune cells that produce NGF also express the specific NGF receptor tyrosine receptor kinase A (TrkA)16 which, on binding to its ligand, activates intracellular pathways and nuclear factors17-20 in a manner similar to what happens in neuronal cells.21 In vitro, the administration of NGF to purified myeloid- or lymphoid-cell populations influences a wide range of functions: the release of inflammatory mediators, chemotaxis, the production of cytokines and immunoglobulins, proliferation, and survival (for a review see Aloe et al22 ).
In spite of the large number of studies on the in vitro effects of NGF, it is still not clear why NGF is produced in vivo during the inflammation process and how its local synthesis, often in an autocrine fashion, can influence the ongoing immune response.
Our recent results on autocrine NGF synthesis in B lymphocytes, which directly regulate the expression and release in these cells23 of calcitonin gene-related peptide (CGRP), a neuropeptide able to inhibit immune response, indicate that NGF may have an anti-inflammatory action.
Specific receptors for CGRP are present in T and B lymphocytes and in macrophages,24-27 and several in vitro studies have demonstrated that CGRP is a potent inhibitor of mitogen and antigen-stimulated proliferation of T-cells,28-31 and of antigen presentation by macrophages.32,33 In vivo data indicate an anti-inflammatory action of CGRP, since its administration inhibits edema formation induced by inflammatory mediators, prevents the induction of contact hypersensitivity, and influences the migration of monocytes and antigen presentation.34-36
Thus, NGF may influence the inflammatory process either directly, by regulating immune-cell functions, or indirectly, by modulating CGRP synthesis, which in turn affects the immune response. To test this hypothesis, we investigated whether NGF can regulate the synthesis of CGRP in monocytes. These antigen-presenting cells express TrkA receptors.37 In agreement with earlier findings in macrophages,15 we found that monocytes synthesize basal levels of NGF and, after lipopolysaccharide (LPS) stimulation, up-regulate NGF expression. We also observed that endogenous NGF affects CGRP and IL-10 synthesis, as well as the expression of molecules involved in T-cell activation, and that these effects can be partially mimicked using a CGRP1 receptor antagonist.
Materials and methods
Chemicals
LPS from Escherichia coli serotype O55:B5 was purchased from Sigma (St Louis, MO). NGF was purified according to Bocchini and Angeletti38 and NGF antibodies were raised in rabbit or goat and purified by affinity chromatography as described by Stoeckel et al.39 Purified rabbit and goat immunoglobulin G (IgG) were from Zymed Laboratories (San Francisco, CA). TrkA polyclonal antibody, specific for an intracellular domain of the receptor, was from Santa Cruz Biotechnology (Santa Cruz, CA). The polyclonal antibody against human CGRP was from Peninsula Laboratories (Bachem Group, San Carlos, CA), as was the CGRP antagonist, CGRP8-37. For the flow cytometry study, the fluoresceine isothiocyanate (FITC)- and phycoerythrin (PE)-conjugated monoclonal antibodies and their isotype controls were from Pharmingen (CD80 and CD86; BD Biosciences Europe, Erembodegem, Belgium) and from Caltag Laboratories (CD14 and human leukocyte antigen-DR [HLA-DR]; Burlingame, CA).
Human monocyte cultures
Peripheral-blood mononuclear cells (PBMCs) were obtained from buffycoats, after centrifugation over Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) gradients. Monocytes were separated from lymphocytes by Percoll gradients as described by Sallusto and Lanzavecchia,40 followed by immunomagnetic negative selection (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of the preparations was assessed by flow cytometry and the fraction of CD14+ cells was greater than 95%.
Monocytes were plated at a concentration of 2 × 106 in 25 cm2 tissue-culture flasks (Nunc A/S, Denmark), cultured at 37°C in a humidified atmosphere enriched with 5% CO2 in RPMI 1640 (Biowhittaker, Walkersville, MD) with 50 U/mL penicillin, 50 μg/mL streptomycin, 2 mM l-glutamine (all from Gibco-BRL Life Technology, Gaithersburg MD) and 10% heat-inactivated and endotoxin-free fetal calf serum (FCS; HyClone Labs, Logan, UT), with or without the addition of 100 ng/mL LPS, 100 ng/mL NGF, 10 μg/mL affinity-purified anti-NGF antibody (abNGF) raised in goat, 10 μg/mL purified goat IgG, and 10 μM CGRP8-37, the CGRP antagonist (Peninsula Laboratories).
RNA extraction
Using the RNeasy mini kit (Qiagen, Hilden, Germany), total RNA was extracted from 5 × 106 cells for each treatment and time-point group (n = 4). In brief, the cells were lysed using lysis buffer and then homogenized using QIAshredder columns (Qiagen). The homogenized lysate was diluted 1:1 vol/vol with ethanol 70% and centrifuged through RNeasy mini columns. To eliminate any genomic DNA contamination before in vitro synthesis of the cDNA, the columns were treated with 1 U/mL DNase (RNase free). After 2 washings, the bound RNA was eluted in 40 μL RNase-free water and the absorbance of all the extracted RNA was measured by spectrophotometer to determine the concentration and purity of the samples.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
A semiquantitative analysis of CGRP and NGF expression was performed as previously described.23,41 In brief, 2 μg total RNA for each sample were incubated with 1 μg oligo dT primer (Gibco, Invitrogen SRL, Milan, Italy), 25 U rRNasin (Promega Italia srl, Milan, Italy), 5 mM deoxynucleoside triphosphate (dNTP; Gibco), and 200 U Moloney murine leukemia virus (M-MLV) reverse transcriptase (Promega, Milan, Italy) in a final volume of 25 μL for 1 hour at 42°C. Duplicate samples, in which only the RT enzyme was omitted, were used as negative control.
The sequences of the primers for CGRP were as follows: sense 5′-TGCCCAGAAGAGAGCCT-3′ corresponding to the 27-43 sequence of CALCA42 and the 68-84 sequence of CALCB43 ; antisense 5′-TGAAGGTCCCTGCGGC-3′ corresponding to the 157-173 sequence of CALCA42 and the 199-214 sequence of CALCB.43
For NGF, the sequences of the primers were as follows: sense 5′-CAGGACTCAAAGGAGCAAGC-3′ and antisense 5′-GCCTTCCTGCTGAGCACACA-3′ corresponding to sequences 511-530 and 860-879 of human NGF mRNA.44
For actin the primers used were as follows: sense 5′-CTACAATGAGCTGCGTGTGG-3′ and antisense 5′-GTGAGGATCTTCATGAGG-3′. For glyceraldehyde-3-phosphate dehydrogenase (GAPDH) the primers used were as follows: sense 5′-CACCACCATGGAGAAGGCC-3′ and antisense 5′-CACCACCATGGAGAAGGCC -3′.
One-tenth cDNA was used as template for PCR amplification in a Gene Amp PCR system (Perkin Elmer, Branchburg, NJ). The PCR reaction mixture in a final volume of 50 μL contained 0.2 mM of each dNTP, 25 pM of each primer, and 3 U Taq polymerase (Promega). The number of cycles was designed to maintain the reaction of the amplification in the exponential phase. The amplification for CGRP cDNA was performed for 35 cycles and each amplification cycle consisted of 1 minute at 95°C (denaturation), 1 minute at 54°C (annealing), and 45 seconds at 72°C (extension). For NGF cDNA each amplification cycle consisted of 1 minute at 95°C (denaturation), 1 minute at 55°C (annealing), and 2 minutes at 72°C (extension).
Analysis of PCR products
PCR products were electrophoresed in 2% agarose gel together with DNA ladder of 100 base pairs (Gibco) and visualized by ethidium bromide. For the Southern blotting, the cDNA was transferred to nylon membranes and the blot was hybridized at 55°C with a radiolabeled oligonucleotide probe (5′-AAGCCTGCCAGCCGATGAGTCACACAGGTG-3′) that recognized the CGRP coding sequence on exon 5, common to CGRP I e II genes. The hybridized membranes were washed at 55°C, twice in 2× standard saline citrate (SSC) for 15 minutes, 1× SSC containing 0.1% sodium dodecyl sulfate (SDS) for 15 minutes, and 0.1× SSC containing 0.1% SDS for 15 minutes. Autoradiography was performed at -80°C with intensifying screening overnight.
Real-time PCR analysis
Total RNA was extracted from monocytes using Trizol reagent (Sigma) according to the manufacturer's instructions. In brief, 10 × 106 cells were rinsed with 1× phosphate-buffered saline (PBS) and lysed with Trizol. RNA was isolated by chloroform extraction, precipitated with isopropanol, and quantified using ultraviolet-visible (UV-VIS) spectrophotometry. One μg total RNA from each sample was reverse-transcribed on DNaseAmpGrade-treated (Gibco-BRL) RNA using M-MLV RT (50 U) in 100 mM Tris-HCl pH 8.3, 500 mM KCl, and 500 pmol random hexamer primers.
Quantitative analysis using the SYBR Green method of CGRP mRNA expression was performed in a fluorescent temperature cycler (Mx3000P Real Time PCR-System; Stratagene Europe, Amsterdam, The Netherlands) in accordance with the manufacturer's instructions. To ensure specific PCR amplification every real-time PCR run was followed by a dissociation phase analysis (Mx3000P software).
CGRP oligonucleotide primers were designed to recognize human CGRPI and CGRP II mRNA. GAPDH primers for endogenous control were as follows: GADPH Fw: 5′-CTG CAC CAC CAA CTG CTT AG-3′; GAPDH Rew: 5′-AGG TCC ACC ACT GAC ACG TT-3′. A 25-μL reaction mixture containing 5 μL cDNA template, 12.5 μL SYBR Master mix, and 0.20 μL of each primer was amplified using the following thermal parameters: denaturing at 95°C for 10 minutes and 45 cycles of the amplification step (denaturation at 95°C for 45 seconds, annealing at 60°C for 45 seconds, extension at 72°C for 45 seconds). All amplification reactions were performed in triplicate and the averages of the threshold cycles (Cts) were used to interpolate curves and to calculate the transcript amounts in samples, using Mx3000P software.
To obtain the expression rate, the CGRP mRNA was normalized with that of GAPDH. The results were expressed as fold increase in arbitrary units.
Flow cytometry analysis
Monocytes obtained from different donors (n = 14), either freshly taken or after 24 hours of culture, were resuspended in cold PBS and incubated for 30 minutes at 4°C with appropriate dilutions of the following FITC- or PE-conjugated monoclonal antibodies: anti-CD14 and anti-HLA-DR (Caltag), anti-CD80 and anti-CD86 (Pharmingen), as well as isotypic FITC- or PE-labeled mouse IgG.
Dual-color flow cytometry was performed after staining monocytes with anti-CD14-PE followed by staining with anti-CGRP antibody (n = 6). The cells were fixed with 2% paraformaldehyde, permeabilized with phosphate buffer solution (PBS) plus 0.1% saponin and incubated with purified rabbit polyclonal antibody against CGRP (Peninsula Laboratories). In all the experiments with single or double staining, permeabilized cells were also incubated with nonspecific rabbit IgG and used as controls for aspecific immunofluorescence. After incubation with anti-rabbit IgG FITC antibodies (Chemicon International, Hofheim, Germany), the CGRP-positive cells were quantified by an EPICS 541 flow cytometer (Coulter Electronics, Hialeah, FL). Fluorescence was excited by the 488-nm line of an Argon-ion laser Innova 90 (Coherent, Palo Alto, CA) with a power of 50 mW and measured with a band-pass filter at 515 nm (FITC) or long-pass 570 nm filter (PE). Both logarithmic and linear signals were acquired from 2 × 104 cells per sample. The mean fluorescence intensity (MFI) of the linear distribution was calculated, the fluorescence of the controls was subtracted, and the results were expressed as increments relative to the controls (ΔMFI = specific MFI - control MFI / control MFI).
Immunofluorescence
Monocytes from different donors (n = 4) after 24 hours of culture were resuspended in cold PBS, fixed in 2% paraformaldehyde in 0.1 M PBS, and, after PBS washing, cytocentrifuged (Shandon, Pittsburg, PA) on slides. All the slides were preincubated with 2% BSA and 10% normal goat serum in PBS solution containing 0.1% saponin for 1 hour and then incubated overnight at 4°C with anti-CGRP rabbit serum (Peninsula Laboratories) diluted 1:100 vol/vol. The specificity of the antibody binding was assessed by incubating cells together with preimmune rabbit antiserum at the same dilution as the specific antibody. After rinsing in 0.1% saponin PBS, slides were incubated for 1 hour at room temperature with FITC-conjugated Fab anti-rabbit IgG (Chemicon International) and analyzed by laser scanning confocal microscopy using a TCS Confocal System (Leica, Germany).
IL-10 ELISA determination
Conditioned media were collected from monocytes obtained from different donors (n = 6) after 24 hours of incubation with LPS with or without the addition of anti-NGF antibodies, purified IgG, and CGRP8-37. After the addition of 1 μL/mL of 0.2 mM phenylmethylsulfonyl fluoride (PMSF) to inhibit proteases, the media were stocked at -70°C and different experiments were analyzed at the same time using an IL-10 enzyme-linked immunosorbent assay (ELISA) Kit (BioSource International, Nivelles, Belgium) following the manufacturer's instructions. The sensitivity of the assay was 5 pg/mL.
Statistical analysis
Statistical analysis of the results was performed using a 2-way analysis of variance (ANOVA) followed by post-hoc Tukey-Kramer test for multiple comparisons.
Results
CGRP expression in monocytes
Total RNA was extracted from freshly isolated human monocytes and in vitro-activated monocytes (n = 4), and reverse-transcribed in cDNA. After PCR amplification using specific exon 5 CGRP primers that recognize homologous regions of CALCA and CALCB, the products were visualized on ethidium bromide-stained gels. Low levels of CGRP gene transcripts were detected in monocytes freshly isolated from blood or after 24 hours in culture without any specific stimulation (unstimulated [Us]). After 24 hours of LPS stimulation of monocytes, the CGRP expression was enhanced (Figure 1A). Two-color flow cytometry analysis showed that all CD14+ cells were positive for CGRP. The expression of CGRP was quantified in monocytes gated for their CD14 expression and the mean fluorescence intensity for CGRP in monocytes (n = 6 for each group) stimulated with LPS for 24 hours was about 4 times higher (P < .01) than the specific fluorescence of unstimulated monocytes (ΔMFI = 4.4 ± 0.4 vs 1.1 ± 0.13). A representative experiment of flow cytometry analysis of CGRP expression in CD14+ cells is shown in Figure 1B.
CGRP induction as LPS response in human monocytes. (A) RT-PCR-amplified CGRP gene transcripts (i) from monocytes after 24 hours of incubation. Us indicates unstimulated monocytes without (-) or with 100 ng/mL NGF (+NGF); LPS, LPS-treated monocytes without (-) or with 10 μg/mL neutralizing goat anti-NGF antibody (+abNGF) or goat IgG as control (+IgG). Southern blotting analysis (ii) and actin expression (iii) of the electrophoresed RT-PCR-amplified samples shown in panel i. These ethidium bromide-stained gels are representative of 4 independent experiments. (B) CGRP expression was quantified in CD14+ cells by flow cytometry analysis. A specific CGRP immunoreactivity was detected in gated CD14+ cells after 24 hours of culture in different conditions. LPS-stimulated monocytes (LPS) showed a higher immunopositivity for CGRP than unstimulated monocytes (Us) but treatment with anti-NGF antibodies (LPS+abNGF) decreased CGRP expression, which was not affected when goat IgG was used (LPS+IgG). The darker area in the graphs represents the unspecific signal obtained in CD14+-permeabilized cells incubated with rabbit IgG and anti-rabbit FITC antibodies. In these graphs, representative of 6 independent experiments, the values of ΔMIF for CGRP positivity are included for each culture condition.
CGRP induction as LPS response in human monocytes. (A) RT-PCR-amplified CGRP gene transcripts (i) from monocytes after 24 hours of incubation. Us indicates unstimulated monocytes without (-) or with 100 ng/mL NGF (+NGF); LPS, LPS-treated monocytes without (-) or with 10 μg/mL neutralizing goat anti-NGF antibody (+abNGF) or goat IgG as control (+IgG). Southern blotting analysis (ii) and actin expression (iii) of the electrophoresed RT-PCR-amplified samples shown in panel i. These ethidium bromide-stained gels are representative of 4 independent experiments. (B) CGRP expression was quantified in CD14+ cells by flow cytometry analysis. A specific CGRP immunoreactivity was detected in gated CD14+ cells after 24 hours of culture in different conditions. LPS-stimulated monocytes (LPS) showed a higher immunopositivity for CGRP than unstimulated monocytes (Us) but treatment with anti-NGF antibodies (LPS+abNGF) decreased CGRP expression, which was not affected when goat IgG was used (LPS+IgG). The darker area in the graphs represents the unspecific signal obtained in CD14+-permeabilized cells incubated with rabbit IgG and anti-rabbit FITC antibodies. In these graphs, representative of 6 independent experiments, the values of ΔMIF for CGRP positivity are included for each culture condition.
The confocal analysis confirmed that LPS-stimulated monocytes display an intense immunopositivity for CGRP when compared with the basal expression of adherent monocytes (Figure 2).
NGF regulates CGRP expression in monocytes
Similarly to other findings in macrophages,15 LPS stimulation enhances NGF mRNA levels in quiescent monocytes (Figure 3). Since CGRP expression is under NGF control in peripheral neurons,45,46 we investigated whether deprivation of endogenous NGF or the addition of exogenous NGF could influence the expression of CGRP in monocytes.
To neutralize the endogenous NGF produced by monocytes, we treated the cells with 10 μg/mL anti-NGF antibodies. As shown in Figure 1A, after anti-NGF antibody treatment the expression of CGRP mRNA decreased in LPS-stimulated monocytes at 24 hours, whereas the addition of purified IgG did not modify CGRP expression (Figure 1A). The inhibitory effect of anti-NGF antibodies on CGRP expression induced by LPS stimulation was further confirmed by real-time PCR analysis (Figure 4A).
In another set of experiments, 100 ng/mL exogenous NGF was added to monocytes in vitro. An enhancement of CGRP mRNA expression was observed in quiescent monocytes as a consequence of NGF administration (Figures 1A and 4B) but no further induction was observed in LPS-stimulated cells (Figure 4B).
The effects of anti-NGF treatment on CGRP synthesis were analyzed in CD14+ cells by flow cytometry (n = 6). A significant difference (P < .05) in CGRP immunoreactivity was observed in LPS-activated monocytes after anti-NGF antibody or control IgG treatment, exhibiting a ΔMFI respectively of 2.3 ± 0.3 and 3.6 ± 0.2 (Figure 1B). The confocal analysis confirmed that the anti-NGF treatment strongly decreases immunopositivity for CGRP in LPS-stimulated monocytes (Figure 2C).
Confocal microscopic analysis of CGRP expression in human monocytes. Monocytes collected from peripheral blood were cultured in vitro for 24 hours and used to test CGRP immunoreactivity. In these images, representative of 4 independent experiments, adherent monocytes showed basal CGRP positivity (A) while LPS-stimulated monocytes showed striking positivity for CGRP (B). When the cells were incubated with LPS and 10 μg/mL anti-NGF antibody, CGRP immunoreactivity strongly decreased (C). Fluorescently labeled samples were imaged under a Leica TCS 4D confocal laser scanning microscope equipped with an argon/krypton laser and 100×/1.3-0.6 oil-immersion objective lenses (Leica, Heidelberg, Germany). The scale bar corresponds to 10 μm.
Confocal microscopic analysis of CGRP expression in human monocytes. Monocytes collected from peripheral blood were cultured in vitro for 24 hours and used to test CGRP immunoreactivity. In these images, representative of 4 independent experiments, adherent monocytes showed basal CGRP positivity (A) while LPS-stimulated monocytes showed striking positivity for CGRP (B). When the cells were incubated with LPS and 10 μg/mL anti-NGF antibody, CGRP immunoreactivity strongly decreased (C). Fluorescently labeled samples were imaged under a Leica TCS 4D confocal laser scanning microscope equipped with an argon/krypton laser and 100×/1.3-0.6 oil-immersion objective lenses (Leica, Heidelberg, Germany). The scale bar corresponds to 10 μm.
Effects of NGF and CGRP deprivation on monocyte functions
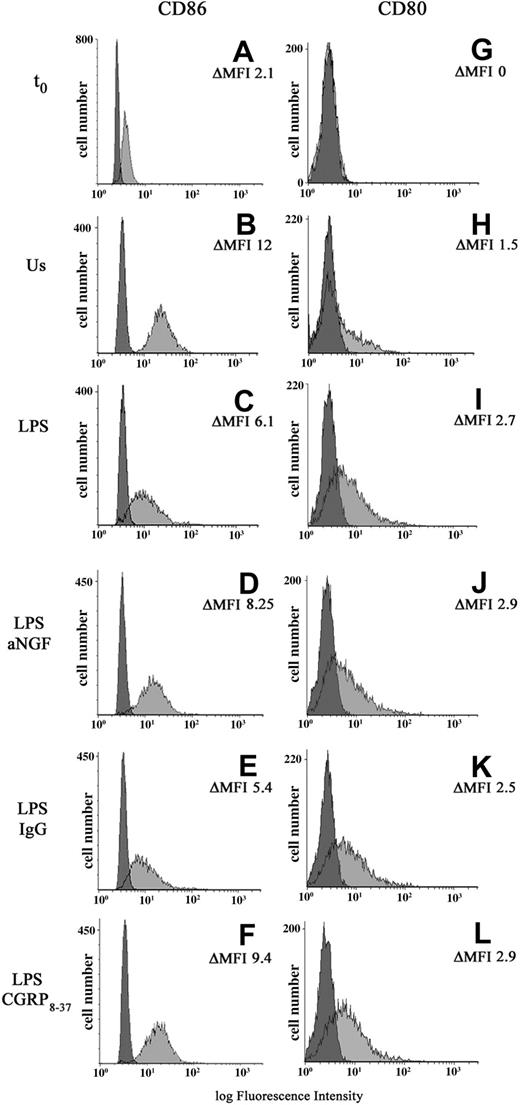
It is known that exogenous CGRP can affect antigen-presenting cell (APC) populations by acting directly on antigen presentation,32 particularly costimulatory molecules.33 In order to study the possible functional effects of the endogenous synthesis of NGF and CGRP, the expression of costimulatory molecules B7.1 (CD80) and B7.2 (CD86) was evaluated in monocytes after anti-NGF treatment or CGRP8-37 administration (n = 8). In freshly taken monocytes, the expression of CD80 is absent; after 24 hours of culture the monocytes show low levels of B7.1 which is up-regulated in LPS-treated monocytes. (ΔMFI 1.6 ± 0.8 vs 4.4 ± 1.1). A basal expression of CD86 characterizes freshly taken monocytes (ΔMFI = 2.9 ± 0.7). After 24 hours of adherence, in comparison with unstimulated cells, the monocyte activation with LPS decreases CD86 expression, with a ΔMFI of 4.5 ± 0.8 for LPS-treated and 9.8 ± 0.6 for untreated cells. LPS-stimulated monocytes treated with either anti-NGF antibodies or 10 μM CGRP8-37 show a significant increase in the expression of CD86. In the same cells, CD80 expression does not change appreciably (Table 1, Figure 5).
B7.1 and B7.2 mean fluorescence intensity in human monocytes
. | t0 . | Us . | LPS . | +CGRP8-37 . | +IgG . | +abNGF . |
|---|---|---|---|---|---|---|
| CD80 | — | 1.5 ± 0.4 | 3.4 ± 0.6 | 2.8 ± 0.5 | 3.2 ± 0.4 | 2.9 ± 0.5 |
| CD86 | 2.9 ± 0.7 | 9.8 ± 0.6 | 3.8 ± 0.8 | 6.7 ± 0.7* | 4.3 ± 0.5 | 7.4 ± 0.6* |
. | t0 . | Us . | LPS . | +CGRP8-37 . | +IgG . | +abNGF . |
|---|---|---|---|---|---|---|
| CD80 | — | 1.5 ± 0.4 | 3.4 ± 0.6 | 2.8 ± 0.5 | 3.2 ± 0.4 | 2.9 ± 0.5 |
| CD86 | 2.9 ± 0.7 | 9.8 ± 0.6 | 3.8 ± 0.8 | 6.7 ± 0.7* | 4.3 ± 0.5 | 7.4 ± 0.6* |
The results are expressed as increments relative to the controls of mean fluorescence intensity plus or minus standard error of the mean (ΔMFI ± SEM).
— indicates not detectable.
LPS+CGRP8-37 vs LPS, and LPS+aNGF vs LPS+IgG: P < .05 according to Tukey-Kramer test
The effects of NGF deprivation on HLA-DR expression were also investigated in LPS-stimulated monocytes (n = 6). Treatment with anti-NGF antibodies resulted in a significant increase (P < .05) in HLA-DR expression in LPS-stimulated monocytes when compared with IgG-treated cells. A similar increase in the expression of HLA-DR was observed after incubating LPS-stimulated monocytes with 10 μM CGRP8-37 (Figure 6).
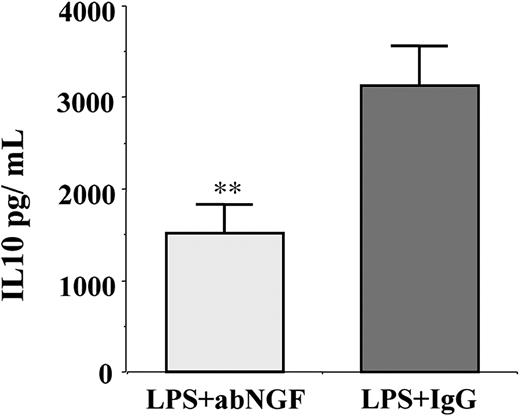
Since in monocytes LPS stimulation induces the synthesis of IL-10, which in turn can affect either HLA-DR47 or B7 expression,48 the conditioned media of previous experiments (n = 6) were collected and the IL-10 concentration was evaluated by specific ELISA. In LPS-stimulated cells, the addition of anti-NGF antibodies by neutralizing the endogenous NGF produced by activated monocytes significantly decreased the synthesis of IL-10 in comparison with the IgG-treated monocytes (Figure 7).
The CGRP1 receptor antagonist was not effective in reducing the IL-10 concentration in LPS-treated monocytes (data not shown).
NGF mRNA expression in peripheral-blood monocytes. Ethidium bromide staining of RT-PCR-amplified NGF gene transcripts of human monocytes obtained from peripheral blood after 24 hours of culture and representative of 4 independent experiments. Us indicates unstimulated monocytes; LPS, LPS-treated cells; + positive control, CD34+ cord-blood cells.41
NGF mRNA expression in peripheral-blood monocytes. Ethidium bromide staining of RT-PCR-amplified NGF gene transcripts of human monocytes obtained from peripheral blood after 24 hours of culture and representative of 4 independent experiments. Us indicates unstimulated monocytes; LPS, LPS-treated cells; + positive control, CD34+ cord-blood cells.41
Discussion
The results of the present study show that, similarly to other APCs,23,49 human monocytes are able to synthesize CGRP, and that CGRP expression is enhanced after LPS stimulation. In addition, we show for the first time that endogenous CGRP synthesis in monocytes is regulated by NGF. Since monocytes produce their own NGF and its synthesis is up-regulated on activation, we found that the neutralization of endogenous NGF using anti-NGF antibodies markedly reduces the synthesis of CGRP induced by LPS.
This regulation of CGRP expression by NGF is also supported by the finding that in vitro, after the addition of exogenous NGF, the enhancement of NGF concentration can modify CGRP production in unstimulated monocytes but does not influence CGRP expression in LPS-treated cells, which already have an up-regulated endogenous synthesis of NGF.
These data confirm our previous results in human B lymphocytes. In these cells, as now similarly observed in monocytes, the enhanced autocrine synthesis of NGF regulates CGRP synthesis23 after specific stimulation.
The NGF control of CGRP expression seems to be a mechanism common to immune cells as well as to the peripheral sensory neurons in which it was first described.45,46 The gene encoding CGRP is induced in neuronal cells by treatment with NGF, which activates the CGRP promoter50 through specific signaling pathways.
Effects of NGF on CGRP expression. (A) NGF deprivation. Using real-time PCR the effects of anti-NGF antibody treatment on CGRP expression were evaluated in LPS-stimulated monocytes after 24 hours of culture. The induction of CGRP expression as a response to LPS (LPS) is abolished by anti-NGF treatment (LPS+abNGF) and is comparable to the basal CGRP expression found in unstimulated monocytes (Us). No similar effect was obtained when using isotypic IgG (LPS+IgG). The results are expressed as fold increase in arbitrary units and represent the mean plus or minus the standard error of the mean (SEM) of 3 independent experiments, each performed in triplicate. (LPS vs Us and LPS+aNGF vs LPS+IgG, *P < .01 according to Tukey-Kramer test). (B) NGF addition. Ethidium bromide staining of RT-PCR-amplified CGRP gene transcripts of unstimulated (Us) and LPS-stimulated human monocytes (LPS) treated for 24 hours with 100 ng/mL NGF. These ethidium bromide-stained gels are representative of 4 independent experiments.
Effects of NGF on CGRP expression. (A) NGF deprivation. Using real-time PCR the effects of anti-NGF antibody treatment on CGRP expression were evaluated in LPS-stimulated monocytes after 24 hours of culture. The induction of CGRP expression as a response to LPS (LPS) is abolished by anti-NGF treatment (LPS+abNGF) and is comparable to the basal CGRP expression found in unstimulated monocytes (Us). No similar effect was obtained when using isotypic IgG (LPS+IgG). The results are expressed as fold increase in arbitrary units and represent the mean plus or minus the standard error of the mean (SEM) of 3 independent experiments, each performed in triplicate. (LPS vs Us and LPS+aNGF vs LPS+IgG, *P < .01 according to Tukey-Kramer test). (B) NGF addition. Ethidium bromide staining of RT-PCR-amplified CGRP gene transcripts of unstimulated (Us) and LPS-stimulated human monocytes (LPS) treated for 24 hours with 100 ng/mL NGF. These ethidium bromide-stained gels are representative of 4 independent experiments.
Sensory neurons innervate the parenchyma of primary and secondary lymphoid organs51 and CGRP binding sites have been found in spleen, thymus, bone marrow, and purified immune-cell populations.24-27,51,52 Thus, in addition to its direct effects on immune-cell functions, NGF may influence inflammatory responses by regulating the synthesis of CGRP in either sensory nerves or immune cells.
A wide range of CGRP effects has been described in in vitro and in vivo experiments,27-36,53,54 showing that CGRP can influence key processes of the immune response and exert an anti-inflammatory action. The main effect of CGRP administration both in vitro and in vivo is to dampen the immune response essentially by affecting antigen presentation in a variety of APCs such as Langerhans cells, dendritic cells, monocytes, and macrophages.32,33,53,55,56 Our results on the functional effect of the autocrine synthesis of CGRP in monocytes indicate that the control of MHC class II and B7 costimulatory molecules is indeed a key feature of the biologic action of CGRP.
Effects of NGF and CGRP8-37 on B7 expression. A representative finding of 8 independent experiments showing CD80 and CD86 expression in peripheral-blood monocytes freshly taken (t0: A,G) or after 24 hours of different culture conditions: unstimulated cells (Us: B,H), LPS-treated monocytes (LPS: C,I) with or without the addition of anti-NGF antibody (LPS abNGF: D,J), control IgG (LPS IgG: E,K), CGRP8-37, the CGRP1 receptor antagonist (LPS CGRP8-37: F,L). Negative controls were cells incubated with isotype mouse IgG (darker area). For each population the values of ΔMFI for CD80 and CD86 positivity are included.
Effects of NGF and CGRP8-37 on B7 expression. A representative finding of 8 independent experiments showing CD80 and CD86 expression in peripheral-blood monocytes freshly taken (t0: A,G) or after 24 hours of different culture conditions: unstimulated cells (Us: B,H), LPS-treated monocytes (LPS: C,I) with or without the addition of anti-NGF antibody (LPS abNGF: D,J), control IgG (LPS IgG: E,K), CGRP8-37, the CGRP1 receptor antagonist (LPS CGRP8-37: F,L). Negative controls were cells incubated with isotype mouse IgG (darker area). For each population the values of ΔMFI for CD80 and CD86 positivity are included.
As previously demonstrated, LPS stimulation strongly up-regulates B7.1 (CD80) in adherent monocytes,57 whereas B7.2 (CD86), which is constitutively expressed, is down-regulated by LPS58,59 as well as HLA-DR.48 In our study we show for the first time that the described LPS-induced reduction in CD86 and HLA-DR expression in human monocytes48,58,60 is mediated by the LPS-induced synthesis of NGF and CGRP. Our data show that in LPS-activated monocytes, the neutralization of endogenous NGF, by reducing the synthesis of CGRP, up-regulates CD86 but does not modify CD80 expression. This effect is specifically mediated by the autocrine production of CGRP, since similar changes in CD86 levels can be observed by treating monocytes before LPS stimulation with the CGRP1 receptor antagonist, CGRP8-37. Previous studies on antigen presentation in monocytes and dendritic cells have shown that exogenous administration of CGRP inhibits APC-driven T-cell proliferative responses by altering CD86 expression in APCs, an effect mediated by the CGRP1 receptors.56
It has been suggested that CD80 and CD86 play a key role in immune activation, tolerance regulation, and skewing of T-helper immune responses in a number of disease models61,62 and alteration of their expression can have profound effects on the development of immune responses.63-65 CD80 and CD86 act as ligands for CD28 and CTLA-4 (now known as CD152) receptors expressed on T lymphocytes and while the binding with CD28 promotes T-cell activation, binding with CD152 inhibits the T-cell response.66 Although a number of studies have shown that the selective blocking of CD80 or CD86 differentially influences the immune response,63-65 it is still a matter of debate whether they mediate redundant signals or have distinct regulatory functions.67 Recent findings have suggested that CD80 and CD86 are not interchangeable costimulators that merely show different kinetics of expression, but that they selectively bind to their receptors and that CD80 is a more potent ligand for CD152.68
Effects of NGF and CGRP8-37 on HLA-DR expression. HLA-DR expression was measured by flow cytometry analysis in LPS-stimulated monocytes after 24 hours of treatement with anti-NGF antibodies, control IgG, and CGRP8-37. NGF neutralization induces a significant increase in the specific HLA-DR immunoreactivity in LPS-stimulated monocytes, and a similar enhancement in HLA-DR expression was observed also in LPS-stimulated cells after CGRP8-37 treatment. The data represent the mean of 6 independent experiments and the results were expressed as increments relative to the controls of ΔMFI plus or minus SEM (LPS+aNGF vs LPS+IgG, *P < .05; LPS+CGRP8-37 vs LPS, **P < .01 according to Tukey-Kramer test).
Effects of NGF and CGRP8-37 on HLA-DR expression. HLA-DR expression was measured by flow cytometry analysis in LPS-stimulated monocytes after 24 hours of treatement with anti-NGF antibodies, control IgG, and CGRP8-37. NGF neutralization induces a significant increase in the specific HLA-DR immunoreactivity in LPS-stimulated monocytes, and a similar enhancement in HLA-DR expression was observed also in LPS-stimulated cells after CGRP8-37 treatment. The data represent the mean of 6 independent experiments and the results were expressed as increments relative to the controls of ΔMFI plus or minus SEM (LPS+aNGF vs LPS+IgG, *P < .05; LPS+CGRP8-37 vs LPS, **P < .01 according to Tukey-Kramer test).
Effects of NGF deprivation on IL-10 production. IL-10 concentration was measured in conditioned media (n = 6) of LPS-treated monocytes incubated for 24 hours with anti-NGF antibody or with goat IgG as control. The neutralization of endogenous NGF using anti-NGF antibodies induced a significant decrease in IL-10 production when compared with IgG treatment (**P < .01 according to the Tukey-Kramer test).
Effects of NGF deprivation on IL-10 production. IL-10 concentration was measured in conditioned media (n = 6) of LPS-treated monocytes incubated for 24 hours with anti-NGF antibody or with goat IgG as control. The neutralization of endogenous NGF using anti-NGF antibodies induced a significant decrease in IL-10 production when compared with IgG treatment (**P < .01 according to the Tukey-Kramer test).
Thus, the selective effect of NGF and CGRP on CD86 expression but not on CD80 can influence the delicate balance between the positive and negative costimulatory signals, having as a final result a down-regulation of the immune response. The possibility that endogenous NGF and CGRP can influence the development of immune responses is further supported by our findings on HLA-DR expression in LPS-stimulated cells. A significant enhancement of HLA-DR expression was observed in NGF-deprived monocytes after LPS activation. In a study on the effect of inflammation in organotypic hippocampal slice culture, a similar enhancement of MHC class II expression has been shown in microglial cells as a consequence of NGF neutralization.69
The LPS-induced endogenous synthesis of CGRP also influences MHC class II expression in monocytes: when treated with CGRP1 receptor inhibitor, LPS-stimulated cells showed an enhanced HLA-DR expression. Complementary to this observation are the findings showing that the administration of exogenous CGRP to monocyte-derived dendritic cells decreased HLA-DR expression and inhibited T-cell proliferative response.56
Furthermore, in septic patients characterized by decreased MHC class II molecule expression in monocytes,70,71 CGRP plasma levels are greatly enhanced and the highest levels are associated with a lethal outcome.72 These findings, together with our in vitro data, suggest that CGRP could be a key mediator of the LPS-induced responses.
The interaction between endogenous NGF and CGRP and the synthesis of IL-10 in LPS-activated monocytes was also investigated. Our data show that, while enhancing HLA-DR and CD86 expression, the inhibition of endogenous NGF resulted in a significant decrease in the concentration of IL-10 in the conditioned media. This finding is consistent with findings in experimental autoimmune encephalitis (EAE) models and in in vitro cultures of microglial cells showing that the administration of exogenous NGF induces the synthesis of IL-1073,74 and changes in B7 expression.75 Induction of IL-10 synthesis is a well-described monocyte response to LPS76 and involves the activation of p38 mitogen-activated protein kinase (MAPK),77 a protein kinase also regulated by the signal transduction pathways activated by the binding of NGF to TrkA in NGF-responsive cells.21,78 As endogenous NGF neutralization results in decreased IL-10 synthesis, it is probable that NGF is directly involved in the regulation of IL-10 production in LPS-stimulated monocytes. This hypothesis is supported by findings in epithelial cells79 showing that NGF has a dose-dependent effect on IL-10 mRNA expression and protein production.
Interestingly, preadministration of the CGRP1 receptor antagonist did not result in a significant inhibition of IL-10 synthesis in LPS-stimulated monocytes. This suggests that the effect of NGF on IL-10 production is not mediated by the endogenous CGRP synthesis. Our results are supported by previous studies showing that Staphyloccocus aureus Cowan strain (SAC)-stimulated mononuclear cells33 and LPS-activated Langerhans cells80 enhance their endogenous synthesis of IL-10 following exogenous addition of CGRP, whereas CGRP8-37 treatment has no effect on IL-10 synthesis induced by LPS activation,80 similarly to what we found in our experimental conditions. Similar results have been obtained in in vivo studies when using exogenous CGRP or when blocking endogenous CGRP production with the CGRP1 receptor antagonist. The intracutaneous injection of CGRP in mice promotes hapten-specific tolerance and enhances IL-10 production.35,81 As ultraviolet treatment increases the number of CGRP nerve fibers and the CGRP content82 in the skin of mice, the use of CGRP8-37 before hapten sensitization only partially reduces immunosuppression.81,82 We cannot rule out the possibility that other newly described receptors83 may be involved in the control of endogenous CGRP functions, thus explaining the lack of action by CGRP8-37.
On the other hand, both exogenous56 and endogenous CGRP regulate the expression of CD86 and HLA-DR through CGRP1 receptors and the fact that CGRP8-37 effectively blocks their expression is suggestive of IL-10-independent regulation. Thus, other mediators than endogenous CGRP, including NGF, may be involved in the direct regulation of IL-10. Our hypothesis is supported by the fact that many of the studies linking CGRP to IL-10-mediated effects have been performed in conditions in which endogenous NGF synthesis is enhanced. For example, in UV-induced tolerance the induction of CGRP synthesis in cutaneous sensory C-fibers after UV radiation is regulated by keratinocyte-derived NGF.84 The data obtained in vitro using neutralizing IL-10 antibodies33,80 were not able to ascertain whether NGF, whose synthesis is induced on activation,7-15 and not CGRP, regulates the effects of IL-10 on CD86 or whether CGRP regulates CD86 through an alternative, IL-10-independent pathway. A recent study on LPS-activated monocytes showed that different MAP kinases are selectively involved in IL-10-dependent and IL-10-independent regulation of CD86.58 Further studies are needed to evaluate whether the effects of endogenous CGRP on antigen presentation can be mediated by the activation of IL-10-dependent or -independent pathways.
Taken together, our results show that the endogenous synthesis of NGF in monocytes has a functional role and that by inducing CGRP and IL-10 production and then influencing HLA-DR and CD86 expression it can reduce the antigen-presenting capacity and costimulatory function of monocytes, possibly contributing to the down-regulation of T-cell responses. These data demonstrate a new immunoregulatory function of NGF mediated by CGRP and IL-10, and suggest the existence of an effector mechanism that is responsible for a protective action of NGF in inflammatory diseases. Thus, the widely described enhancement of NGF in body fluids and in the tissue of patients and animal models of inflammatory diseases might represent a physiologic mechanism to dampen the immune response.
This hypothesis is supported by studies showing the anti-inflammatory effects of NGF in experimental models of EAE. Administration of NGF delays the onset of clinical EAE and prevents the full development of EAE lesions in the marmoset.73 The local enhancement of NGF in EAE models can modify the T-helper balance, influence the migration of monocytes, down-regulate interferon γ (IFN-γ) synthesis, and enhance IL-10 production.73,74,85 Complementary effects are observed in an EAE rat model, in which NGF deprivation results in an exacerbated brain inflammation and more severe clinical signs.86
Similar results were obtained in an animal model of colitis, where endogenous NGF neutralization with anti-NGF treatment increased the severity of the experimental inflammation and, it is worth mentioning, significantly reduced the CGRP content in the gut.87 Recently, topical applications of NGF have been successfully used for the clinical treatment of human immune corneal ulcers and pressure ulcers.88,89 However, because of the wide range of effects that NGF can have on the nervous, endocrine, and immune systems,2 its therapeutic use has been limited. One of the key findings of the present study is that induction of CGRP synthesis by NGF seems to be responsible, at least in part, for the effects of NGF in monocytes. Thus, some of the functions ascribed to NGF may in fact be mediated by CGRP, whose NGF-induced synthesis in either immune or neuronal cells23,45,46 can influence other cell functions in a paracrine way and dampen the immune response. Since the synthesis of other neuropeptides with immunomodulatory functions is controlled by NGF not only in neuronal cells90 but also in immune cells,91 more studies are needed to clarify which of the effects of NGF are mediated by the induction of neuropeptide synthesis. The discovery of other intermediate mediators controlled by NGF may provide a better therapeutic approach than NGF administration in inflammatory diseases.
Prepublished online as Blood First Edition Paper, August 11, 2005; DOI 10.1182/blood-2004-10-4055.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Prof Anna Maria Teti, Prof Edoardo Alesse, Dr Angelo Fratticci, and Dr Nadia Rucci of the Department of Experimental Medicine, University of L'Aquila, for the use of their facilities and assistance in performance of real-time PCR experiments. The authors are deeply indebted to Dr Stefano Cencioni of Dompé SPA and Dr Paolo Anghileri of Sigma for primer design and synthesis.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal