Abstract
Multipotent adult progenitor cells (MAPCs) are bone marrow-derived stem cells that have extensive in vitro expansion capacity and can differentiate in vivo and in vitro into tissue cells of all 3 germinal layers: ectoderm, mesoderm, and endoderm. The origin of MAPCs within bone marrow is unknown. MAPCs are believed to be derived from the bone marrow stroma compartment as they are isolated within the adherent cell component. Numerous studies of bone marrow chimeras in the human and the mouse point to a host origin of bone marrow stromal cells. Mesenchymal stem cells (MSCs), which coexist with stromal cells, have also been proven to be of host origin after allogeneic bone marrow transplantation in numerous studies. We report here that following syngeneic bone marrow transplants into lethally irradiated C57BL6 mice, MAPCs are of donor origin.
Introduction
Stromal cells constitute the cell population that supports the hematopoietic system. They play a key role in the maintenance of hematopoietic stem cells (HSCs) by modulating their self-renewal, proliferation, commitment, maturation, and apoptosis.1 Stromal cells are defined in vitro as the adherent cell component that supports hematopoietic cells in long-term cultures. This population is very heterogonous and includes adipocytes, fibroblasts, endothelial cells, osteoblasts, and osteocytes.1-4
Because of the close relationship between the stroma and the hematopoietic compartments, the idea of a common precursor has been contemplated for more than half a century. However, numerous studies of bone marrow (BM) chimeras demonstrate that BM stromal cells, including mesenchymal stem cells (MSCs), are host derived after BM transplantation.1,4-10
Multipotent adult progenitor cells (MAPCs) are stem cells obtained from BM that can differentiate into mesodermal (eg, osteoblast, chondrocytes, adipocytes, myoblasts, and endothelial cells), endodermal (eg, hepatocytes), and ectodermal (eg, neurons, oligodendrocytes, and astrocytes) cells.11-15 MAPCs are isolated within the BM adherent cell component, but their origin is still unknown.
MAPCs have extensive expansion capacity in vitro, are easily transduced, and can differentiate both in vitro and in vivo into cells of the 3 germinal layers; thus, MAPCs hold great promise for cell therapy. Here we studied if MAPCs are transplantable within unfractionated BM transplants into irradiated recipients. Two million mononuclear BM cells from green fluorescent protein (GFP) congenic mice were transplanted into lethally irradiated mice. BM cells from chimeras were analyzed 1, 2, and 3 months after transplant and MAPCs were isolated. Surprisingly, we consistently found that chimeric BM contained both host- and donor-derived MAPCs.
Study design
BM transplants
MAPC culture and differentiation
MAPCs were isolated from the BM of C57BL6 mice and expanded in culture as described previously.12 MAPCs were expanded in serum-free (SF) medium, (60% low-glucose Dulbecco modified Eagle medium [DMEM-LG]; 40% MCDB-201 (a modification of Ham nutrient mixture F-12); insulin, transferrin, and selenic acid [ITS]; linoleic acid [LA]-bovine serum albumin [BSA]; 10 nM dexamethasone; 0.1 μM ascorbic acid 2-phosphate; 100 U penicillin; and 1000 U streptomycin) with 2% fetal calf serum (Hyclone Laboratories, Logan, UT), 10 ng/mL human platelet-derived growth factor-BB (hPDGF-BB; R&D Systems, Minneapolis, MN), 10 ng/mL mouse epidermal growth factor (mEGF; Sigma, Saint Louis, MO), and 1000 U/mL mouse leukemia inhibitory factor (mLIF; Chemicon, Temecula, CA). Differentiation medium contained SF supplemented with respective growth factors.
Flow cytometry and immunofluorescence analysis
Flow cytometry and immunofluorescence were performed as described previously.11,12 Antibodies against CD73-phycoerythrin (PE), CD45-PE, CD14-PE, CD44-PE, Flk-1-PE (VEGF-R2), Sca-1-PE, and CD13-PE were from BD Pharmingen (San Diego, CA). Antibody against von Willebrand factor (VWF) was from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody against neurofilament 200 (NF-200), smooth muscle actin-Cy-3, and albumin were from Sigma. Antibody against CD105 was from eBiosciences (San Diego, CA). Cells were fixed with 4% paraformaldehyde or methanol, and incubated sequentially for 30 minutes each with primary and secondary antibodies. Immunofluorescence staining was analyzed with a Nikon Eclipse E-1000 fluorescence microscope (Nikon, Tokyo, Japan), using a Plan Apochromat 20 ×/0.75 numeric aperture objective (Carl Zeiss, Thornwood, NY). Images were captured with a SPOT 1.4.0 camera and SPOT Advanced 3.5.8 acquisition software (both from Diagnostic Instruments, Sterling Heights, MI).
Karyotypes
For karyotyping, 50% confluent cultures were exposed to 0.1 μg/mL colcemid (Sigma) for 12 hours, harvested, washed, and exposed to 75 mM KCl hypotonic solution for 5 minutes. Hypotonic solution was washed out by centrifugation and cells were fixed in 5 mL methanol/acetic acid (3:1) fixative. Fixed cells were collected by centrifugation and resuspended in 0.5 mL methanol/acetic acid (3:1) fixative. Cells were dropped onto a hot glass slide. Chromosomes were stained using 10% Giemsa solution. Twenty chromosomal spreads were read per population to rule out mosaicisms more than 14% with a confidence interval of 95%.
Fluorescent in situ hybridization (FISH)
Cells were fixed onto slides as per the karyotype protocol specified in “Karyotypes,” followed by dehydration with serial ethanol washes and denaturation with a 70% formamide and 30% 2× standard saline citrate (SSC) solution. Nuclei were stained with a whole mouse Y chromosome fluorescein isothiocyanate (FITC) probe (purchased from Cambio, Cambs, United Kingdom) overnight, washed with phosphate-buffered saline (PBS) and coverslipped with DAPI (4′,6-diamidino-2-phenylindole) mounting medium.
Results and discussion
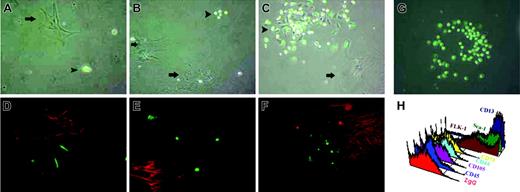
Mouse BM MAPCs were isolated by adhesion of unfractionated BM mononuclear cells to a Petri dish, followed by removal of nonadherent cells after 24 hours. In early cultures, MAPCs coexisted with other types of adherent cells including stromal cells, macrophages, and endothelial cells. When MAPCs were isolated from BM chimeras (n = 12, 4 to 12 weeks after syngeneic BM transplant from a transgenic mouse ubiquitously expressing GFP under the control of the constitutively active cytomegalovirus [CMV]/β-actin promoter), a mixture of large and small GFP-positive and GFP-negative cells were seen (Figure 1A-C). After 3 weeks, colonies of small cells resembling MAPCs emerged adjacent to larger cells (Figure 1C).
Mononuclear cells from BM chimeras were replated in MAPC medium at a density of 106 cells/cm2. (A) After 1 week, large GFP-negative cells (arrows) and small GFP-positive and GFP-negative cells were seen. (B) After 2 weeks, colonies of the small GFP-positive cells began to be observed adjacent to larger cells. (C) After 3 weeks, colonies of small, MAPC-like cells were seen (arrowheads). (D) Staining for CD73-PE, a marker for mesenchymal cells. (E) Staining for smooth muscle actin-Cy-3, which is a marker for activated stromal cells. (F) Staining for CD14-PE, a marker for macrophages. Notice the presence of small GFP-positive spindle-shaped cells that resemble MAPCs, which were negative for the above markers. (G) MAPC colony seen after 5 weeks. (H) MAPC immunophenotype: negative for CD45, CD105, CD44, CD73, but positive for Flk-1, Sca-1, CD13. Original magnification, ×20 for panels A-G. Arrowheads indicate MAPC-like cells; arrows, large GFP-negative cells.
Mononuclear cells from BM chimeras were replated in MAPC medium at a density of 106 cells/cm2. (A) After 1 week, large GFP-negative cells (arrows) and small GFP-positive and GFP-negative cells were seen. (B) After 2 weeks, colonies of the small GFP-positive cells began to be observed adjacent to larger cells. (C) After 3 weeks, colonies of small, MAPC-like cells were seen (arrowheads). (D) Staining for CD73-PE, a marker for mesenchymal cells. (E) Staining for smooth muscle actin-Cy-3, which is a marker for activated stromal cells. (F) Staining for CD14-PE, a marker for macrophages. Notice the presence of small GFP-positive spindle-shaped cells that resemble MAPCs, which were negative for the above markers. (G) MAPC colony seen after 5 weeks. (H) MAPC immunophenotype: negative for CD45, CD105, CD44, CD73, but positive for Flk-1, Sca-1, CD13. Original magnification, ×20 for panels A-G. Arrowheads indicate MAPC-like cells; arrows, large GFP-negative cells.
These colonies were composed of GFP-positive and GFP-negative cells. Some of the medium-sized cells stained positive for CD73 (Figure 1D) and other MSC markers (not shown). Other medium-sized cells stained positive for smooth muscle actin (Figure 1E), which is a marker for activated stromal cells. These medium-sized cells were always GFP negative, indicating that stromal cells and MSCs were of host origin in our culture system from BM chimeras. The largest cells, which contained both GFP-positive and -negative cells, were identified as macrophages as they stained positive for CD45, a pan-hematopoietic marker (not shown), and CD14 (Figure 1F), a macrophage-specific marker. After 5 weeks of culture, MAPCs outgrew the stromal cells (including MSCs and macrophages) emerging as colonies of homogeneous cells, some of which were 100% GFP positive (Figure 1G).
Interestingly, an increased percentage of GFP-positive MAPCs was seen in cultures derived from mice at longer intervals after transplant, suggesting that the BM cells responsible for the generation of MAPCs when cultured in vitro may be susceptible to irradiation (Table 1, Figure 1). After 30 cell doublings, these cells displayed the classic phenotype of MAPCs, as they did not display immunoreactivity for CD45 or MSC markers, such as CD105, CD44, or CD73, but they stained positive for FLK-1 (vascular endothelial growth factor receptor 2 [VEGFR2]), Sca-1, and CD13 (Figure 1H).
Percentage of successful MAPC cultures obtained from primary and secondary BM recipients and age-matched controls
. | 1st recipient 1 month after transplantation, n = 4 . | 1st recipient 2 months after transplantation, n = 4 . | 1st recipient 3 months after transplantation, n = 4 . | 2nd recipient 2 months after transplantation, n = 9 . |
|---|---|---|---|---|
| Successful MAPC cx in age-matched mice | 2-mo-old mice 4/6, 66% | 3-mo-old mice 5/11, 45% | 4-mo-old mice 3/7, 42% | 5- to 6-mo-old mice 1/10, 10% |
| Successful MAPC cx in transplant recipients | n = 3, 75% | n = 2, 50% | n = 2, 50% | n = 3, 30% |
| 40.6 | ||||
| % GFP MAPC | 32 ± 7 | 49 ± 9 | 74 ± 13 | 30 ± 5 |
. | 1st recipient 1 month after transplantation, n = 4 . | 1st recipient 2 months after transplantation, n = 4 . | 1st recipient 3 months after transplantation, n = 4 . | 2nd recipient 2 months after transplantation, n = 9 . |
|---|---|---|---|---|
| Successful MAPC cx in age-matched mice | 2-mo-old mice 4/6, 66% | 3-mo-old mice 5/11, 45% | 4-mo-old mice 3/7, 42% | 5- to 6-mo-old mice 1/10, 10% |
| Successful MAPC cx in transplant recipients | n = 3, 75% | n = 2, 50% | n = 2, 50% | n = 3, 30% |
| 40.6 | ||||
| % GFP MAPC | 32 ± 7 | 49 ± 9 | 74 ± 13 | 30 ± 5 |
Percentage of successful MAPC cultures, defined as cultures grown for more than 50 cell doublings, capable of differentiating into endothelial cells, hepatocytes, and neurons, in first and second recipient isolations and percentage of GFP-positive cells (determined by FACS analysis) within those populations. Compare to the percentage of successful MAPC cultures in age-matched control mice (nontransplanted, nonirradiated). Cx indicates cultures.
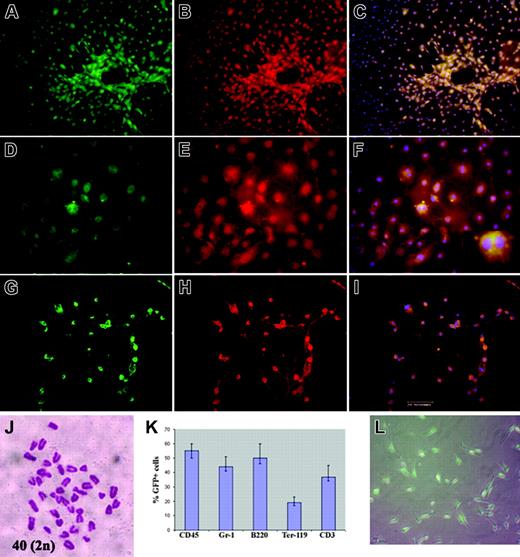
In a second experiment, BM obtained 1 month after BM transplant (n = 3) was harvested and mononuclear cells were sorted as GFP-positive and GFP-negative cells and were cultured in MAPC expansion medium. MAPCs grew from the GFP-positive fraction. These GFP-positive cells displayed the typical MAPC-like immunophenotypes and were expanded for more than 50 cell doublings and differentiated into endothelial cells, hepatocytes, and neurons (Figure 2A-I). Karyotypic analysis performed on randomly selected clones (n = 5) that were expanded for more than 50 cell doublings revealed diploid karyotypes (Figure 2J). Although stromal cells and MSCs were seen in the early cultures of the GFP-negative fraction, no MAPCs grew out of this population.
MAPCs may require support or assistance from other types of hematopietic cells in early culture, which were absent in the GFP-negative population. This possibility might explain why mouse MAPCs cannot be expanded if the mononuclear cells are depleted of CD45-positive cells, in contrast to human MAPC isolation, which requires upfront depletion of CD45-positive cells.14
To rule out the possibility that MAPCs are the product of cell fusion between a host and a donor cell either in vivo or in our in vitro culture conditions, we performed sex-mismatched transplants of female GFP donor BM cells into a male host. In this setting, if fusion occurs we should find Y chromosome-positive cells in the donor population even if reduction from a tetraploid cell to a triploid or diploid cell occurred. If fusion did not occur, no Y chromosome-positive cells would be present in the donor population.
MAPCs derived from sorted GFP-positive BM mononuclear cells were cultured for more than 50 cell doublings, then induced to differentiate into endothelial cells (mesodermal lineage), hepatocytes (endodermal lineage), or neurons (ectodermal lineage). (A,D,G) GFP-positive MAPCs treated with VEGF (A-C; notice vascular tubelike structures), FGF-4/HGF (D-F), or bFGF (G-I) for 14 days. Cultures were stained with anti-VWF labeled with Cy3 (B), anti-albumin-Cy3 (E), or anti-NF200-Cy3 (H). (C,F,I) An overlay of Cy3 staining with green fluorescence and DAPI. (A,C,D,F, G,I) 100% of these cells were eGFP positive. (J) Cytogentic analysis of selected clones revealed a diploid karyotype. (K) BM from secondary recipients showed trilineage hematopoietic differentiation (% GFP-positive cells determined by FACS). (L) MAPCs from secondary recipients contained GFP-positive cells. Inset: Binucleated cells. Bar equals 200 μm.
MAPCs derived from sorted GFP-positive BM mononuclear cells were cultured for more than 50 cell doublings, then induced to differentiate into endothelial cells (mesodermal lineage), hepatocytes (endodermal lineage), or neurons (ectodermal lineage). (A,D,G) GFP-positive MAPCs treated with VEGF (A-C; notice vascular tubelike structures), FGF-4/HGF (D-F), or bFGF (G-I) for 14 days. Cultures were stained with anti-VWF labeled with Cy3 (B), anti-albumin-Cy3 (E), or anti-NF200-Cy3 (H). (C,F,I) An overlay of Cy3 staining with green fluorescence and DAPI. (A,C,D,F, G,I) 100% of these cells were eGFP positive. (J) Cytogentic analysis of selected clones revealed a diploid karyotype. (K) BM from secondary recipients showed trilineage hematopoietic differentiation (% GFP-positive cells determined by FACS). (L) MAPCs from secondary recipients contained GFP-positive cells. Inset: Binucleated cells. Bar equals 200 μm.
BM from 5 chimeras were harvested 4 weeks after transplant and MAPC cultures were established from either fresh BM sorted as GFP-positive cells or unsorted BM cells. After 5 weeks, colonies of MAPCs were seen. Those derived from the sorted GFP-positive fraction were prepared for karyotyping and Y chromosome FISH analysis. Cytogenetic analysis of the GFP-positive cells revealed a diploid female karyoptype (40, XX). MAPC colonies derived from unsorted BM cells were then sorted as GFP positive and GFP negative and analyzed for the presence of the Y chromosome by FISH analysis. We analyzed more than 1 million cells per population by FISH in 5 different chimeras. As expected, all GFP-negative (host cells) contained the Y chromosome, whereas all GFP-positive cells (donor cells) were negative for the Y chromosome by FISH (Supplemental Figure S1, available at the Blood website; click on the Supplemental Figure link at the top of the online article). This result proves that MAPCs are not derived from an in vitro or in vivo fusion event.
In a third study, BM mononuclear cells from mice that had been previously undergone BM transplantation with syngeneic GFP-positive donors (n = 3) were transplanted into a second set of syngeneic recipients (n = 9). Two months after the second transplant, BM was harvested and mononuclear cells were cultured in MAPC medium. The secondary recipients showed multilineage engraftment, as GFP-positive cells were present in all 3 hematopoietic lineages (Figure 2K). Interestingly, the MAPC population isolated from the secondary recipients also contained GFP-positive cells (Table 1, Figure 2L).
The frequency of successful MAPC cultures from transplant recipients is very similar to the frequency obtained from previous MAPC isolations of age-matched, nonirradiated, nontransplanted mice, indicating that the capacity to generate MAPC cultures is age-dependent and dramatically decreases with increasing age (Table 1). Thus, we demonstrated that MAPCs were transplantable within unfractionated BM transplants, and were able to engraft, self-renew, and repopulate secondary recipients.
This study is the first demonstration that ex vivo-cultured MAPCs are physiologically different from stromal cells and MSCs, suggesting they may be unrelated ontologically, even though they all form part of the BM adherent cell component defined in culture. This is also the first demonstration that BM transplantation leads to the transfer of cells, that upon isolation in vitro generate MAPCs and, whatever the identity of this cell, is eliminated by irradiation.
Numerous studies indicate that hematopoietic stem cells (HSCs), or BM populations highly enriched for HSCs, can give rise to nonhematopoietic cells, particularly vascular cells, including endothelial and smooth muscle cells.18-21 Additional studies from single HSC transplants may help to elucidate whether MAPCs and HSCs have a common origin.
Studies by Jiang et al12 demonstrated that when in vitro-cultured MAPCs were intravenously transplanted into nonobese diabetic-severe combined immunodeficient mice, engraftment was observed in multiple tissues, including hematopoietic cells.
When BM cells from these initial recipients were transplanted into secondary recipients, similar engraftment in multiple tissues was observed. However, in these studies MAPCs were not isolated for culturing in vitro from either the primary or secondary recipients, so it could not be determined whether MAPCs engrafted and/or self-renewed, or whether they instead differentiated into progenitor cells that were responsible for the observed engraftment. Here we show that following unfractionated BM cell transplant, MAPCs or the cell type that generates MAPCs when cultured in vitro, exists in the mononuclear BM fraction in sufficient quantities to be transplantable (MAPCs are donor derived in BM chimeras), enabling self-renewal (both in vitro and in vivo) and repopulation in secondary recipients. Whether MAPCs are involved in the plasticity phenomena seen following BM transplants remains to be determined.22-25 Nonetheless, this finding has many implications for the use of BM transplants to deliver and reconstitute the MAPC population, which in turn could be of potential use for many clinical applications.
Prepublished online as Blood First Edition Paper, August 11, 2005; DOI 10.1182/blood-2004-12-4603.
Supported by the National Institutes of Health and the Muscular Dystrophy Association.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal