Warfarin is the most widely prescribed anticoagulant for thromboembolic therapy, despite a 20-fold interindividual difference in dose requirement and a narrow therapeutic range. Incorrect dosage, especially during the initial phase of treatment, carries a high risk of either severe bleeding or failure to prevent thromboembolism.1 Knowledge of biochemical mechanisms, site of drug action, and the human genome enable discovery of genetic factors that cause variable drug response. Warfarin acts through interference with vitamin K epoxide reductase that is encoded by VKORC1.2-4 Reduced vitamin K is an essential cofactor for the activation of clotting factors by gamma-glutamyl carboxylase, which is encoded by GGCX.5-7
Recently, Shikata et al described a microsatellite marker in intron 6 of the GGCX gene that was associated with warfarin dose.8 Ten, eleven, and thirteen (CAA) repeats were detected in 45 warfarin-treated Japanese patients. Three individuals heterozygous for 13 repeats (10/13 or 11/13) required higher maintenance doses than patients with fewer repeats. We typed this microsatellite in 201 Swedish warfarin-treated patients from Uppsala University Hospital anticoagulation clinic. Details on patient characteristics, blood collection, and DNA extraction have been published previously.9 Genotyping of the GGCX microsatellite (chromosome 2: 85693236-85693265 bp, NCBI 35) was performed by polymerase chain reaction (PCR) amplification using fluorescently labeled primers and electrophoretic separation of PCR products on an ABI PRISM Genetic Analyzer. Allele calling was performed with the Genotyper Software v 3.7. Statistics were calculated with analysis of variance (ANOVA).
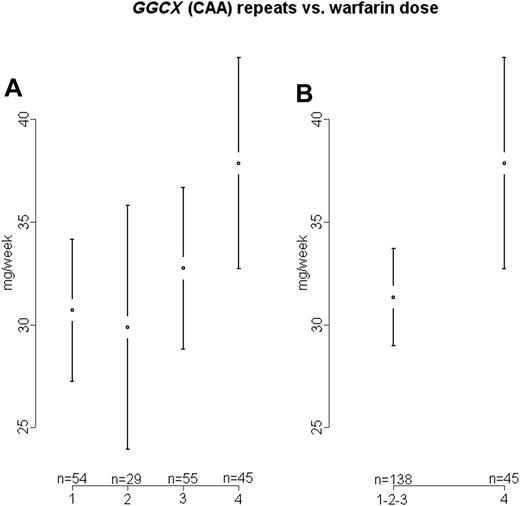
Individuals are divided into 4 groups according to GGCX genotype. Group 1: 10/10 (CAA) repeats; group 2: 10-11/11 repeats; group 3: 10-11/13 repeats; group 4: 13/13 or x/14-16 repeats. (A) Mean weekly warfarin doses for varying GGCX (CAA) repeats with 95% confidence intervals (groups 1, 2, 3, and 4; ANOVA P = .064). (B) Mean and 95% confidence interval of mean weekly warfarin doses for varying GGCX (CAA) repeats (groups 1-3 combined vs 4; ANOVA P = .011).
Individuals are divided into 4 groups according to GGCX genotype. Group 1: 10/10 (CAA) repeats; group 2: 10-11/11 repeats; group 3: 10-11/13 repeats; group 4: 13/13 or x/14-16 repeats. (A) Mean weekly warfarin doses for varying GGCX (CAA) repeats with 95% confidence intervals (groups 1, 2, 3, and 4; ANOVA P = .064). (B) Mean and 95% confidence interval of mean weekly warfarin doses for varying GGCX (CAA) repeats (groups 1-3 combined vs 4; ANOVA P = .011).
We detected a wider range of (CAA) repeats than the Japanese cohort: 10, 11, 13, 14, 15, and 16 repeats, with 10 being the most common. In analogy with the Shikata study, we divided patients into groups according to genotype: (1) 10/10 repeats, (2) 10/11 or 11/11 repeats, and (3) 10/13 or 11/13 repeats. In addition, we had a fourth group of patients with more (CAA) repeats, that is, homozygous for 13 or heterozygous for 14, 15, or 16 repeats (Figure 1A-B).
We could not confirm the Japanese finding of higher doses in individuals with 10/13 or 11/13 (CAA) repeats compared to patients with lower numbers of repeats (Figure 1A; groups 1, 2, and 3; P = .616). However, when we included the fourth group in the analysis, we observed a difference of near nominal significance (Figure 1A; groups 1, 2, 3, and 4; P = .064). When the fourth group was compared with all patients with fewer repeats, a significantly higher warfarin dose requirement was detected (Figure 1B; groups 1-3 vs 4; P = .011).
In our study, warfarin dose requirement tends to increase with the number of microsatellite repeats. This corresponds with the Japanese finding; however, in Swedes the effect is only apparent in patients with higher numbers of repeats. We have previously observed a GGCX polymorphism in intron 2 that increases warfarin dose requirement (P = .036).10 In univariate models, the GGCX microsatellite explains 3.5% of the variance in dose, while polymorphisms in GGCX, VKORC1, and CYP2C9 explain 3.3%, 29.9%, and 11.2%, respectively (Table 1).10 In a multiple model, the polymorphisms all show significant association to dose, but due to high linkage disequilibrium within the GGCX gene, no information is gained by adding the microsatellite to the model. Larger studies with different ethnicities will be needed to further elucidate the influence of GGCX variability on warfarin dosing.
Univariate regression models for warfarin maintenance dose
Variables . | P . | r2 . |
|---|---|---|
| GGCX microsatellite | .011 | 0.035 |
| GGCX rs12714145 | .036 | 0.033 |
| VKORC1 rs9923231 | < .001 | 0.299 |
| CYP2C9 alleles *2 and *3 | < .001 | 0.112 |
Variables . | P . | r2 . |
|---|---|---|
| GGCX microsatellite | .011 | 0.035 |
| GGCX rs12714145 | .036 | 0.033 |
| VKORC1 rs9923231 | < .001 | 0.299 |
| CYP2C9 alleles *2 and *3 | < .001 | 0.112 |
The GGCX microsatellite groups 1-3 combined and 4, GGCX rs12714145 G > A, VKORC1 rs9923231 G > A, and CYP2C9 alleles *2 and *3 are tested for covariance with warfarin maintenance dose using univariate analysis in SAS.
Kristina Sörlin is acknowledged for going through the medical records and Ralph McGinnis for critical reading of the manuscript.
Supported by the Wellcome Trust, the Swedish Society of Medicine, the Swedish Foundation for Strategic Research, the Swedish Heart and Lung Foundation, the Tore Nilson Foundation, and the Clinical Research Support (ALF) at Uppsala University.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal