Abstract
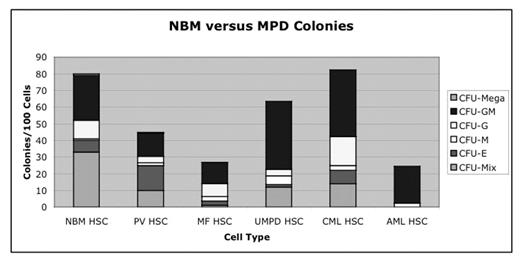
Myeloproliferative disorders (MPD) are clonal hematopoietic disorders characterized by a hypercellular marrow, an overabundance of distinct lineages of terminally differentiated progeny and a propensity to transform to acute myelogenous leukemia (AML). Recent reports revealed that a specific (V617F) mutation in JAK2 resulted in constitutive cytokine signaling and increased sensitivity to cytokines in a large proportion of patients with MPD. However, the stage of hematopoiesis at which this mutation occurs and whether additional mutations contribute to the evolution of MPD into AML has yet to be determined. We performed phenotypic and functional analyses of hematopoeitic stem cells (HSC), common myeloid progenitors (CMP), granulocyte-macrophage progenitors (GMP) and megakaryocyte-erythroid progenitors (MEP) in 63 MPD peripheral blood or bone marrow samples donated by patients with PV (n=15), essential thrombocythemia (ET; n=8), post-polycythemic myeloid metaplasia/myelofibrosis (PPMM/MF; n=5), chronic myelogenous leukemia (CML; n=7), atypical CML/myeloproliferative disease unspecified (aCML/UMPD; n=2), chronic eosinophilic leukemia (CEL; n=1), chronic myelomonocytic leukemia (CMML; n=13) and AML (n=12) in order to identify the stage of hematopoiesis at which mutations arose as well as to provide prognostic information on patients with a propensity to transform to AML. Of the MPD mononuclear cells sequenced, 12 of 15 PV, 2 of 5 ET, 3 of 3 post-polycythemic myeloid metaplasia, and 2 of 2 aCML samples were positive for the JAK2 V617F mutation. With regard to phenotypic changes in progenitor profiles, PV samples had an increase in CMP together with a distinctive IL-3Ra high population that distinguished it from all other MPD. Conversely, aCML, AML and proliferative CMML had a preponderance of GMP. Functional changes in progenitors were assessed using hematopoietic progenitor assays (Figure 1A and B). This methodology demonstrated that the differentiation potential of PV was already skewed toward the erythroid lineage at the HSC level in contrast to CMML, CML, aCML and AML HSC which produced a preponderance of myeloid colonies. In addition, the aberrant erythroid potential of PV HSC could be potently inhibited with a specific JAK2 inhibitor - AG490 suggesting that JAK2 plays a role in enhancing the erythroid differentiation potential of PV at the stem cell level. Finally, other molecular abnormalities were detected at the progenitor level in MPD including changes in GATA-1 and PU.1 expression, bcl-2 overexpression and beta-catenin activation that were associated with progression to AML. Thus, molecular progenitor profiling may provide prognostic information in a variety of MPD and could be a useful adjunct to current diagnostic methods.
NBM versus MPD Colonies
Normal BM vs PV Progenitor Colonies
Author notes
Corresponding author


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal