Abstract
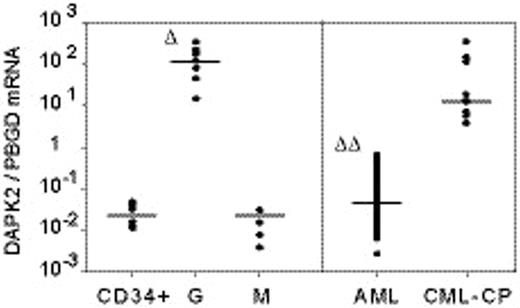
DAPK2 is a 42-kDa Ca2+/Calmodulin-regulated serine/threonine kinase involved in apoptosis. In gene expression profiles derived from in vitro differentiated myeloid leukemic NB4 cells treated with all-trans retinoid acid (ATRA), we found that DAPK2 was decisively induced during differentiation towards neutrophils. DAPK1, a close relative of DAPK2, is inactivated in a number of hematopoietic malignancies (AML, lymphoma, myeloma), and it may play a role during normal and leukemic myeloid cell differentiation. We therefore investigated DAPK2 for its possible role in both normal and leukemic myelopoiesis. Real time quantitative RT-PCR (RQ-PCR) and Western blot analysis of DAPK2 gene expression in primary myeloid cells revealed significantly higher DAPK2 expression in granulocytes (G; n=9) compared with monocytes/macrophages (M; n=8) and CD34+ progenitor cells (CD34+; n=6) (Δ, p< 0.001; figure, left panel). Moreover, significantly increased DAPK2 mRNA levels were also seen when cord blood CD34+ progenitor cells were induced to differentiate towards neutrophils with human recombinant G-CSF (hrG-CSF). In addition, ATRA-induced neutrophil differentiation of two leukemic cell lines, NB4 and U937, showed significantly higher DAPK2 mRNA expression paralleled by DAPK2 protein induction. However, during differentiation of CD34+ cells (with hrM-CSF) and U937 cells (with PMA) towards monocytes/macrophages, DAPK2 mRNA levels remained low. DAPK2 expression in primary leukemic cells revealed significantly lower DAPK2 expression levels in AML blasts (AML; n=100) than in samples from chronic myeloid leukemia patients in chronic phase (CML-CP; n=9) (ΔΔ, p< 0.001; figure, right panel).
Stable lentiviral-mediated expression of wild-type DAPK2 enhanced ATRA-induced granulocytic differentiation of NB4 cells as shown by morphology and by increased CD11b expression. Furthermore, upregulation of mRNA levels of key regulator genes for terminal differentiation, such as C/EBPe, the G-CSF receptor and the secondary granule protein lactoferrin, was also enhanced. Expression of a kinase-inactive DAPK2 mutant did not show these effects, a finding consistent with a role of DAPK2 in granulopoiesis.
Conclusion: we demonstrate for the first time, that DAPK2 expression levels correlate with the degree of granulocytic differentiation, and that DAPK2 upregulation is restricted to granulopoiesis. Furthermore lentiviral-mediated DAPK2 expression enhances granulocytic differentiation. The finding that DAPK2 expression is low in AML and high in CML-CP patients suggests that suppressed DAPK2 expression may contribute to the differentiation block in AML.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal