Abstract
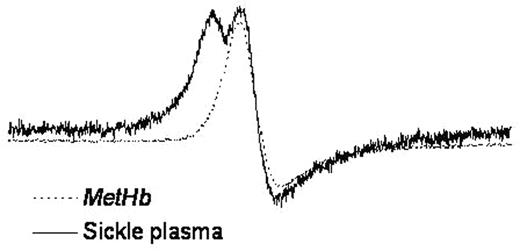
It has previously been shown that red blood cell-free oxyhemoglobin (oxyHb) can affect endothelial function in Sickle Cell Disease (SCD) by virtue of its NO scavenging properties (Reiter et al, Nat Med, 2002). This reaction may have down-stream consequence via formation of metHb which has been demonstrated to have pro-oxidant properties and can oxidize low-density lipoprotein in vitro. In this study we have examined the fate of metHb in SCD vs normal plasma by examining the characteristic single line EPR signature of metHb in the g = 6 region. We show that the EPR signature of metHb differs in SCD compared with normals and exhibits a split EPR line indicative of a more rhombic heme geometry (See Figure).
Addition of purified metHb to SCD plasma results in a conversion of the single line EPR spectrum to the rhombic form. By addition of hemin and hemoglobin to plasma constituents we have determined that the rhombic EPR spectrum is consistent with heme bound to serum albumin. In addition, immunoprecipitation of serum albumin from SCD plasma with EPR analysis of the IP fraction revealed the presence of albumin associated metHb. Interestingly, the transfer of heme from metHb to albumin is accelereated by the presence of low-density lipoprotein and inhibited by the addition of haptoglobin suggesting that plasma components can modulate heme release from metHb and that the lack of haptoglobin in SCD plasma allowed the transfer of heme from metHb to albumin. To confirm this we added metHb to SCD plasma in the presence and absence of haptoglobin and observed that the addition of haptoglobin prevented conversion of the metHb spectrum to the albumin associated split (rhombic) species. From these studies we conclude that plasma haptoglobin not only binds hemoglobin but also limits heme release from metHb. To examine potential oxidative consequences of this effect, we incubated LDL with metHb in the presence and absence of haptoglobin. Haptoglobin inhibited LDL oxidation in a dose-dependent manner. Interestingly, the 1-1 isoform of haptoglobin was much more effective than the 2-2 isoform, in agreement with published studies on the efficacy of hemoglobin binding to the two haptoglobin varieties. Additionally, we have measured levels of 8-isoprostanes in the plasma of SCD patients as an index of lipid oxidation and show that 8-isoprostane levels are significantly elevated in SCD patients (295 209 pg/ml, mean S.D., n= 13, vs. 69 37 pg/ml, mean S.D. n= 5 for normal controls). In conclusion, these studies provide evidence that the saturation of the haptoglobin clearance mechanisms in hemolytic disorders such as SCD can not only affect the NO-axis of endothelial function due to increases in cell-free hemoglobin, but can also lead to altered heme metabolism leading to lipid oxidation and formation of pro-inflammatory lipids which have the potential to alter both endothelial and red cell function.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal