Abstract
To improve ABVD results we first developed a protocol which adds G-CSF to the standard ABVD treatment. From March 1997 to May 2004 69 patients with HL were treated with ABVD+G-CSF and 22 with a standard ABVD. Recently we designed a new dose-dense and dose-intensity ABVD scheme (escABVD-21) for advanced HL; in this new schedule the adriamycin was escalated from 25 to 35 mg/m2 (cycles 1,2,3,4) and the inter-cycle period was shortened from 28 to 21 days (for all 6 cycles); primary G-CSF was administered from d3 to d8 and drugs were delivered at d10 and d21 of every cycle. From June 2004, 19 patients were treated with this protocol.
Relative dose-intensity (RDI) was calculated for any of these 110 newly diagnosed HL patients treated with ABVD. The results were also compared with a historical group of 70 patients who had undergone hybrid MOPP/ABVD.
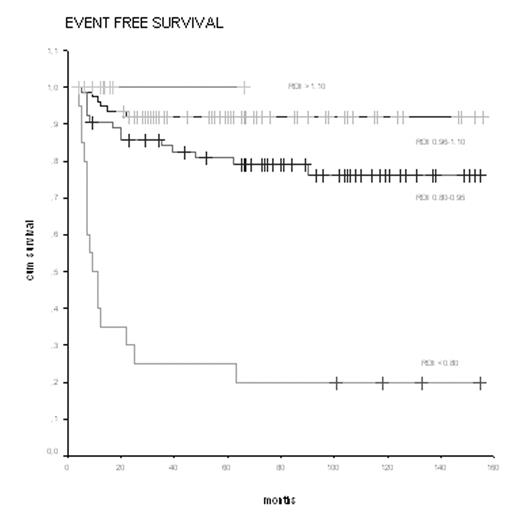
HL patients received from 4 to 8 cycles of CT +/− IF-RT. Patients were divided in 4 groups according to the RDI. The first group included 20 (11%) pts with RDI less than 0.80; the 2nd group, 64 (36%) pts with RDI values between 0.80 and 0.95, the 3rd group, 74 (42%) pts with RDI values between 0.96 and 1.10 and, finally, the 4th group included pts with RDI values of more than 1.10. In Tab 2 we report the CR, EFS and OS rates according to the 4 levels of RDI. Figures 1 shows EFS curve according to Kaplan-Meyer.
Response and survival rates of groups 1,2,3 and 4 were: 50%vs91%vs97% vs 100%, for CR (p<0.0001); 20%vs78%vs92%vs100% for EFS (p< 0.0001); and 25%vs91%vs99%vs100% for OS (p< 0.0001).
The best progression rates of CR, EFS and OS were seen in patients with RDI >1.10. In particular, the new dose-dense and dose-intensity escABVD-21 protocol seems very promising in terms of complete response and toxicity profile: 19/19 pts (100%) obtained an early CR; (PET negative at the end of the 2nd cycle), and, as on 8th August 2005, all these19 patients were disease-free. The dose-escalation of adriamycin and the dose-density of the schedule were well-tolerated; toxicity was mild. These results show that subptimal RDI may compromise outcomes proportionally to the level of RDI reduction. On the contrary, Primary G-CSF permits to deliver dose-dense and dose intense schedules such as escABVD-21 maintaining the same profile of toxicity of standard ABVD, higher RDI levels, and consequently, a significant impact on complete response and survival rates.
Presentation features
| n. of pts . | male sex . | age>45 yrs . | advanced stage . | E sites>1 . | bulky . | B symptoms . | IPI score ≥ 3 . |
|---|---|---|---|---|---|---|---|
| 180 | 92 | 43 | 148 | 23 | 80 | 83 | 56 |
| % | 51% | 24% | 82% | 13% | 44% | 46% | 31% |
| n. of pts . | male sex . | age>45 yrs . | advanced stage . | E sites>1 . | bulky . | B symptoms . | IPI score ≥ 3 . |
|---|---|---|---|---|---|---|---|
| 180 | 92 | 43 | 148 | 23 | 80 | 83 | 56 |
| % | 51% | 24% | 82% | 13% | 44% | 46% | 31% |
Response and Survival according to 4 RDI levels
| RDI level (range 0.5–1.5) . | N. of pts. (%) . | CR rate . | Fisher’s Exact test (2-sided) . | EFS rate . | log-rank statistic . | 0S rate . | log-rank statistic . |
|---|---|---|---|---|---|---|---|
| overall | 180(100%) | 90% | 80% | 88% | |||
| <0.80 | 20 (11%) | 50% | 20% | 25% | |||
| 0.80–0.95 | 64 (36%) | 91% | 28.391 | 78% | 74.99 | 91% | 96.76 |
| 0.96–1.10 | 76 (42%) | 97% | 92% | 99% | |||
| >1.10 | 20 (11%) | 100% | 100% | 100% | |||
| significance | <0.0001 | <0.0001 | <0.0001 |
| RDI level (range 0.5–1.5) . | N. of pts. (%) . | CR rate . | Fisher’s Exact test (2-sided) . | EFS rate . | log-rank statistic . | 0S rate . | log-rank statistic . |
|---|---|---|---|---|---|---|---|
| overall | 180(100%) | 90% | 80% | 88% | |||
| <0.80 | 20 (11%) | 50% | 20% | 25% | |||
| 0.80–0.95 | 64 (36%) | 91% | 28.391 | 78% | 74.99 | 91% | 96.76 |
| 0.96–1.10 | 76 (42%) | 97% | 92% | 99% | |||
| >1.10 | 20 (11%) | 100% | 100% | 100% | |||
| significance | <0.0001 | <0.0001 | <0.0001 |
Figure
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal