Abstract
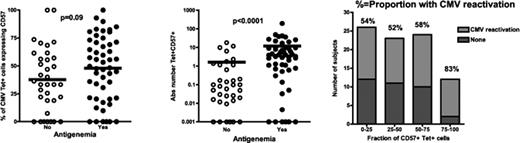
CMV reactivation remains a major cause of morbidity after after allogeneic stem cell transplantation. Using HLA-peptide tetramer staining and cytokine flow cytometry, we previously demonstrated that higher CD4+ and CD8+ T cells specific for CMV were found in the peripheral circulation of individuals who experienced CMV reactivation, but that more of these cells were dysfunctional in subjects experiencing viral reactivation. Recent studies have examined the role of maturation status of virus-specific T cells in the setting of of cleared, persistent and progressive viral infections. CD57 is a marker of replicative senescence that is expressed on a subset of human CD8+ memory T cells that generally lacks CD28 expression, and that is characterized by downregulation of the naïve and central memory T cell markers CD45RA and CCR7. To examine whether CMV reactivation after SCT was associated with late-stage differentiation of CMV-specific CD8+ T cells specific for viral proteins, we first examined the fraction of CD57-expressing CD8+ T cells in patients stratified by the occurrence of post-SCT viral reactivation. In 87 patients examined with HLA-peptide tetramer staining at approximately 3 months after allogeneic SCT, we found that individuals experiencing CMV reactivation within the first 100 days after SCT had significantly higher absolute numbers of circulating CD57+ tetramer-stained CD8+ T cells specific for CMV than those who did not experience viral reactivation (mean 11.8 vs. 1.6 CD57+ tetramer-stained cells/μl, median 3.3 vs. 0.08 cells/μl, p<0.0001). In individuals in whom >75% of tetramer-stained CD8+ T cells were CD57+, the incidence of CMV reactivation was 83%, in contrast to 52% of others. In other experiments, we found that CD57+ tetramerstained CD8+ T cells failed to expand following co-culture with autologous monocyte-derived dendritic cells pulsed with CMV peptide pools, confirming that CD57+ CMV-specific CD8+ T cells may have impaired proliferative capacity. Taken together, these results suggest that late-stage differentiation of memory CD8+ T cells may be indicative of an exhausted antigen-specific immune response, and that optimal post-SCT immune restoration should consider the differentiation status and not simply the numbers of antigen-specific T cells targeted.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal