Abstract
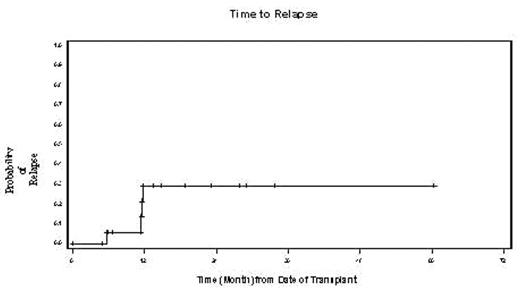
The treatment of high-risk MCL remains a challenge. High dose chemotherapy (HDC) with autologous stem cell transplantation (ASCT) has been used for some of these patients (pts). However, relapse rates remain high. RIT with single agent 90Yttrium(90Y) ibritumomab tiuxetan (Zevalin®) has demonstrated activity in pts with relapsed MCL. Therefore, we postulated that the combination of 90Y with HDC and ASCT may reduce relapse rates of pts with high- risk MCL. Between 5/00 and 9/04 eighteen pts with MCL were enrolled on one of two RIT ASCT trials. The first was a phase I/II dose escalation trial of high-dose 90Y + cyclophosphamide 100mg/kg (day -4) and etoposide 60mg/kg(day-2). Pts underwent dosimetry day-21 with 5mCi Indium111 followed a week later by 90Y to deliver a maximum target dose of 1000cGy to normal organs. The second study was a phase I/II trial of standard dose 90Y (0.4mCi/kg) administered day-14 and BEAM (day-8 to day-2, BCNU300mg/m2, cytarabine 800mg/m2, etoposide 800mg/m2, melphalan 140mg/m2). Ten pts who were>60yrs or had received prior dose-limiting radiation were placed on the 90Y BEAM trial while the other 8 received high-dose 90Y. The median age at ASCT was 58 years (range 44–72). Disease status at ASCT included 1st CR -9(high or high intermediate IPI score), 1st PR-4, 1st relapse -4, 2nd CR-1. Fifty percent had received HyperCEVAD chemotherapy and 56% had Rituximab prior to ASCT. Thirteen pts had stage IV disease, 5 had stage III disease. The median 90Y dose administered was 40mCi (range 27–100). Treatment was well tolerated. Engraftment to anc>500 occurred at a median of 10 days (range 9–26). Reversible grade 3 pulmonary toxicity occurred in 5 pts. This included steroid responsive pneumonitis in four and acute respiratory distress related to sepsis in one. One pt with a prior history of heavy alcohol use died of liver failure at four months post ASCT. Four pts have relapsed and two have died of disease progression. With a median follow up of 19 months (range 6–60), the estimated overall survival and disease free survival at two years are 79% (CI 55–91) and 59% (CI 43–73) respectively. By univariate analysis 90Y dose did not correlate with risk of relapse. To date, no relapses were seen beyond the first twelve months (see figure). In conclusion 90Y based transplant conditioning regimens are well tolerated, even in older pts. The apparent plateau in the relapse rate is encouraging and suggests that this approach may lead to durable remissions in pts with high-risk MCL.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal