Abstract
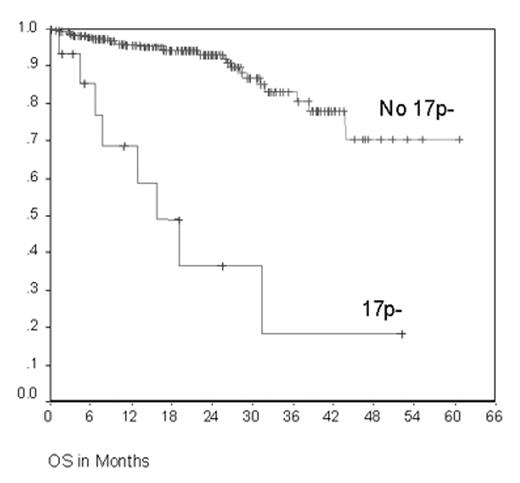
The CLL4 trial evaluated first line treatment with fludarabine (F) or F + cyclophosphamide (FC) among 375 CLL patients up to 65 years enrolled between 1999 and 2003. FC resulted in a higher overall response rate (94% vs. 83%; P=0.001), longer median progression free survival (PFS) (48 vs. 20 months (m); P=0.001), but no difference in overall survival (OS) (Eichhorst et al., ASH 2003 and submitted). At enrollment, genomic aberrations and the VH mutation status have been analyzed for 307 and 280 cases thus far. The most frequent genomic aberrations were 13q-: 50.3%, 13q- single: 34.1%, 11q-: 20.3%, +12q: 13.7%, 6q-: 8.7%, 14q-: 6.1%, and 17p-: 4.9%. VH was mutated in 33.9% and unmutated in 66.1% of cases. The aberrations 13q- and 13q- single were more frequently observed in the VH mutated subgroup (45.4% vs. 32.7% and 35.5% vs. 18.8%, respectively), while 11q- (26.9% vs. 12.2%) and 6q- (14.2 vs. 1.7%) were more common in the VH unmutated subgroup. Clinical outcome was evaluated in subgroups defined by genomic aberrations and VH status for both treatment arms combined. In univariate analyses, significant associations were found for the following parameters: the overall response rate was significantly lower in the subgroup with 17p- (53.8% vs. 89.6%, P=0.001), the median PFS was significantly shorter in the subgroups with 11q- (17.4 vs. 26.8 m, P=0.044), 14q- (14.5 vs. 24.5 m, P=0.018), and 17p- (11.0 vs. 24.1 m, P=0.002), and the median OS was significantly shorter in the subgroup with 17p- (15.9 m vs. not reached, 75% survival at 43.8 m, P<0.001, Figure 1). Multivariate analysis was performed including the treatment arms, specific genomic aberrations, and the VH mutation status as possible prognostic factors: the parameters with significant adverse impact were F monotherapy (HR 1.70, 95%CI 1.12-2.58, P=0.013) and 17p- (HR 3.67, 95%CI 1.66-8.14, P=0.001) regarding PFS, but only 17p- (HR 7.32, 95%CI 2.44-21.90, P<0.001) regarding OS. In conclusion, this first analysis of the prospective CLL4 trial shows that 17p- CLL is characterized by a short OS after F-based first line therapy. In future trials, alternative primary treatment strategies of proven efficacy in 17p- CLL such as alemtuzumab should be considered for these patients.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal