Comment on Hiddemann et al, page 3725
Hiddemann and colleagues demonstrate improved response duration and overall survival with the addition of rituximab to CHOP, compared with CHOP alone, in symptomatic patients with untreated advanced-stage follicular lymphoma.
The arrival of rituximab (R) onto the therapeutic landscape heralded a new era in the treatment of B-cell lymphoma and provided a rational basis for its combination with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone). Czuczman et al1 first demonstrated the benefits of R-CHOP in indolent lymphomas, but the broad applicability of their findings was uncertain due to limited sample size and absence of a control group. A recent 9-year follow-up of their study and the results of the present report, however, provide new evidence for the merits of R-CHOP and reshape treatment recommendations.2 FIG1
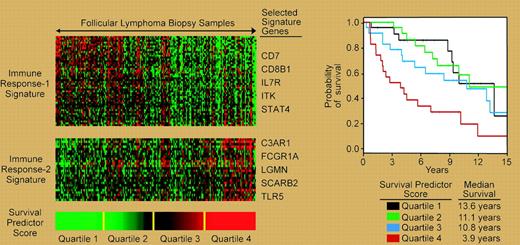
Molecular prognostic model of follicular lymphoma based on signatures of infiltrating “immune” cells.
Molecular prognostic model of follicular lymphoma based on signatures of infiltrating “immune” cells.
In this issue of Blood, Hiddemann and colleagues report the results of a randomized study of CHOP versus R-CHOP in 428 patients with advanced-stage follicular lymphoma of grade I or II histology and requiring treatment. Responding patients younger than 60 years of age were offered a second randomization following CHOP or R-CHOP of either intensification with stem cell support or long-term interferon-α maintenance, whereas older patients received interferon-α maintenance. At the median follow-up time of 18 months (range, 1-38 months), the risk of treatment failure was reduced by 60% in the R-CHOP arm with benefit observed in patients younger or older than 60 years and with low or high international prognostic index scores. The time to next therapy also demonstrated an advantage for R-CHOP. Of great interest was the significant improvement in overall survival with R-CHOP, albeit only modest at the relatively short follow-up time. A justifiable caveat to a broad embrace of these findings is found in the secondary treatment requirement. It is reassuring that the secondary randomizations were stratified for risk and that the R-CHOP benefit was irrespective of interferon-α maintenance. It is notable though that the R-CHOP benefit was not observed among patients randomized to stem cell consolidation, suggesting that enhanced treatment efficacy and not the unique biologic attributes of rituximab may underlie the benefits of R-CHOP. It should also not escape notice that differential access to rituximab containing salvage treatment for patients on the 2 arms could account for the survival advantage of R-CHOP. Nevertheless, these results as well as those of Czuczman et al,2 where the observation time is quite mature, raise the specter of survival advantage, if not cure, with R-CHOP in follicular lymphoma. While many opinions might favor the notion that rituximab combined with CHOP delays relapse in follicular lymphoma, the prospect of cure should be considered with appropriate circumspection.
These results are of sufficient measure to reshape the debate from “if” to “when” patients should receive R-CHOP for the initial treatment of advanced-stage disease, not to mention its role in early-stage disease. Questions of who will benefit from treatment with R-CHOP, versus less “hair-razing” regimens like R-CVP (rituximab–cyclophosphamide, vincristine, and prednisone), will occupy the minds of oncologists and patients.3 Gene expression profiling, while providing insight into tumor biology, is also a powerful prognostic tool that may help identify beneficiaries of such treatment. This technology has revealed a central biologic role for infiltrating “reactive” cells, termed the immune reaction (IR), within the malignant lymph node on the survival of follicular lymphoma.4 The relative expression of 2 signatures, termed IR-1 and IR-2, that differentially express genes related to T-cell markers, macrophages, and/or dendritic cells, can be used to predict survival (see figure). It is not unreasonable, given the early benefits of rituximab on survival, to hypothesize that rituximab may impact tumors that predominantly express IR-2, as this signature is associated with short survival. Indeed, a recent study on gene expression associated with rituximab responsiveness suggested the relevance of immune cells albeit as negative predictors.5 The prospective application of these technologies to future trials is vital for understanding outcome biology and to guide the rational clinical development and use of new treatments. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal