The primary clinical manifestations of paroxysmal nocturnal hemoglobinuria (PNH) are hemolytic anemia, marrow failure, and thrombophilia. However, PNH is not a simple binary diagnosis and both flow cytometric characterization of glycosyl phosphatidylinositol–anchored protein expression on peripheral blood cells and marrow analysis are required for comprehensive disease classification. For optimum management, the contribution of both hemolysis and marrow failure to the complex anemia of PNH should be determined. Complement inhibition by eculizumab is a promising new approach to treating the hemolytic anemia. Stem cell transplantation is potentially curative, but the decision on use is best made on a case-by-case basis because of the heterogeneous natural history of the disease. PNH clone size and ethnic/geographic factors appear to influence thrombophilic propensity, but a consensus on prophylactic anticoagulation has not been reached. Involvement of unusual sites (hepatic, mesenteric, cerebral, dermal veins) is characteristic of the thrombophilia of PNH. Indefinite anticoagulation is recommended following a thromboembolic event and thrombolytic therapy should be considered for acute hepatic vein thrombosis (Budd-Chiari syndrome). Pregnancy in a patient with PNH is complicated and requires careful management including prophylactic anticoagulation. To obtain a broad overview of the natural history, approaches to management, and outcome, the International PNH Registry was recently established.
Definition and diagnostic criteria
Definition
PNH is a consequence of nonmalignant clonal expansion of one or several hematopoietic stem cells that have acquired a somatic mutation of PIGA. Progeny of affected stem cells are deficient in glycosyl phosphatidylinositol–anchored proteins (GPI-APs). Deficiency of the GPI-anchored complement regulatory proteins CD55 and CD59 accounts for the intravascular hemolysis that is the primary clinical manifestation of the disease. PNH frequently arises in association with disorders of bone marrow failure, particularly aplastic anemia. Thrombophilia is a major cause of morbidity and mortality in PNH.
Classification
A scheme that incorporates variations in the presenting features, clinical manifestations, and natural history among patients with PNH was developed (Table 1). Continued investigation may necessitate modification of the proposed 3 subcategories, but for now, they serve as a working classification that provides a common language for the field.
Classification of PNH
|
|
Classic PNH. Patients with classic PNH have clinical evidence of intravascular hemolysis (reticulocytosis, abnormally high concentration of serum lactate dehydrogenase [LDH] and indirect bilirubin, and abnormally low concentration of serum haptoglobin) but have no evidence of another defined bone marrow abnormality. A cellular marrow with erythroid hyperplasia and normal or near-normal morphology, but without nonrandom karyotypic abnormalities, is consistent with classic PNH.
PNH in the setting of another specified bone marrow disorder. The patients in this subcategory have clinical and laboratory evidence of hemolysis but also have concomitantly, or have had a history of, a defined underlying marrow abnormality. Bone marrow analysis and cytogenetics are used to determine if PNH arose in association with aplastic anemia, myelodysplastic syndrome (MDS), or other myelopathy (eg, myelofibrosis). Standard criteria are used for diagnosis of the bone marrow abnormality (eg, aplastic anemia, MDS, or myelofibrosis). Finding nonrandom karyotypic abnormalities that are associated with a specific bone marrow abnormality may contribute diagnostically (eg, abnormalities of chromosomes 5q, 7, and 20q are associated with MDS).
Subclinical PNH (PNH-sc). Patients with PNH-sc have no clinical or laboratory evidence of hemolysis. Small populations of GPI-AP–deficient hematopoietic cells (peripheral blood erythrocytes, granulocytes, or both) are detected by very sensitive flow cytometric analysis. PNH-sc is observed in association with bone marrow failure syndromes, particularly aplastic anemia and refractory anemia-MDS.
Minimal essential diagnostic criteria
Flow cytometry is recommended to identify the GPI-AP deficient peripheral blood cells that are diagnostic of PNH (see “Diagnosis of PNH”). Assessment of hemolytic parameters and bone marrow analysis are required to determine the subcategory into which each patient falls (“Classification,” Table 1, Table 2).
Minimal essential criteria required for diagnosis and categorization
|
|
FLAER (fluorescently labeled aerolysin). Aerolysin is a bacterial protein that binds selectively to GPI-APs. When used for flow cytometric analysis, leukocytes that express GPI-APs bind the fluorescently labeled reagent. PNH leukocytes do not bind FLAER because they do not express GPI-APs. Therefore, PNH leukocytes are identified in the flow cytometric histogram as a population of cells with absent or dim fluorescence. FLAER cannot be used for analysis of erythrocytes because, for unknown reasons, the reagent does not bind well to human red cells under the conditions required for the assay.
Recommended ancillary studies
Appropriate management requires determination of a number of factors that influence clinical manifestations and prognosis (Table 3).
Recommended supporting studies
|
|
Hemolysis depends on both the proportion and type of abnormal erythrocytes (see “Management of the anemia of PNH”).
Determining the contribution of PIGA mutant hematopoiesis may influence therapeutic decisions such as prophylactic anticoagulation.
The association between HLA type and subclinical PNH may have prognostic and therapeutic implications (see “Subclinical PNH”).
Management of PNH
Who should be screened for PNH?
Essentially all patients with classic PNH report gross hemoglobinuria at some point during the course of their illness, but this symptom may be absent in patients with PNH/aplastic anemia or PNH/refractory anemia-MDS because the clone size is often relatively small. In a series of 80 unclassified patients, only 26% reported hemoglobinuria at presentation (Table 4).1 As the hemolysis is a complement-mediated, intravascular process, all patients with clinically significant hemolysis have an abnormally high serum LDH. Therefore, in the absence of elevated LDH, screening for clinical forms of PNH (eg, classic PNH or PNH/aplastic anemia) is unwarranted. However, this diagnostic criterion is not applicable for PNH-sc, because, by definition, clinical evidence of hemolysis is absent in these patients. Screening for PNH in patients with aplastic anemia, even in the absence of clinical evidence of hemolysis, is recommended at diagnosis and at least yearly during follow-up (Table 5). Identifying PNH-sc appears clinically relevant, as recent studies2 suggest that patients with a small population of PNH cells, in combination with either aplastic anemia or refractory anemia-MDS, have a high probability of responding to immunosuppressive therapy (IST). Routine screening of patients with subcategories of MDS other than refractory anemia or with other clonal myelopathies (eg, myelofibrosis) who have no clinical or biochemical evidence of hemolysis is not recommended.
Presenting features in 80 patients with PNH
Signs and symptoms . | No. of patients (%) . |
|---|---|
| Symptoms of anemia | 28 (35) |
| Hemoglobinuria | 21 (26) |
| Hemorrhagic signs and symptoms | 14 (18) |
| Aplastic anemia | 10 (13) |
| Gastrointestinal symptoms | 8 (10) |
| Hemolytic anemia and jaundice | 7 (9) |
| Iron-deficiency anemia | 5 (6) |
| Thrombosis or embolism | 5 (6) |
| Infections | 4 (5) |
| Neurologic signs and symptoms | 3 (4) |
Signs and symptoms . | No. of patients (%) . |
|---|---|
| Symptoms of anemia | 28 (35) |
| Hemoglobinuria | 21 (26) |
| Hemorrhagic signs and symptoms | 14 (18) |
| Aplastic anemia | 10 (13) |
| Gastrointestinal symptoms | 8 (10) |
| Hemolytic anemia and jaundice | 7 (9) |
| Iron-deficiency anemia | 5 (6) |
| Thrombosis or embolism | 5 (6) |
| Infections | 4 (5) |
| Neurologic signs and symptoms | 3 (4) |
Modified from Dacie and Lewis.1
Who should be screened for PNH?
|
|
Patients with PNH and thrombosis usually have a relatively large PNH clone. Therefore, most PNH patients with thrombosis will have clinically apparent evidence of intravascular hemolysis. Patients with primarily or exclusively PNH type II cells, however, may represent an exception. In this case, patients could have a large PNH clone with only subtle clinical evidence of spontaneous hemolysis. Arterial thrombosis has been observed in patients with PNH but is uncommon compared with venous thrombosis.
Patients with aplastic anemia should be screened even in the absence of evidence of intravascular hemolysis to identify those with subclinical PNH (PNH-sc/aplastic anemia). Screening of patients with refractory anemia-MDS is also recommended even in the absence of clinical evidence of hemolysis. Routine screening of patients with other forms of MDS or with myeloproliferative disease that have no evidence of intravascular hemolysis is not recommended outside of a research setting.
A thromboembolic event as the presenting manifestation of PNH is uncommon (∼5%; Table 4). Routine screening for PNH of all patients with thrombosis is not recommended, but patients who present with thrombosis at unusual sites (Table 5) should be screened, especially if coexistent cytopenias, intravascular hemolysis, or both are noted.
Gastrointestinal complaints (episodic abdominal pain and dysphagia/odynophagia, see “Dysphagia, male impotence, abdominal pain”) may be the initial manifestation of the disease in approximately 10% of patients (Table 4). Due to chronic intravascular hemolysis, urinary iron loss is high in patients with PNH, and iron deficiency is common.
Diagnosis of PNH
Flow cytometry. Flow cytometric analysis3-5 using antibodies directed against GPI-AP is the most sensitive and informative assay available for diagnosis of PNH (Figures 1, 2, 3; Table 6). For initial studies, quantitation of at least 2 GPI-APs is recommended to exclude the possibility that the clinical process is a consequence of an inherited, isolated deficiency of a single GPI-AP.6,7
Recommendations for flow cytometric analysis in diagnosis and management of PNH
|
|
Best performed prior to transfusion or, if clinically feasible, during a period of transfusion abstinence. The results of the analysis for erythrocytes should include the percentage type I, type II, and type III cells.
Analysis of granulocytes is needed for the following 2 reasons: (1) analysis provides the best estimate of the size of the PNH clone(s); (2) analysis is unaffected by red cell transfusion.
Regardless of marrow cellularity.
For patients with established, stable disease, yearly analysis of peripheral blood GPI-AP expression is recommended, however, a change in clinical parameters (worsening or more frequent hemolysis or a thromboembolic event) warrants immediate re-evaluation (Table 6). Clinical improvement should also prompt an interim analysis as the mutant clone sometimes becomes significantly smaller or undetectable.8 A reduction in the percentage of GPI-AP–deficient granulocytes might influence the decision about prophylactic anticoagulation, as thrombophilic proclivity appears related to PNH clone size9-11 (see “Thrombosis”).
Flow cytometric analysis is more than binary (ie, positive or negative). In addition to identifying a population of GPI-AP–deficient cells, the analysis can both determine the percentage of cells that are abnormal and identify discrete populations with different degrees of deficiency (particularly on erythrocytes; Figure 1). Erythrocytes with complete deficiency of GPI-APs are called PNH III, those with subtotal deficiency (usually ∼10% of normal expression) are called PNH II, and those with normal expression are called PNH I (Figure 1). Knowing both the percentage and type of deficient red cells is helpful in managing the anemia of PNH (see “Management of the anemia of PNH”). Recent red cell transfusion is unlikely to obscure the diagnosis of PNH, but the flow cytometric histogram will be affected, as transfusion will increase the proportion of cells with normal expression of CD55 and CD59. Therefore, to obtain accurate information about the percentage of GPI-AP–deficient erythrocytes, analysis should be performed prior to transfusion or during a period of transfusion abstinence (at least 1 month, but longer if clinically safe).
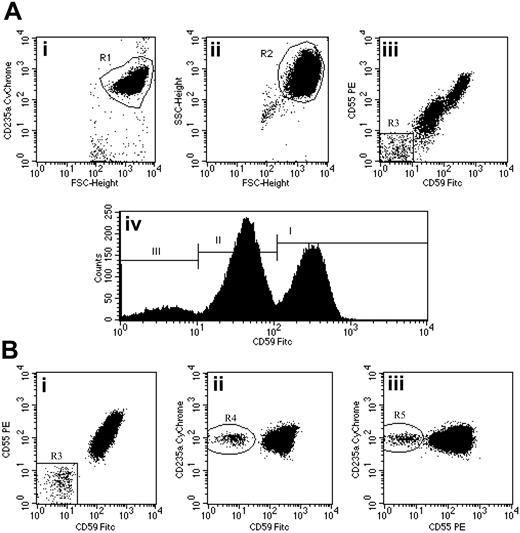
Flow cytometric analysis of red cells in PNH using a combination of anti-CD59 FITC, anti-CD55 PE, and anti-CD235a CyChrome. (Ai-ii) Flow cytometry plots show the gating strategy for identifying a pure population of red cells. An initial region (R1) is set on CD235a+FSCHigh events. A second region (R2) is then drawn around the logFSC/SSC characteristics of these cells. Only events that meet both these criteria are analyzed. (Aiii) CD59 and CD55 bivariate expression on these erythroid events. A small population of complete deficiency cells (type III) are detected (region R3 = 8.0%). (Aiv) Histogram analysis of CD59 expression also shows a major population of partially deficient cells (type II) comprising 53.9% of the total. Type I (normal expression) cells comprise 38.1% of the total. (Bi-iii) Detection of a very small red cell clone in a patient with PNH-sc/aplastic anemia. The red cell PNH clone comprises 0.4% of the total red cells (Bi). Confidence that this population is not an artifact is shown by simultaneous CD59 (Bii) and CD55 (Biii) deficiency and strong expression of CD235a. Illustration enhanced by A. Y. Chen.
Flow cytometric analysis of red cells in PNH using a combination of anti-CD59 FITC, anti-CD55 PE, and anti-CD235a CyChrome. (Ai-ii) Flow cytometry plots show the gating strategy for identifying a pure population of red cells. An initial region (R1) is set on CD235a+FSCHigh events. A second region (R2) is then drawn around the logFSC/SSC characteristics of these cells. Only events that meet both these criteria are analyzed. (Aiii) CD59 and CD55 bivariate expression on these erythroid events. A small population of complete deficiency cells (type III) are detected (region R3 = 8.0%). (Aiv) Histogram analysis of CD59 expression also shows a major population of partially deficient cells (type II) comprising 53.9% of the total. Type I (normal expression) cells comprise 38.1% of the total. (Bi-iii) Detection of a very small red cell clone in a patient with PNH-sc/aplastic anemia. The red cell PNH clone comprises 0.4% of the total red cells (Bi). Confidence that this population is not an artifact is shown by simultaneous CD59 (Bii) and CD55 (Biii) deficiency and strong expression of CD235a. Illustration enhanced by A. Y. Chen.
Analysis of expression of GPI-AP on granulocytes provides additional clinically relevant information. In contrast to GPI-AP–deficient red cells,12 the life span of PNH granulocytes is normal.13 Therefore, the proportion of abnormal granulocytes more accurately reflects the PNH clone size and is unaffected by red cell transfusion (Figure 2).
Subclinical PNH. Standard single-color flow cytometry is sufficiently sensitive to detect accurately 3% GPI-AP–deficient cells.5 By using 2-color analysis and careful gating, sensitivity can be increased by 3 orders of magnitude (Figure 3).2,14 Patients with small PNH clones (< 10% GPI-AP–deficient granulocytes) have little or no clinical evidence of hemolysis. Most of these patients have aplastic anemia or refractory anemia-MDS.2,14,15 As discussed in “Who should be screened for PNH?” finding small populations of GPI-AP–deficient cells in the setting of aplastic anemia or refractory anemia-MDS appears to have important prognostic and therapeutic implications.2,14,15 For this reason, high-sensitivity flow cytometric analysis is recommended for these patients both at diagnosis and yearly during and after treatment, even in the absence of clinical or biochemical evidence of hemolysis (Table 6).
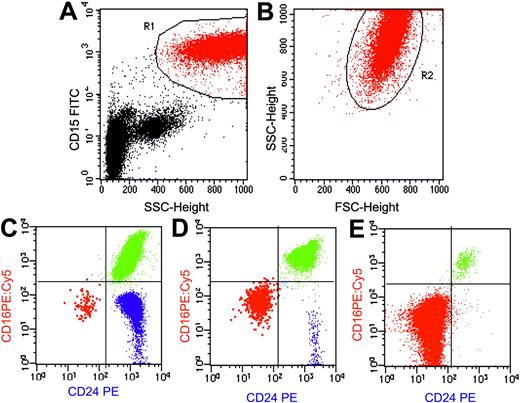
Flow cytometric analysis of granulocytes in PNH using a combination of anti-CD15 FITC, anti-CD24 PE, and anti-CD16 PE:Cy5. Flow cytometry plots (A-B) show the gating strategy for identifying a pure population of granulocytes based on light scatter characteristics and a transmembrane lineage marker (CD15). An initial region (R1) is set on CD15a+SSCHigh events (A). A second region (R2) is then drawn around the FSC/SSC characteristics of these cells to further refine the gating procedure (B). Only events that meet both these criteria are analyzed. (C-E) Using this combination, normal eosinophils (blue) can be clearly separated from PNH cells (red) and normal granulocytes (green). Plots are from 3 representative patients with PNH. (C) A small PNH granulocyte clone of 0.18% of total granulocytes in a patient with aplastic anemia (PNH-sc/aplastic anemia). (D) A larger PNH granulocyte clone of 8.7% in a patient with aplastic anemia (PNH/aplastic anemia). (E) Plot is from a patient with classic PNH and shows a large PNH granulocyte pool (98.8%). In this instance, the majority of hematopoiesis is derived from PNH stem cells. There is some residual normal granulocyte production (the 1% of cells that are CD16+CD24+) and this population serves as an internal positive control for antibody staining. Illustration enhanced by A. Y. Chen.
Flow cytometric analysis of granulocytes in PNH using a combination of anti-CD15 FITC, anti-CD24 PE, and anti-CD16 PE:Cy5. Flow cytometry plots (A-B) show the gating strategy for identifying a pure population of granulocytes based on light scatter characteristics and a transmembrane lineage marker (CD15). An initial region (R1) is set on CD15a+SSCHigh events (A). A second region (R2) is then drawn around the FSC/SSC characteristics of these cells to further refine the gating procedure (B). Only events that meet both these criteria are analyzed. (C-E) Using this combination, normal eosinophils (blue) can be clearly separated from PNH cells (red) and normal granulocytes (green). Plots are from 3 representative patients with PNH. (C) A small PNH granulocyte clone of 0.18% of total granulocytes in a patient with aplastic anemia (PNH-sc/aplastic anemia). (D) A larger PNH granulocyte clone of 8.7% in a patient with aplastic anemia (PNH/aplastic anemia). (E) Plot is from a patient with classic PNH and shows a large PNH granulocyte pool (98.8%). In this instance, the majority of hematopoiesis is derived from PNH stem cells. There is some residual normal granulocyte production (the 1% of cells that are CD16+CD24+) and this population serves as an internal positive control for antibody staining. Illustration enhanced by A. Y. Chen.
Not all flow cytometry laboratories have the capacity to perform high-sensitivity analysis. To diagnose and follow patients with PNH-sc, physicians are encouraged to identify laboratories that can consistently and reproducibly detect less than 1.0% GPI-AP–deficient erythrocytes and granulocytes.
Other approaches to diagnosis. The FLAER (fluorescently labeled aerolysin) assay takes advantage of binding of a bacterial protein, aerolysin, to GPI-AP.16-19 This assay is useful for analyzing leukocytes for expression of GPI-AP but cannot be used for analysis of erythrocytes (Table 6).
Although they have much biologic and historic importance,20 the acidified serum lysis test (Ham test) and the sucrose lysis test (sugar water test) have largely been abandoned as diagnostic assays because they are both less sensitive and less quantitative than flow cytometry. Testing for expression of other GPI-AP using functional or immunohistochemical assays (eg, erythrocyte acetylcholine esterase or neutrophil alkaline phosphatase) can be used as part of the screening process or as ancillary studies to support the diagnosis, but they should not be used in place of flow cytometry.
Bone marrow analysis. While deficiency of GPI-AP can be demonstrated on CD34+ bone marrow cells using 2-color flow cytometry,21 this type of analysis is unnecessary for standard diagnosis of PNH.
Morphologic analysis of the bone marrow aspirate and biopsy and cytogenetics are needed for proper classification, as PNH is often observed in association with marrow failure syndromes22-24 and sometimes with clonal myelopathies2,15,25-27 (Tables 1 and 2). Although nonrandom karyotypic abnormalities associated with PNH have not been identified,28,29 cytogenetic analysis is recommended for the following 2 reasons: (1) by analyzing a large number of samples using modern technology, disease-specific abnormalities may be identified; and (2) nonrandom karyotypic abnormalities associated with other underlying disease processes may help in categorizing individual patients (Table 2).
PIGAmutations. Deficiency of GPI-AP in patients with PNH is due to an acquired somatic mutation in PIGA.30 Therefore, documentation of the PIGA mutation(s) would confirm the diagnosis of PNH.31 Because of technical challenges, identification of PIGA mutations has been limited to the research setting; however, new technology may eventually make routine clinical determination of PIGA mutations feasible.32-34
Management of the anemia of PNH
The anemia of PNH is complex. Coombs-negative hemolytic anemia is the clinical hallmark of PNH, but because the disease usually arises in the setting of an underlying abnormality of the bone marrow, hemolysis may account for only part of a patient's anemia. Further, the erythrocytes of PNH are a mosaic of normal and abnormal cells, and the portion of GPI-AP–deficient red blood cells (RBCs) varies among patients (Figures 1, 2, 3, 4).35,36 For example, in hypothetical patient 1, only 15% of the circulating RBCs may be GPI-AP deficient, whereas in hypothetical patient 2, 75% GPI-AP–deficient erythrocytes may be observed.35 In the former, hemolysis would contribute modestly to an observed anemia, whereas in the latter, a significant hemolytic component would be expected (Figure 4).35 Another complicating factor is that deficiency of GPI-AP may be partial rather than complete (Figures 1 and 4), and partial expression of CD55 and CD59 is sufficient to protect PNH II cells from spontaneous complement-mediated lysis in vivo.35,37 Therefore, even if a patient has a high proportion of PNH II cells, only modest evidence of spontaneous hemolysis is usually observed (Figures 1 and 4). But brisk hemolysis may occur in patients with predominantly PNH II erythrocytes in a situation where complement activation is enhanced (eg, by infection, trauma, surgery, pregnancy, unusual stress).
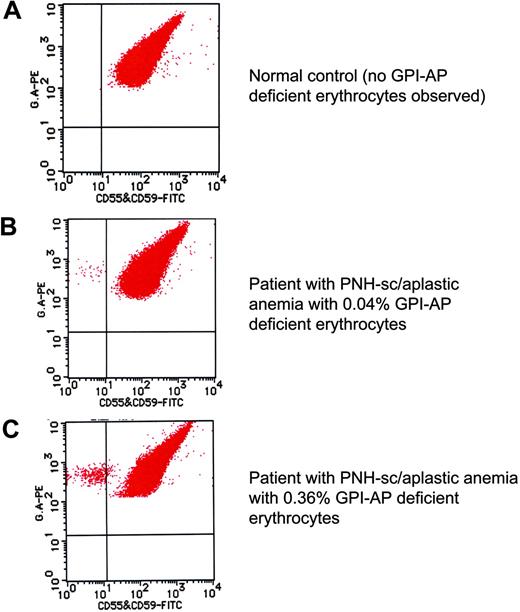
High-sensitivity flow cytometric analysis of erythrocytes. By careful gating and by using dual-color flow cytometry, GPI-deficient cells that comprise less than 1% of the total erythrocyte population can be reliably and reproducibly demonstrated (B-C).2 Using this technique, GPI-AP–deficient cells are not identified in the peripheral blood of the controls (volunteer donors; A). A combination of FITC-labeled anti-CD55 and anti-CD59 was used along with phycoerythrin (PE)–labeled anti–glycophorin A for the dual staining. These data were kindly provided by Dr Shinji Nakao and Dr Chiharu Sugimori, Kanazawa University, Japan, and are used with their permission. Illustration enhanced by A. Y. Chen.
High-sensitivity flow cytometric analysis of erythrocytes. By careful gating and by using dual-color flow cytometry, GPI-deficient cells that comprise less than 1% of the total erythrocyte population can be reliably and reproducibly demonstrated (B-C).2 Using this technique, GPI-AP–deficient cells are not identified in the peripheral blood of the controls (volunteer donors; A). A combination of FITC-labeled anti-CD55 and anti-CD59 was used along with phycoerythrin (PE)–labeled anti–glycophorin A for the dual staining. These data were kindly provided by Dr Shinji Nakao and Dr Chiharu Sugimori, Kanazawa University, Japan, and are used with their permission. Illustration enhanced by A. Y. Chen.
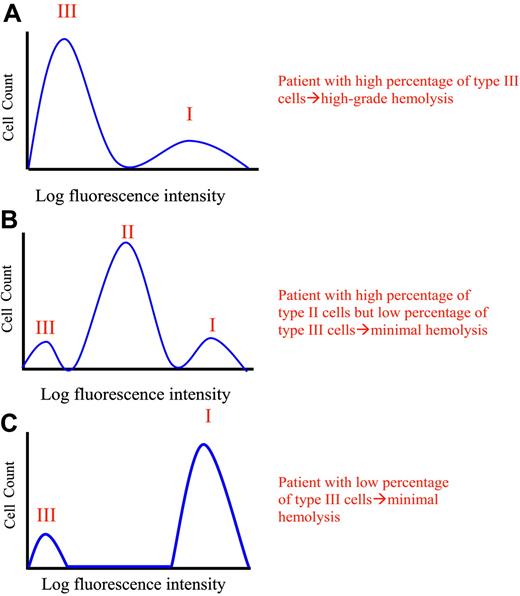
Phenotypic mosaicism in PNH. Hypothetical histograms of erythrocytes from patients with PNH stained with anti-CD59 are illustrated. The proportion and type of abnormal erythrocytes varies greatly among patients with PNH and these characteristics are important determinants of clinical manifestations. In general, patients with a high percentage of type III erythrocytes have clinically apparent hemolysis (A). If the erythrocytes are partially deficient in GPI-AP, hemolysis may be modest even if the percentage of the affected cells is high (B). A patient may have a diagnosis of PNH, but if the proportion of type III cells is low, only biochemical evidence of hemolysis may be observed (C). Illustration enhanced by A. Y. Chen.
Phenotypic mosaicism in PNH. Hypothetical histograms of erythrocytes from patients with PNH stained with anti-CD59 are illustrated. The proportion and type of abnormal erythrocytes varies greatly among patients with PNH and these characteristics are important determinants of clinical manifestations. In general, patients with a high percentage of type III erythrocytes have clinically apparent hemolysis (A). If the erythrocytes are partially deficient in GPI-AP, hemolysis may be modest even if the percentage of the affected cells is high (B). A patient may have a diagnosis of PNH, but if the proportion of type III cells is low, only biochemical evidence of hemolysis may be observed (C). Illustration enhanced by A. Y. Chen.
What is the contribution of hemolysis to the anemia? Prior to initiating therapy, an effort should be made to determine how much of the anemia is a consequence of hemolysis and how much is due to impaired erythropoiesis (Table 7). Review of the complete blood count is informative because evidence of thrombocytopenia, leukopenia, or both suggests stem cell dysfunction. The capacity of the marrow to respond to the anemia can be inferred from the reticulocyte count. An element of marrow failure is likely a contributing factor in a patient with PNH who has anemia with an inappropriately low reticulocyte count.
Information useful for managing anemia in patients with PNH
|
|
Indicative of chronic hemolysis but provides no quantitative information.
Biochemical parameters of hemolysis should be assessed (Table 7). Normal or minimal elevation of LDH argues against hemolysis as a major contributing factor to the anemia. The presence of urine hemosiderin suggests chronic intravascular hemolysis but provides no quantitative information, while gross hemoglobinuria indicates clinically significant intravascular hemolysis.
The concentration of erythropoietin should be determined as renal dysfunction may complicate PNH.38 Iron deficiency due to hemosiderinuria/hemoglobinuria is common.39,40
Is treatment of hemolysis necessary? If the assessment in “What is the contribution of hemolysis to the anemia?” suggests that hemolysis contributes significantly to the anemia, treatment is indicated for the following reasons: (1) patients with chronic hemolysis complain of lethargy, malaise, myalgia and loss of sense of well being that diminishes significantly quality of life; (2) there is evidence that chronic hemolysis has an untoward effect on renal function38 ; (3) the dysphagia and male impotence of PNH appear related to hemolysis41 ; (4) a correlation between thrombosis and hemolysis may exist.
Treatment of the hemolysis of PNH. If a modality with a favorable therapeutic index were available, there would be little debate about whether treatment of hemolysis is appropriate. Although such an agent appears to be forthcoming in the form of eculizumab,42 current options are limited, and the outcome of treatment is often unsatisfactory because of inconsistent response rates and unfavorable toxicity profiles.
Corticosteroids. Corticosteroids as treatment, for both chronic hemolysis and acute hemolytic exacerbations,39,43-45 is a subject of debate, and some members of the International PNH Interest Group do not advocate the use of steroids in PNH in any circumstances. Part of the debate is fueled by the empiric nature of the therapy, as there are no experimental data that provide a plausible explanation for why steroids should ameliorate the hemolysis of PNH. Nonetheless, some patients appear to respond rapidly and dramatically to glucocorticoids (given in the dosage range of 0.25-1.0 mg/kg per day of prednisone). The rapid response (often within 24 hours of initiating therapy) suggests that complement inhibition accounts for antihemolytic activity of glucocorticoid therapy. Such an effect could be direct (the result of inhibition of the activity of some component of the alternative pathway of complement) or indirect (the result of dampening a process, such as inflammation, that stimulates activation of complement).
The main value of corticosteroids may be in attenuating acute hemolytic exacerbations. Under these circumstances, brief pulses of prednisone may reduce the severity and duration of the crisis while avoiding the untoward consequences associated with long-term use. The value of steroids in treating chronic hemolysis is limited by toxicity, and the harm that can accrue from long-term use cannot be overemphasized. An every-other-day schedule may attenuate some of the adverse effects of chronic steroid use,39 but patients may note worsening of symptoms on the off day. Careful follow-up is essential and both bacterial prophylaxis and prophylaxis against steroid-induced osteopenia are recommended. Awareness of the potentially debilitating effects of steroid myopathy and sensitivity to the disfiguring consequences of iatrogenic Cushing syndrome are essential for proper management.
Androgens. Androgen therapy, either alone or in combination with steroids, has been used successfully to treat the anemia of PNH.39,40 As with glucocorticoids, the mechanism by which androgenic steroids ameliorate the anemia of PNH is not fully understood, although the rapid onset of action is consistent with complement inhibition.40
Potential complications of androgen therapy include liver toxicity (cholestatic jaundice, peliosis hepatis), prostatic hypertrophy, and virilizing effects. The toxicity profile is more favorable for attenuated synthetic androgens such as Danazol, making long-term use of this drug a reasonable management option. A starting dose of 400 mg twice a day is recommended, but a lower dose (200-400 mg/d) may be adequate to control chronic hemolysis. Monitoring of liver function studies during therapy is mandatory. Whether androgen therapy increases the risk of developing Budd-Chiari syndrome in patients with PNH is unknown.
Iron replacement. Patients with PNH frequently become iron deficient as a result of both hemoglobinuria and hemosiderinuria.39,40 Clinically important iron loss from hemosiderinuria can occur even in the absence of gross hemoglobinuria. Replacement is often associated with exacerbation of hemolysis, regardless of the route of administration.39,40 Compared with parenteral replacement, oral administration of iron may be accompanied by less severe hemolytic exacerbations, but urinary iron loss may be so great that repletion cannot be achieved through this mechanism.39 Parenteral repletion is generally safe. Concern for inducing a hemolytic exacerbation should not deter iron repletion, as iron deficiency not only limits erythropoiesis but also exacerbates the hemolysis of PNH.40 If a hemolytic exacerbation occurs in the setting of iron repletion, the episode can be controlled by treatment with corticosteroids or androgens or by suppression of erythropoiesis by transfusion.
Transfusion. In addition to increasing the hemoglobin concentration, transfusion may ameliorate hemolysis by suppressing erythropoiesis. Concerns about inducing a hemolytic exacerbation as a consequence of infusion of small amounts of donor plasma that may contaminate red cell preparations appear unwarranted.46 However, hemofiltration is recommended to prevent transfusion reaction arising from the interaction between donor leukocytes and recipient antibodies. Iatrogenic hemochromatosis from chronic transfusion may be delayed in patients with PNH due to iron loss from hemoglobinuria/hemosiderinuria.39 In fact, iron overload in patients with classic PNH is rare. But iron overload remains a concern in patients who require chronic transfusion when the anemia is primarily a consequence of marrow failure rather than hemolysis.
Splenectomy. The role of splenectomy in the management of patients with PNH is unclear. Reports of amelioration of hemolysis and improvement in cytopenias following splenectomy are anecdotal. Concerns about lack of proven efficacy and the potential for postoperative complications, particularly thrombosis, have led some members of the International PNH Interest Group to argue that splenectomy has no role in the management of PNH.
Folate. Supplemental folate (5 mg/d) is recommended to compensate for increased utilization associated with heightened erythropoiesis that is a consequence of hemolysis.
Complement inhibitors. As the hemolysis of PNH is a consequence of complement-mediated cytolysis, inhibition of complement is a logical approach to therapy.47 Recently, the results of a phase 2 study using a humanized monoclonal antibody against complement C5 (eculizumab) to treat patients with PNH were reported.42 This reagent was effective in controlling the symptoms and signs of the hemolysis, and a marked improvement in quality-of-life parameters was documented in most patients. Significant adverse events were not observed. A phase 3 transfusion avoidance trial in which the efficacy of eculizumab is compared with placebo began enrolling patients in the late fall of 2004.
Management of nonhemolytic anemia. Treatment of anemia that is primarily due to bone marrow failure should be aimed at the underlying disease (eg, aplastic anemia, MDS). If absolute or relative erythropoietin deficiency is felt to contribute to the anemia, replacement with the recombinant protein is warranted, but patients should be closely monitored as erythropoietin supplementation could exacerbate hemolysis by increasing production of GPI-AP–deficient erythrocytes.
Androgens may also be beneficial in patients with PNH who have a hypoproliferative component to their anemia.39
Stem cell transplantation
The issues that confound recommendations for transplantation for PNH center both on the heterogeneous presentation and natural history and on its close association with marrow failure syndromes. To date, the 220 patients with PNH from the registry of the French Society of Hematology represents the largest natural history study group.48 Although the French cohort had a median survival of 12 years, the study identified risk factors associated with a worse outcome. While some of the risk factors are of little help for transplant decision-making (eg, age older than 55 years; relative risk of death [RR] = 4.0), others may provide guidance. There were 4 factors associated with worse survival: (1) occurrence of thrombosis (regardless of location; RR = 10.2); (2) progression to pancytopenia (RR = 5.5); (3) transformation to MDS or acute leukemia (RR = 19.1); and (4) thrombocytopenia at diagnosis (RR = 2.2).
Data from 67 patients from single centers experience and from 2 registries studies were reviewed.49-72 The details of the literature review can be found in Document S1 (see the Supplemental Document link at the top of the online article, at the Blood website). The conclusions that were derived from the reviewed literature on transplantation for PNH are shown in Table 8. We are also left with several unresolved questions related to PNH and transplantation. Given the unpredictable course of the disease, including the possibility of spontaneous remission, what is the optimal time for stem cell transplantation (SCT)? Should a conventional or a nonmyeloablative conditioning regimen be proposed given that the latter may be associated with less early transplant-related mortality (although graft-versus-host disease [GVHD] is still a problem)? What is the place of transplantation using stem cells from an unrelated donor in the era of HLA molecular matching that appears to produce outcomes similar to those observed for transplantation using stem cells from HLA-identical sibling donors? How will new treatment options (eg, eculizumab) influence recommendations for SCT?
Bone marrow/stem cell transplantation for patients with PNH
Indications for consideration of transplantation |
| PNH-specific transplant-related issues |
|
Indications for consideration of transplantation |
| PNH-specific transplant-related issues |
|
If the dominant clinical abnormalities are a consequence of bone marrow failure (eg, hypoproliferative anemia, neutropenia with recurrent infections, thrombocytopenia with major bleeding complications) the decision to recommend transplantation should be based on guidelines for management of the specific marrow failure syndrome.
Factors including age, comorbid conditions, availability of an HLA-matched sibling donor, and time from initial diagnosis should be considered before recommending transplantation for major complications of PNH.
The availability of new treatment options (eg, eculizumab) may influence the decision to recommend transplantation.
Radiation- or busulfan-based.
By establishing a worldwide PNH registry (see “PNH registry and future directions”) that will allow comparison of the outcome of patients who have not undergone transplantation to those who undergo SCT, these questions can begin to be answered.
Thrombosis
Overview of thromboembolic disease in PNH. Thrombophilia is the leading cause of mortality in PNH.8,48 But, in contrast to our thorough understanding of the basis of the hemolysis, much less is known about the mechanism that underlies the thrombophilia of PNH (Table 9).73
Thrombosis and PNH
|
|
The size of the PNH clone is determined by flow cytometric analysis of expression of GPI-APs on peripheral blood granulocytes.
Standard-intensity warfarin therapy (INR 2.0-3.0) is recommended for chronic therapy.
Long-term anticoagulation should be reassessed in any patient who undergoes a spontaneous remission or in whom the PNH clone size falls to below 50%.
Recent clinical studies support the hypothesis that the probability of a thromboembolic event is directly related to the size of the PNH clone.9-11 In the study by Hall and colleagues,10 the 10-year risk of thrombosis in patients with more than 50% GPI-AP–deficient granulocytes was 44% compared with 5.8% for patients with less than 50%. Using a logistic regression model, Moyo et al9 calculated that the odds ratio for thrombosis was 1.64 for each 10% increase in the percentage of GPI-AP–deficient granulocytes. According to that study, a patient with more than 70% GPI-AP–deficient granulocytes had an 11.8-fold greater risk of thrombosis than a patient with a PNH clone of 20%.
Prophylaxis. Prophylaxis against thromboembolic events in patients with PNH is an issue of debate among members of the International PNH Interest Group. Current estimates of the risk are based on retrospective analysis.9-11,73 The relatively high risk of a thromboembolic complication led Hall and colleagues to advocate prophylaxis with warfarin for patients with PNH with more than 50% GPI-AP–deficient granulocytes who have no contraindications to anticoagulation.10 Those investigators reported no thromboembolic events in 39 patients who received warfarin prophylaxis compared with a 36.5% 10-year risk of thrombosis for 56 patients who did not. In that study, there were 2 serious hemorrhages in approximately 100 patient years of warfarin therapy.
Even when differences in clone size are taken into account,11 the prevalence of thromboembolism (∼2%) among patients from Japan, China, and Mexico11,74-76 appears lower than in patients from the United States and Europe, making anticoagulant prophylaxis for patients with PNH from these ethnic/geographic backgrounds difficult to justify.
Due to the lack of randomized, prospective studies, evidence-based guidelines for antithrombotic prophylaxis cannot be formulated satisfactorily. Available data (and its limitations) should be discussed with patients, and factors such as age, activity level, compliance, and comorbid conditions (in addition to the size of the PNH clone) should enter into decisionmaking about anticoagulant prophylaxis. The role of reagents that inhibit platelet function in prophylaxis against the thrombophilia of PNH is undefined. Newer pharmacologic agents (eg, oral thrombin inhibitors) with a more favorable therapeutic index than warfarin may liberalize recommendations for prophylactic anticoagulation in patients with PNH.
Management of thromboembolic disease. Although arterial thrombosis may be observed, thromboembolic events in patients with PNH usually involve the venous system. Acute thrombotic events require anticoagulation with heparin. Thrombolytic therapy77,78 or radiologic intervention79 should be strongly considered in patients with acute onset of Budd-Chiari syndrome. Thrombocytopenia often complicates PNH, and this issue must be addressed when formulating an anticoagulation management plan. Thrombocytopenia is a relative but not an absolute contraindication to anticoagulation, and transfusions should be given to maintain the platelet count in a safe range rather than withholding therapy.80
Patients with PNH who experience a thromboembolic event should be anticoagulated indefinitely (Table 9). There are no satisfactory guidelines for management of patients who fail standard-intensity warfarin therapy. High-intensity warfarin therapy (International Normalized Ratio [INR], 3.0-4.0) or subcutaneous low-molecular-weight heparin are options in this setting. In the absence of proven efficacy, the risk of hemorrhage makes the addition of antiplatelet agents to therapeutic anticoagulation difficult to support. Recurrent, life-threatening thrombosis merits consideration for bone marrow transplantation (see “Stem cell transplantation”). The role of complement inhibitors such as eculizumab in the management of the thrombophilia of PNH is currently undefined.
Pregnancy
Women with PNH can have serious morbidity and increased mortality during pregnancy.81 A retrospective review showed that, in 25% of the cases, the diagnosis of PNH was first made during pregnancy.80 In that study, a high mortality rate was reported (5 deaths in 24 women), with an all-cause mortality of 20.8% (confidence interval [CI] 7.3-39.0). Three deaths were attributed to venous thromboembolism and 2 to infections.
In women with PNH, anemia from hemolysis, underlying bone marrow failure, or both frequently worsens during pregnancy.80 Because of concerns about fetal/maternal risks from exposure to potentially toxic therapy, transfusion is the mainstay of management (Table 10). There is no experience with complement inhibitors such as eculizumab42 in this setting. Moderate to severe thrombocytopenia may complicate the pregnancy, and clinically significant bleeding in this setting necessitates platelet transfusion.
PNH and pregnancy
|
|
Low-molecular-weight heparin may be advantageous because it is associated with a lower incidence of heparin-induced thrombocytopenia compared with unfractionated heparin. Regardless of the form of heparin used, frequent (at least every other week) monitoring of the platelet count is needed.
Warfarin can be used for anticoagulation in the postpartum period.
The incidence of clinically apparent venous thromboembolism during pregnancy in women with PNH is about 10%,80 and these events are associated with a high risk of mortality.81 Similar to nonpregnant patients with PNH, cerebral and hepatic veins are commonly involved sites of thrombosis. Patients who develop thrombosis should be therapeutically anticoagulated, and thrombolytic therapy should be considered for those with Budd-Chiari syndrome. Concurrent thrombocytopenia may necessitate platelet transfusion in patients who require anticoagulation.
The role of prophylactic anticoagulation for pregnant women with PNH has not been studied systematically; however, because of the significant morbidity and mortality associated with thromboembolism in this setting, prophylaxis is recommended. Coumadin is contraindicated because of teratogenic potential in the first trimester and hemorrhagic risks later in gestation. Anticoagulation with heparin should begin immediately once the combination of pregnancy and PNH is documented. Low-molecular-weight heparin has a hypothetical advantage over unfractionated heparin because of a lower incidence of drug-induced thrombocytopenia. Careful monitoring of the platelet count is required because thrombocytopenia may worsen during the period of anticoagulation (Table 10). Anticoagulation can be discontinued briefly around the time of delivery. However, it should be restarted as soon as is feasible and continued for at least 6 weeks postpartum, as thrombosis during the puerperium is a major concern.81
Most deliveries can be accomplished vaginally, although premature delivery may be necessary. Ray et al80 identified 3 infant deaths (2 still births and 1 neonatal) among 34 births for a perinatal mortality rate of 8.8% (CI 1.9-23.7). All surviving infants had normal growth and development after delivery.
In summary, there are both maternal and fetal risks when PNH complicates pregnancy. Untoward events can be expected in at least half of the mothers and in some of the infants. Counseling female patients with PNH about pregnancy should take into account age, overall health, extent of hemolysis, degree of bone marrow failure (especially thrombocytopenia), previous thrombosis, and comorbid conditions. Despite the many concerns, successful outcomes appear to be the rule rather than the exception.11,80 But management is complicated and should involve the combined efforts of an experienced hematologist and an obstetrician specializing in high-risk pregnancy.81
Pediatric PNH
PNH can occur in the young (about 10% of patients are younger than 21)8,11,48 but is often misdiagnosed and mismanaged.82,83 A retrospective analysis of 26 cases82 underscored the many similarities between childhood and adult PNH. Signs and symptoms of hemolysis, bone marrow failure, and thrombosis dominate the clinical picture, although hemoglobinuria may be less common in young patients. A generally good response to immunosuppressive therapy (6 of 9 patients) was observed. However, based on the lack of spontaneous remissions and poor long-term survival (80% at 5 years, 60% at 10 years, and only 28% at 20 years), sibling-matched stem cell transplantation is the recommended treatment of childhood PNH. A recent Dutch study confirmed the common presentation of bone marrow failure in 11 children with PNH,83 and reported that 5 patients eventually underwent bone marrow transplantation (BMT; 3 matched unrelated donors and 2 matched family donors), of whom 4 are alive. Mortality appears high in young patients with PNH treated with transplantation using unrelated donors although surviving cases have been reported.72,83
Dysphagia, male impotence, abdominal pain
Many patients with PNH are troubled by dysphagia and odynophagia, especially during hemolytic exacerbations.41,84 These symptoms appear to be a consequence of esophageal spasm. The cause of the spasm is speculative, but may be due to acquired deficiency of nitric oxide (NO), a bioactive gas that mediates smooth muscle relaxation.85 Males with PNH may experience episodes of impotence, particularly during hemolytic exacerbations.41 The cause of the impotence may also be a consequence of decreased bioavailability of NO. Sildenafil citrate has shown efficacy in the treatment of hypercontractile motility disorders of the esophagus, including idiopathic achalasia, where the mechanism of disease appears to be impaired NO production similar to that reported for erectile dysfunction.86-88 Therefore, sildenafil citrate and pharmacologically related compounds are candidate therapies for both the dysphagia/odynophagia and male impotence of PNH. Agents such as oral or dermal nitroglycerine that supply NO pharmacologically have also shown efficacy.
Some patients with PNH are debilitated by recurrent episodes of colicky abdominal pain. The etiology of the abdominal pain is largely speculative, but thrombosis of mesenteric vessels appears to play a role in some cases.39 Vascular spasm may also contribute to this process. Vigorous hydration and pain control are the mainstays of management, but mesenteric vein thrombosis can result in intestinal infarction necessitating surgical intervention. Still to be determined are the roles of anticoagulation, complement inhibition, and NO supplementation in the management of abdominal pain of PNH.
Geographic and ethnic differences
The natural history of PNH appears different for Americans and Europeans compared with Asian/Pacific Islanders and Hispanics.8,11,48,74-76,82,89,90 In general, the manifestations of bone marrow failure are more common in Asians/Pacific Islanders and Hispanics. In contrast, thrombosis and infection appear more common in American and European patients. The basis of these phenotypic differences is unknown, but the relationship of ethnicity and geography to the natural history of PNH should be considered when formulating a management plan.
PNH registry and future directions
The International PNH Interest Group supported the development of a worldwide patient registry in order to generate more detailed epidemiologic data and to gain greater insight into both the natural history of the disease and the outcomes of therapy. The registry also has the potential to provide the framework upon which controlled, randomized clinical studies can be organized. Enrollment began in the fall of 2004. Physicians who follow patients with PNH are encouraged to enter patients into the registry. Information about registry participation can be found on the World Wide Web (http://www.pnhregistry.com).
The International PNH Interest Group encourages clinical and basic research. Some potential topics for investigation are shown in Table 11.
Future directions for clinical and laboratory studies in PNH
|
|
Appendix
The International PNH Interest Group is composed of approximately 60 physicians and investigators who have a special interest in the disease. This paper represents the efforts of the group to develop guidelines for the diagnosis and management of PNH. The members of the International PNH Interest Group are as follows:
Fiorella Alfinito, Division of Hematology, Federico II University, Naples, Italy; Leslie Andritsos, Washington University School of Medicine, St Louis; David J. Araten, Memorial Sloan Kettering Cancer Center; Han Bing, Peking Union Medical College; Morey Blinder, Departments of Internal Medicine and Pathology, Washington University School of Medicine, St Louis; Michael Bombara, Alexion Pharmaceuticals Inc; Christopher Bredeson, International Bone Marrow Transplant Registry; Robert A. Brodsky, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Division of Hematologic Malignancies; Tatsuya Chuhjo, Protected Environment Unit, Kanazawa University Hospital; Matthew Cullen, Haematological Malignancy Diagnostic Service, Leeds General Infirmary; Carlos M. de Castro, Duke University Medical Center; Lisa DeBoer, Alexion Pharmaceuticals Inc; Steven M. Devine, Washington University School of Medicine, St Louis; Modupe Elebute, Hematology and Transfusion Medicine, St George's Health Care NHS Trust; Xingmin Feng, Cellular Transplantation Biology, Kanazawa University Graduate School of Medical Science; Paul Finnegan, Alexion Pharmaceuticals Inc; Norbert Gattermann, Heinrich-Heine University Department of Hematology/Oncology and Clinical Immunology; Ulrich Germing, Heinrich-Heine University Department of Hematology/Oncology and Clinical Immunology; Susan Harris,Alexion Pharmaceuticals Inc; Robert Hayashi, Department of Pediatrics, Division of Hematology-Oncology, Washington University School of Medicine, St Louis; Anita Hill, Academic Unit of Haematology, Leeds General Infirmary; Thad Howard, St Jude Children's Research Hospital; Eric Hsi, Section of Hematopathology, Director of Flow Cytometry, Cleveland Clinic Foundation; Norimitsu Inoue, Osaka Medical Center for Cancer and Cardiovascular Diseases; Yuzuru Kanakura, Osaka University Graduate School of Medicine; Akihisa Kanamaru, Kinki University School of Medicine; Tatsuya Kawaguchi, Departments of Hematology & Infectious Diseases, Kumamoto University School of Medicine; Peter Keller, Division of Hematology, Inselspital Bern, University of Berne; Masahiro Kizaki, Division of Hematology, Keio University School of Medicine; Seiji Kojima, Department of Pediatrics, Nagoya University Graduate School of Medicine; Elisabeth Korthof, Leiden University Medical Center; Loree Larratt, University of Alberta Hospitals; Jaroslaw P. Maciejewski, Taussig Cancer Center, The Cleveland Clinic Foundation; Philip J. Mason, Department of Internal Medicine and Department of Genetics, Washington University School of Medicine, St Louis; Gabrielle Meyers, University of Utah School of Medicine; Christopher Mojcik, Alexion Pharmaceuticals Inc; Shoichi Nagakura, Kumamoto National Hospital; Hideki Nakakuma, Wakayama Medical University; Shinji Nakao, Cellular Transplantation Biology, Kanazawa University Graduate School of Medical Science; Rosario Notaro, National Institute for Cancer Research (IST); Keiya Ozawa, Professor and Chairman, Division of Hematology, Department of Medicine, Division of Cell Transplantation and Transfusion, Division of Genetic Therapeutics, Center for Molecular Medicine, Jichi Medical School; Andrew Rawstron, Haematological Malignancy Diagnostic Service, Leeds General Infirmary; Stephen Richards, Haematological Malignancy Diagnostic Service, Leeds General Infirmary; Antonio M. Risitano, Division of Hematology, Federico II University, Naples; Wendell F. Rosse, Duke University; Russell P. Rother, Alexion Pharmaceuticals Inc; Bruno Rotoli, Federico II University, Naples; Hubert Schrezenmeier, Institute for Clinical Transfusion Medicine and Immunogenetics; Joerg Schubert, Internal Medicine I, Saarland University Medical School; Tsutomu Shichishima, First Department of Internal Medicine, Fukushima Medical University; Elaine M. Sloand, National Heart, Lung, and Blood Institute, National Institutes of Health; Chiharu Sugimori, Cellular Transplantation Biology, Kanazawa University Graduate School of Medical Science; Akiyoshi Takami, Cellular Transplantation Biology, Kanazawa University Graduate School of Medical Science; David B. Wilson, Department of Pediatrics, Division of Hematology-Oncology, Washington University School of Medicine, St Louis; Paul Woodard, Hematology/Oncology, Division of Stem Cell Transplantation, St Jude Children's Research Hospital.
Prepublished online as Blood First Edition Paper, July 28, 2005; DOI 10.1182/blood-2005-04-1717.
Supported in part by the Health and Labor Sciences Research Grants for Research on Measures for Intractable Diseases, Ministry of Health, Labor, and Welfare, Japan (M.O. and T.K.); by the Japan Intractable Diseases Research Foundation and the Japan Health Sciences Foundation (J.N.); by National Institutes of Health grant RO1-CA89 091 and by the Bursary Award from the Aplastic Anemia (AA) & Myelodysplastic Syndrome (MDS) International Foundation (M.B.); and by an unrestricted educational grant from Hémoglobinurie Paroxystique Nocturne (HPN) France Association (G.S.).
A complete list of the members of the International PNH Interest Group appears in “Appendix.”
The online version of this article contains a data supplement.
The expert advice and support of members of the International PNH Interest Group are gratefully acknowledged. We also thank Dr David E. Jenkins Jr, Hummelstown, PA, for informative discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal