The aim of the present randomized trial was to compare high-dose therapy (HDT) with continued conventional chemotherapy in patients with multiple myeloma (MM) who responded to the initial treatment. From May 1994 to October 1999, 216 patients (122 men/94 women; stage II or III; Eastern Cooperative Oncology Group [ECOG] score less than 3) entered the study. Initial chemotherapy consisted of 4 cycles of alternating vincristine, BCNU, melphalan, cyclophosphamide, prednisone/vincristine, BCNU, Adriamycin, dexamethasone (VBMCP/VBAD). Responding patients were randomly assigned to receive 8 additional cycles of VBMCP/VBAD, intensification with melphalan 200 mg/m2, or melphalan 140 mg/m2 plus 12 Gy fractionated total body irradiation (TBI). One-hundred sixty-four patients were randomly assigned, 83 to continued chemotherapy and 81 to HDT. The complete remission (CR) rate was significantly higher with HDT (30% vs 11%; P = .002). However, progression-free survival (PFS) was not significantly different between HDT and conventional therapy (median, 42 vs 33 months; P = not significant [NS]), and overall survival (OS) was similar in both groups (median, 61 vs 66 months). Finally, survival after relapse was identical in the 2 arms (15.9 vs 16.4 months). In conclusion, these results show that HDT intensification, when given to myeloma patients who have responded to the initial chemotherapy, significantly increases the CR rate but has no significant impact on PFS or OS.
Introduction
Treatment of patients with multiple myeloma (MM) is disappointing. In this regard, the Southwest Oncology Group (SWOG) experience on standard dose therapy in 7 consecutive, large phase 3 studies has shown median survival times of less than 3 years, irrespective of the treatment given.1 In addition, the Programa para el Estudio de la Terapéutica en Hemopatía Maligna (PETHEMA) group found that increased doses of conventional chemotherapy did not result in significant prolongation of survival.2 Finally, meta-analysis of 6633 patients from 27 randomized trials failed to show any superiority of combination chemotherapy over melphalan/prednisone in terms of duration of response and survival.3 Furthermore, the Nordic Myeloma Study Group reported no survival improvement in conventionally treated younger patients with myeloma in the past 2 decades.4 These limitations led to the current tendency to offer high-dose therapy (HDT)/stem cell support to MM patients as part of the frontline treatment.5 Two randomized trials conducted by the Intergroup Français du Myeloma (IFM) and the Medical Research Council (MRC) showed that HDT significantly increased complete remission (CR), event-free survival (EFS), and overall survival (OS) compared with conventional chemotherapy.5,6 However, 2 other randomized trials, reported in abstract form, failed to show any superiority of autologous transplantation.7,8 The objective of the present study was to investigate, in a prospective randomized trial, the efficacy of HDT intensification therapy compared with continuation with conventional chemotherapy in patients with MM who had responded to the initial chemotherapy. It should be noted that the design of this study is slightly different from that of the IFM and MRC trials because patients with primary refractory disease were specifically excluded. The rationale for excluding this group of patients was that early disease progression during initial chemotherapy results in either early death or poor performance status, precluding HDT in many patients, and that the benefit of HDT in nonresponding patients with nonprogressive disease is doubtful because their prognoses are relatively good with conventional chemotherapy.9
Patients, materials, and methods
Patients and diagnostic criteria
Patients with newly diagnosed and untreated symptomatic stage II or III MM who were younger than 65 years and had a performance status from 0 to 2 were eligible to enter the study. From 1997 the upper age limit was extended to 70 years. Two hundred sixteen patients from 29 Spanish institutions were enrolled in the study between May 1, 1994, and October 31, 1999. Diagnosis of MM was made according to the criteria of the Chronic Leukemia-Myeloma Task Force.10 The study was approved by the National Health Service and by all the local institutional ethics committees. All patients gave written informed consent.
Study design
All patients were given alternating courses of vincristine, BCNU, melphalan, cyclophosphamide, prednisone/vincristine, BCNU, Adriamycin, dexamethasone (VBMCP/VBAD) as initial chemotherapy. The VBMCP regimen consisted of BCNU 0.5 mg/kg administered intravenously on day 1, vincristine 0.03 mg/kg (upper limit, 2 mg) intravenously on day 1, melphalan 0.25 mg/kg by mouth on days 1 to 4, and oral prednisone 1 mg/kg on days 1 to 4, 0.5 mg/kg on days 5 to 8, and 0.25 mg/kg on days 9 to 12. The VBAD regimen consisted of vincristine 1 mg intravenously on day 1, BCNU 30 mg/m2 intravenously on day 1, doxorubicin 40 mg/m2 intravenously on day 1, and dexamethasone 40 mg by mouth on days 1 to 4, 9 to 12, and 17 to 20. The VBMCP/VBAD courses were administered at 5-week intervals. Responding patients were randomly assigned to receive 8 additional courses of VBMCP/VBAD chemotherapy rather than HDT intensification followed by peripheral blood progenitor cell support. Patients who were in response after the completion of the 12 courses of chemotherapy and those in response after the HDT intensification procedure were given maintenance therapy with interferon (3 MU subcutaneously 3 times per week) and dexamethasone (40 mg by mouth 4 consecutive days every 4 weeks) until relapse. For patients entering the HDT arm, peripheral blood progenitor cells were collected after mobilization with granulocyte–colony-stimulating factor (G-CSF) (10 μg/kg subcutaneously for 5 days) or with an intermediate dose of cyclophosphamide (1.5 g/m2) plus G-CSF. The intensive regimens consisted of melphalan 200 mg/m2 given intravenously in 2 divided doses on days –3 and –2 or total body irradiation (12 Gy in 4 fractions of 3 Gy) from day –6 to day –3 plus melphalan 140 mg/m2 intravenously in a single dose on day –2. All patients receiving HDT were given G-CSF at a dose of 5 μg/kg per day from day +7 until granulocyte recovery.
Criteria of response
CR required the disappearance of the M-protein in serum and urine electrophoresis and less than 5% of bone marrow plasma cells. Objective or partial response (PR) was defined as a reduction of 50% or more of the monoclonal protein level in serum or urine (or both), an improvement in performance status, and a decrease greater than 50% in a measured cross-sectional area of plasmacytoma. Furthermore, the size and number of lytic bone lesions must not have increased, and there also must have been correction of hypercalcemia, anemia, and hypoalbuminemia. Patients who fulfilled all these criteria but who had less than 50% reduction in the M-protein level were considered to have had a minimal response (MR). When the criteria of CR, PR, or MR were not accomplished, the case was considered a treatment failure.10 Very short, transient responses observed during the first courses of treatment with subsequent relapse before the completion of the 4 courses were also considered treatment failures.
Statistical methods
The χ2 test was used to assess the statistical significance of multiple comparisons. OS was calculated from the start of the initial treatment to the date of death or last visit. Progression-free survival (PFS) was calculated from the day of initiation of treatment to the date of relapse or disease progression. Survival curves were plotted according to the method of Kaplan and Meier11 and were statistically compared by means of the log-rank test.12 Relationships between prognostic factors were determined by the Cox proportional hazards model for covariate analysis of censored survival date using SPSS software version 11.0 (SPSS, Chicago, IL).13 Variables at diagnosis included in the regression model were age, β2-microglobulin level, serum albumin level, hemoglobin level, M-protein type (immunoglobulin A [IgA] vs others) and treatment arm.
Results
Response to initial chemotherapy
One-hundred eighty-five of 216 (85%) patients responded to the initial chemotherapy. CR, PR, and MR rates were 12%, 59%, and 14%, respectively. Thirty-one of 216 patients did not respond to the induction treatment. Among the nonresponders, 7 died within the first months of the initiation of therapy, 14 received different salvage chemotherapy regimens, and 10 underwent HDT/stem cell transplantation (SCT) (9 autologous, 1 allogeneic). Outcomes of the 9 patients who underwent autologous transplantation were PR in 6, failure in 2, and toxic death from hemorrhage in 1. The patient who received an allogeneic graft achieved a transient CR. After relapse she underwent donor lymphocyte infusion, and she is now in prolonged CR.
Randomization and comparability of treatment groups
One-hundred sixty-four of the 185 responders were randomly assigned to receive 8 additional courses of VBMCP/VBAD chemotherapy (arm A, 83 patients) compared with HDT/SCT intensification (arm B, 81 patients; 24 received melphalan-140 [MEL-140]/TBI and 57 received MEL-200). Twenty-one responding patients were not randomly assigned because of patient refusal (8 patients), comorbid conditions (8 patients), and allogeneic transplantation (5 patients).
Pretreatment characteristics of patients according to treatment arm are shown in Table 1. Presenting features were similar in both groups, except for a significantly higher proportion of patients with hemoglobin levels lower than 100 g/L (10 g/dL) in the chemotherapy arm. Patients with hemoglobin levels lower than 100 g/L (10 g/dL) had significantly shorter survival times than those with higher levels in both treatment arms. Both groups were well balanced for β2-microglobulin serum levels and percentage of cells in S-phase. However, there was an imbalance in the M-protein type, with a significantly higher number of patients with IgA myeloma in the HDT arm. In the present series, the survival of patients with IgA myeloma was not significantly different from that of patients with other myeloma types. Presenting features showing prognostic significance in univariate analysis were serum β2-microglobulin (cutoff 296.6 nM [3.5 mg/L]; P = .009), hemoglobin level (cutoff 100 g/L [10 g/dL]; P = .001), and age (cutoff 56 years; P = .005). There was a trend toward shorter survival for patients with lactate dehydrogenase (LDH) serum levels higher than 450 IU/L (P = .08). Cox regression analysis included the following parameters: age, β2-microglobulin level, serum albumin level, hemoglobin level, M-protein type (IgA vs others), and treatment arm. Only age (cutoff 56 years; relative risk [RR], 1.87; 95% confidence interval [CI], 1.19-3.19; P = .016) and hemoglobin level (cutoff 100 g/L [10 g/dL]; RR, 1.88; 95% CI, 1.12-3.12; P = .015) emerged as independent prognostic factors at logistic regression analysis.
Patient characteristics according to treatment arm
Characteristic . | Chemo, n = 83 . | HDT/SCT, n = 81 . | P . |
|---|---|---|---|
| Median age, y | 56 | 57 | NS |
| Sex, M/F | 43/40 | 52/29 | NS |
| Hemoglobin level, less than 100 g/L, % | 47 | 30 | .03 |
| Calcium level, greater than or equal to 2.87, % | 10 | 15 | NS |
| Creatinine level, greater than or equal to 176.8 μM, % | 10 | 15 | NS |
| Serum albumin level, less than 35 g/L, % | 31 | 34 | NS |
| LDH, greater than 350 IU/L, % | 12 | 10 | NS |
| Lytic bone lesions, % | 67 | 70 | NS |
| β2-microglobulin, greater than 339 nM, % | 37 | 30 | NS |
| Phase-S, greater than 2, % | 40 | 37 | NS |
| M-protein type, no. | |||
| IgG | 65 | 47 | .02 |
| IgA | 11 | 30 | .003 |
| Light chain | 23 | 21 | NS |
| Others | 1 | 2 | NS |
Characteristic . | Chemo, n = 83 . | HDT/SCT, n = 81 . | P . |
|---|---|---|---|
| Median age, y | 56 | 57 | NS |
| Sex, M/F | 43/40 | 52/29 | NS |
| Hemoglobin level, less than 100 g/L, % | 47 | 30 | .03 |
| Calcium level, greater than or equal to 2.87, % | 10 | 15 | NS |
| Creatinine level, greater than or equal to 176.8 μM, % | 10 | 15 | NS |
| Serum albumin level, less than 35 g/L, % | 31 | 34 | NS |
| LDH, greater than 350 IU/L, % | 12 | 10 | NS |
| Lytic bone lesions, % | 67 | 70 | NS |
| β2-microglobulin, greater than 339 nM, % | 37 | 30 | NS |
| Phase-S, greater than 2, % | 40 | 37 | NS |
| M-protein type, no. | |||
| IgG | 65 | 47 | .02 |
| IgA | 11 | 30 | .003 |
| Light chain | 23 | 21 | NS |
| Others | 1 | 2 | NS |
LDH indicates lactate dehydrogenase; NS, not significant.
The degree of response to the initial chemotherapy, immediately before randomization, was virtually identical in both groups (Table 2).
Response to initial chemotherapy according to treatment arm
Response . | Chemotherapy, %; n = 83 . | HDT/SCT, %; n = 81 . |
|---|---|---|
| CR | 15 | 14 |
| PR | 68 | 68 |
| MR | 17 | 18 |
Response . | Chemotherapy, %; n = 83 . | HDT/SCT, %; n = 81 . |
|---|---|---|
| CR | 15 | 14 |
| PR | 68 | 68 |
| MR | 17 | 18 |
Six patients in arm A did not complete the assigned treatment: 5 underwent autologous transplantation, and 1 underwent intensity dose-reduced allogeneic transplantation. Conversely, 8 patients in arm B did not receive the planned HDT followed by autologous stem cell support: 4 underwent allogeneic transplantation (conventional conditioning in 2 and intensity dose-reduced in 2), whereas 4 did not receive any type of intensive therapy (2 continued alternating VBMCP/VBAD chemotherapy, and 2 had aggressive disease progression before the planned HDT procedure could be applied). As previously mentioned, the response to therapy and survival were analyzed on an intention-to-treat basis.
Response after completion of chemotherapy and after HDT
Eight (9.6%) patients in arm A experienced disease progression before the completion of the 12 courses of chemotherapy. On the other hand, 6 (7.4%) patients in arm B experienced disease progression within the 6 months of HDT intensification. In addition, 3 patients in arm A died in response before the completion of 12 courses of chemotherapy: 1, who had received HDT, died of pneumonia and respiratory failure 19 days after transplantation, while the other 2 died of myocardial infarction and neutropenic sepsis after 10 and 12 courses of chemotherapy, respectively. In addition, 3 patients in arm B died before the response evaluation at 6 months after HDT, 1 died of toxicity from cerebral hemorrhage, and the 2 patients who underwent allogeneic transplantation with conventional conditioning died, 1 of graft versus-host-disease and 1 of multiorgan failure.
The CR rate 6 months after HDT was significantly higher than after the completion of the 12 courses of chemotherapy (30% vs 11%; P = .002). CR rates for patients receiving MEL-140/TBI and MEL-200 were 37% and 28% (P = not significant [NS]), respectively.
Progression-free and overall survival
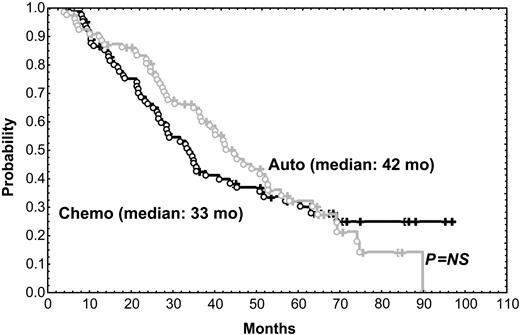
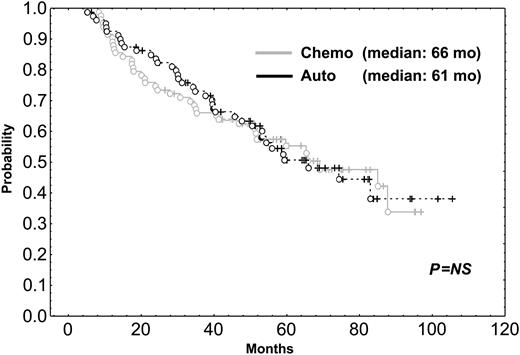
After a median follow-up of 56 months, the median survival time for the 216 patients included in the trial was 58 months. The median survival time of the 31 patients who did not respond to the initial VBMCP/VBAD chemotherapy was 22 months. With a median follow-up time of 66 months, the median survival time for the 164 randomly assigned responding patients was 65 months. As shown in Figure 1, PFS was not significantly different between HDT/SCT and conventional chemotherapy (median, 42 vs 33 months; P = .57). OS was also similar in both groups (Figure 2) (median, 61 months for HDT vs 66 months for chemotherapy; P = .89). To evaluate the impact of CR status at the time of randomization, subgroup analysis was performed but no significant differences in PFS and OS between patients given HDT and those who were continued on standard dose therapy were observed. PFS and OS were not significantly different between the 2 HDT regimens (ie, MEL-140/TBI and MEL-200). One patient in the chemotherapy arm acquired myelodysplastic syndrome (refractory anemia with excess of blasts associated with monosomy 7) after 10 cycles of chemotherapy and eventually died of acute myeloid leukemia while in PR of her myeloma. One patient in arm 1 died of adenocarcinoma of the hypophysis, and 2 patients in arm 2 developed urinary bladder carcinoma and small lung cell cancer, respectively.
Survival after relapse
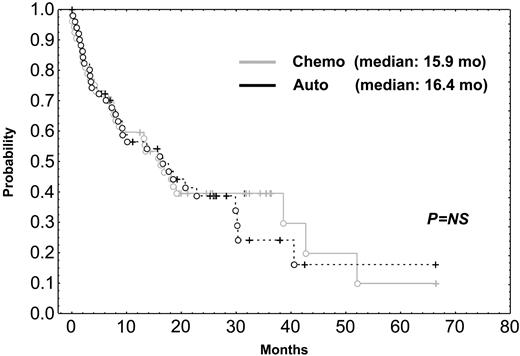
Most patients received conventional rescue chemotherapy after relapse. In fact, only 10 patients in the chemotherapy arm underwent rescue transplantation, and 7 patients in the HDT group underwent second transplantation as salvage regimen. Finally, survival after relapse was identical in the 2 arms (median, 15.9 vs 16.4 months; Figure 3).
Discussion
Despite the relative efficacy of the current antimyeloma agents, the outcome of patients with MM (median survival, less than 3 years) is still unsatisfactory.14 In addition, the proportion of long-term survivors with conventional chemotherapy is disappointingly small.15,16 Multiple myeloma is characterized by a high degree of resistance to therapy that results in a low CR rate with conventional chemotherapy. The fact that HDT can overcome tumor resistance, resulting in a CR rate ranging from 25% to 50%, has stimulated the use of HDT followed by stem cell rescue as part of the up-front therapy in patients with MM. In this regard, 1 case-control study17 and 2 population-based control studies18,19 have shown that patients given HDT survived significantly longer than those who received conventional chemotherapy. However, data from randomized studies are limited. Thus, only 2 prospective randomized trials have shown the superiority of HDT/SCT in terms of EFS and OS,5,6 whereas 2 randomized studies, reported in abstract form, have shown that myeloablative therapy does not prolong survival compared with conventional chemotherapy.7,8
PFS in patients given HDT intensification compared with those who were continued on conventional dose chemotherapy. Chemo indicates chemotherapy; auto, autologous transplantation.
PFS in patients given HDT intensification compared with those who were continued on conventional dose chemotherapy. Chemo indicates chemotherapy; auto, autologous transplantation.
OS of patients undergoing HDT intensification compared with those who were continued on conventional dose chemotherapy. Chemo indicates chemotherapy; auto, autologous transplantation.
OS of patients undergoing HDT intensification compared with those who were continued on conventional dose chemotherapy. Chemo indicates chemotherapy; auto, autologous transplantation.
In line with almost all the reported studies on HDT compared with conventional chemotherapy,5-7 we found that the high-dose regimen significantly increased the CR rate. However, we did not obtain a significant prolongation in EFS and OS with HDT when compared with continuing conventional treatment in patients responding to the initial chemotherapy. The main difference between our study and the other randomized trials is that in the present trial only patients with chemosensitive disease (ie, those who had CR, PR, or MR after 4 courses of chemotherapy) were included. In our study, it was considered that the crucial question concerning the possible impact of HDT as part of the initial therapy in patients with MM is whether those with MM sensitive to initial therapy, which involves most of the myeloma population, might benefit from HDT intensification. Although it is true that response to the initial chemotherapy selects by itself patients with good prognosis, whether randomization at diagnosis could have resulted in significant differences in our trial is uncertain. In fact, the finding that in the present series β2-microgloblin serum levels did not show prognostic influence at multivariate analysis might be at least attributed to the fact that only patients who showed chemosensitive disease were randomly assigned. Although it has been claimed that patients with primary resistant disease are the most likely to benefit from early HDT,20 it is important to recognize the heterogeneity of the 2 categories of patients with refractory MM: those who have primary unresponsive progressive disease and those who show no significant change in M-protein size and no clinical progression (also called nonresponders or nonprogressors). Our rationale for excluding patients with primary refractory disease from randomization was that early disease progression during initial chemotherapy usually precludes HDT because of early death or poor performance, whereas patients with nonresponding, nonprogressive disease have good outcomes, likely because of the more indolent natural history of the disease, irrespective of the treatment given.9 In fact, in all the studies in which the randomization was performed at the initiation of induction therapy, approximately one fourth of the patients allocated to the HDT therapy arm did not receive such treatment mainly because of disease progression or poor performance status.5-7 In the present trial, in which only responding patients were randomly assigned, less than 10% of the patients in each arm did not receive the allocated treatment.
Aside from time of randomization, other possible factors could have accounted for the differences between our results and those of trials reporting a significant benefit in favor of HDT. First, the dose intensity of our conventional arm, using the multialkylating-based regimen alternated with the noncross-resistant high-dose dexamethasone-containing VBAD regimen, was higher than in other trials. In the MRC trial,6 patients allocated to the conventional arm received a median of only 6 cycles of Adriamycin, BCNU, cyclophosphamide, melphalan (ABCM), whereas patients allocated to HDT were given a median of 5 cycles of cyclophosphamide, vincristine, Adriamycin, methylprednisolone (C-VAMP), which is by itself more intensive than ABCM, plus HDT intensification with MEL-200. In the IFM study,5 the chemotherapy of the control arm consisted of alternating VCMP/VBAP, which contains lower doses of melphalan than the VBMCP regimen and does not include high-dose dexamethasone. Supporting the possible impact of the chemotherapy arm, the authors of the US Intergroup study, in which no difference between HDT and continuation of conventional chemotherapy with VBMCP was found, claimed that the dose intensity of VBMCP, which was higher than that of standard regimens, could be the responsible for the lack of significant differences in results between HDT intensification and conventional chemotherapy.8 Second, our maintenance therapy included not only interferon but also monthly high-dose dexamethasone. This more intensive maintenance therapy could have resulted in the longer PFS observed in our series compared with the other randomized studies,4-7 thus diluting the possible influence of HDT. This notion is reinforced by the fact that the median survival time after relapse was only 16 months in both arms compared with a median survival of approximately 2 years in patients who had relapses after HDT in most series. Third, although it seems that the achievement of CR is the crucial step for a long-lasting disease control and overall survival in MM,21-23 the less than 20% difference in CR rate between the 2 arms in our series could have contributed to the lack of difference between HDT intensification and conventional chemotherapy in our study. Finally, the finding that the different events observed in each of the steps of our trial, including outcome after relapse, were virtually identical in the 2 arms is an additional argument supporting the equivalent value of both treatment approaches. In fact, the median survival of approximately 5 years in the present trial is similar to that reported by the PETHEMA Group24 and by Alexanian et al25 in patients with MM responding to initial chemotherapy who met the criteria for HDT intensification but who did not receive such treatment.
Survival after relapse in patients undergoing HDT compared with survival in those given standard dose chemotherapy. Chemo indicates chemotherapy; auto, autologous transplantation.
Survival after relapse in patients undergoing HDT compared with survival in those given standard dose chemotherapy. Chemo indicates chemotherapy; auto, autologous transplantation.
In summary, our results show that HDT intensification, when given to myeloma patients who have responded to initial chemotherapy, significantly increases the CR rate but has no significant impact on PFS and OS. A meta-analysis based on the individual patient data of all the randomized trials on HDT and conventional therapy is ongoing. It seems that the contribution of HDT by itself in the outcome of patients with MM treated with the conventional chemotherapy regimens is modest. However, the potential of HDT as a tool to further reduce tumor burden/minimal disease must be explored in the current context of the incorporation of novel agents with new mechanisms of action such as thalidomide, immunomodulatory drugs (IMiDs), and bortezomib in the pretransplantation setting.26-28
Appendix
Following is the complete list of the members of PETHEMA: Hospital Clínic Universitari Barcelona (Joan Bladé, Laura Rosiñol, Montserrat Fontanillas); Hospital Clínico Universitario Salamanca (Jesús San Miguel, M. Victoria Mateos); Hospital Germans Trias i Pujol Badalona (Josep M. Ribera); Hospital General de Especialidades Jaén (Antonio Alcalá); Hospital Els Camils Sant Pere de Ribes (Antoni Asensio); Hospital del Insalud Avila (Abelardo Barez); Hospital Son Dureta Palma de Mallorca (Joan Besalduch, Andrés Novo); Hospital Del Mar Barcelona (Carles Besses); Hospital Clínico Universitario Valladolid (Javier Fernández Calvo, Daniel Borrego, Luciano Guerras); Hospital Vall d'Hebrón Barcelona (Manuel Callís, Andrés López); Hospital General Valencia (Félix Carbonell); Hospital Central de Asturias Oviedo (Dolores Carrera); Hospital Clínico San Carlos Madrid (Joaquín Díaz Mediavilla, Rafael Martínez); Hospital Verge de la Cinta Tortosa (Llorenç Font); Hospital Clínico Universitario Valencia (Javier Gacía-Conde, Mar Tormo, M. José Terol); Hospital Ramón y Cajal Madrid (José García Laraña, Jesús Odriozola); Hospital Dr Trueta-ICO Girona (Santiago Gardella); Hospital Miguel Servet Zaragoza (Pilar Giraldo, Manuel Giralt); Hospital Universitario de Canarias la Laguna (Gloria González Brito, Luis Hernández Nieto); Hospital Virgen de la Vega Salamanca (Ramiro Jiménez Galindo); Hospital Pesset Valencia (Pilar León); Hospital Carlos Haya Málaga (Juan Maldonado, Jesús Trujillo); Hospital Mútua de Terrassa (Josep M. Martí); Hospital General de Murcia (José M. Moraleda); Hospital Virgen Blanca León (M. Jesús Moro, Fernando Ortega); Hospital Clínico Universitario Zaragoza (Luis Palomera); Hospital Arnau de Vilanova Valencia (Reyes Sancho-Tello); Hospital Taulí Sabadell (Juan Alfonso Soler); Hospital Sant Pau Barcelona (Anna Sureda, Salut Brunet); Hospital General de Segovia (José Hernández).
Prepublished online as Blood First Edition Paper, August 16, 2005; DOI 10.1182/blood-2005-03-1301.
A complete list of the members of PETHEMA appears in “Appendix.”
Supported in part by grants FIS 95/0828 and 2003-V-REDG-136-O from the Fondo de Investigaciones Sanitarias de la Seguridad Social.
J.B. and J.S.M. created the initial concept/design of the trial, analyzed and interpreted the data, wrote the manuscript, modified subsequent drafts, and finalized the manuscript; L.R. and A. Sureda contributed to analysis and interpretation of the data and to drafting the manuscript; J.B., J.S.M., L.R., and A. Sureda brought a significant number of patients to the study; M.F. participated in the analysis of the data and in the updating process throughout the study period; and J.M.R., J.D.-M., J.G.-L., M.V.M., L.P., J.F.-C, J.M.M., P.G., F.C., M.C., J.T., S.G., M.J.M., A.B., A. Soler, and L.F. participated in the conception/design of the study, included patients, provided the PETHEMA database with the patient data and periodic updating, and actively discussed the progress of the trial at the annual PETHEMA meetings. All the authors are members of PETHEMA and reviewed and approval the final version of the manuscript.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Jordi Esteve for his help in the statistical analysis and M. José Sánchez-Melero for her outstanding secretarial support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal