The phagocyte nicotinamide adenine dinucleotide phosphate (NADPH) oxidase plays an instrumental role in host defense and contributes to microbicial killing by releasing highly reactive oxygen species. This multicomponent enzyme is composed of membrane and cytosolic components that assemble in the plasma membrane or phagolysosome. While the guanosine S′-triphosphatase (GTPase) Rac2 has been shown to be a critical regulator of NADPH oxidase activity and assembly, the role of its effector, p21-activated kinase (Pak), in oxidase function has not been well defined. Using HIV-1 Tat-mediated protein transduction of Pak inhibitory domain, we show here that Pak activity is indeed required for efficient superoxide generation in intact neutrophils. Furthermore, we show that Pak translocates to the plasma membrane upon N-formyl-methionyl-leucyl-phenylalanine (fMLF) stimulation and colocalizes with translocated p47phox and with p22phox, a subunit of flavocytochrome b558. Although activated Pak phosphorylated several essential serine residues in the C-terminus of p47phox, direct binding to p47phox was not observed. In contrast, active Pak bound directly to p22phox, suggesting flavocytochrome b was the oxidase-associated membrane target of this kinase and this association may facilitate further phosphorylation of p47phox in the assembling NADPH oxidase complex.
Introduction
Phagocytes represent the first line of cellular host defense against microorganisms. This function relies in part on the ability of these cells to generate large amounts of reactive oxygen species, including superoxide anion (O2·-), hydroxyl radical (OH-), and hydrogen peroxide (H2O2). This phenomenon, known as the respiratory burst, results from activation of a multicomponent, O2·--producing enzyme known as the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. In resting phagocytes, the NADPH oxidase complex is dormant, separated into individual cytosolic and membrane-bound components.1,2 Upon activation by a variety of stimuli, such as chemotactic peptides, phorbol esters, or opsonized particles, the cytosolic factors translocate to the membrane and associate with flavocytochrome b558, a heterodimer composed of gp91phox and p22phox. The 4 minimally required cytosolic factors of the NADPH oxidase include p67phox, p47phox, p40phox, and the Rho guanosine triphosphatase (GTPase) Rac.1
Cell-free assay systems, in vivo experiments in Rac2 knock-out mice, as well as the clinical phenotype caused by the presence of a Rac2 mutation in an immunocompromised patient have confirmed the crucial role of Rac2 in O2·- generation by phagocytes.3-6 Stimulation of Rac activity, followed by association of the active GTPase with the NADPH oxidase complex, coordinates essential steps of the electron flow and facilitates activation of downstream targets. NADPH oxidase activation is highly regulated, involving a number of phosphorylation events, conformational changes in oxidase components, and protein-protein interactions.1 While all of the oxidase components have been reported to be targets of phosphorylation and several protein kinases have been implicated in various assay systems, the actual role of all these events in regulating the oxidase in intact neutrophils remains unclear.
We have previously shown that several isoforms of p21-activated kinases (Paks) are rapidly and transiently activated in chemoattractant-stimulated neutrophils.7,8 Furthermore, we found Pak activation was dependent on heterotrimeric G-protein coupling to the N-formyl-methionyl-leucyl-phenylalanine (fMLF) receptor and occurred in concert with Rac activation. Subsequently, Dharmawardhane et al reported that Pak localizes to membrane ruffles and lamellipodia at the leading edge of polarized cells in fMLF-stimulated leukocytes.9 This localization closely resembles the pattern of Rac redistribution during NADPH oxidase activation. Pak activity and protein-protein interactions facilitated by proline-rich regions in the N-terminal domain of Pak are considered regulatory elements for actin reorganization and are involved in important neutrophil functions, such as chemotaxis and phagocytosis.10 For example, Pak regulates a number of cellular functions through phosphorylation of multiple targets, predominantly on serine residues surrounded by lysine or arginine residues. These targets include mitogen-activated protein kinase kinase-1 (MEK-1), Raf-1, LIM kinase, myosin light chain kinase, stathmin, merlin, Bad, RhoGDI (Rho guanosine diphosphate dissociation inhibitor), phosphoglycerate mutase B, and phosphoglucomutase.10
While several isoforms of Pak are highly expressed and rapidly activated by chemoattractants in neutrophils, participation of Pak in regulation of O2·- production by the NADPH oxidase has not been firmly established. Previously, we reported that the NADPH oxidase component p47phox might represent a potential target for regulatory phosphorylation by activated Pak and that Pak-induced phosphorylation of the glycolytic enzyme phosphoglycerate mutase B (PGAM-B) increases O2·- generation.11 However, further understanding of the role of Pak in NADPH oxidase function has been limited due to the absence of Pak-specific, small molecule inhibitors and the technical difficulties associated with efficient gene transfer into phagocytes. Thus, to address this issue, we used an HIV-Tat–mediated protein transduction approach to investigate the role of Pak in regulating the neutrophil NADPH oxidase in intact cells and provide direct evidence to substantiate a regulatory role for Pak in oxidase function. Furthermore, our data suggest that the basis of Pak-mediated regulation of the NADPH oxidase involves regulatory phosphorylation of the oxidase component p47phox and direct association with flavocytochrome b558.
Materials and methods
Materials
The chemoattractant peptide fMLF was purchased from Sigma Chemical (St Louis, MO) and Molecular Probes (Eugene, OR). [γ-32P] adenosine triphosphate (ATP) and [32P] H3PO4 were from ICN (Irvine, CA) and Amersham-Pharmacia (Piscataway, NJ), respectively. Anti-p47phox antibody was from BD Biosciences (San Diego, CA), anti–phospho Pak antibody was from Cell Signaling Technology (Beverly, MA), and anti-Pak antibodies,7,8 anti-p22phox, and anti-gp91phox antibodies12 have been described previously. Anti–epitope tag antibodies or secondary antibodies were purchased from Covance (Berkeley CA), Promega (Madison, WI), and Molecular Probes. Peptides encompassing C-terminal sequences of p47phox were synthesized and purified by the Scripps Research Institute Peptide facility.
Protein and plasmid preparations
The pTAT-HA bacterial expression vector was provided by S. Dowdy (Howard Hughes Medical Institute and University of California at San Diego) (Vocero-Akbani et al13 ) and used to insert sequences for green fluorescent protein (GFP), Pak–Pak-inhibitory domain (PID) (amino acids [aa] 83-149), PID L107F, and Rac2 T17N by polymerase chain reaction (PCR). The plasmids were validated by sequencing and used for transformation of Escherichia coli BL21(DE3) LysS competent cells (Invitrogen, Carlsbad, CA). Expression and Tat protein induction were essentially as described.13 Recombinant proteins were isolated from the bacterial pellet by sonication and denaturation in 8 M urea. Proteins were purified over a nickel-nitrilotriacetic acid (Ni2+-NTA)–agarose affinity column, followed by a fast performance liquid chromatography (FPLC) purification and/or desalting gel filtration. To assist in protein refolding, the urea concentration of Tat-PID and Tat-PID L107F was slowly lowered during the last 2 purification steps. Identity and purity of the proteins were assessed by Coomassie blue staining and immunoblotting. For some experiments, Tat proteins were labeled with fluorescein isothiocyanate (FITC) for 2 hours at room temperature (RT), and unbound FITC was removed with a Sephadex G-25 column. Expression and purification of glutathione-S–transferase (GST) fusion proteins, including GST-Pak1 and GST-p47phox, have been described previously.7 Cytochrome b558 was purified from human neutrophil membranes as described previously.14 Myc-tagged Pak1 plasmids for mammalian expression were prepared as reported in Shalom-Barak and Knaus.11 Single and multiple mutations of p47phox were introduced with the Quikchange Site-directed mutagenesis kit (Stratagene, La Jolla, CA) and verified by sequencing. Wild-type and mutant p47phox were also subcloned into pJ3H for mammalian expression. Endotoxin levels in the purified protein preparations were less than 0.01 EU/mL when measured using a commercially available limulus amebocyte lysate assay (Bio-Whittaker, Walkersville, MD).
Cell isolation and cell culture
Neutrophils were isolated under endotoxin-free conditions from heparinized venous blood of healthy volunteers by 2-step sedimentation on dextran and Ficoll-Hypaque.3 The purity was consistently higher than 95%. Monocyte isolation from human blood was performed as described.15 Monocytes were allowed to attach to coverslips for 2 hours, and nonadherent cells were removed by gentle washing. Adherent monocytes were cultured for 10 days in RPMI 1640 containing 10% heat-inactivated fetal bovine serum (FBS; Invitrogen) and recombinant macrophage colony-stimulating factor (rM-CSF, 10 g/mL), and medium was changed every 3 days. HL60 cells were obtained from American Type Culture Collection (ATCC, Rockville, MD) and differentiated with 15 ng/mL phorbol myristate acetate in RPMI 1640 containing 20% FBS for 4 days.
Protein transduction and O2·- generation
Neutrophils (1-5 × 105) were suspended in RPMI and preincubated with Tat proteins or bovine serum albumin (BSA) for 10 minutes and washed with phosphate-buffered saline (PBS) before stimulation with fMLF (1 μM) or phorbol myristate acetate (PMA, 100 ng/mL). O2·- generation was measured using luminol-based chemiluminescence. Neutrophils were preincubated in 25 mM Hepes (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.5), 118 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 5.5 mM glucose, and 1.0 mM CaCl2 (KRHG) or in RPMI at 37°C for 5 minutes before adding 71 μM luminol and 50 μg/mL horseradish peroxidase. Stimulation with fMLF or PMA was initiated after 1 to 2 minutes of baseline recording by injecting the stimulus. Chemiluminescence was recorded every 20 seconds for up to 15 minutes, and results are expressed as relative light units (RLU). Experiments were performed 3 times in triplicates using a Wallac Berthhold plate luminometer (Gaithersburg, MD).
Transient transfection and immunoprecipitation
Cos7gp91phox/p22phox cells or Cos7p22phox cells were maintained in Dulbecco modified Eagle medium (DMEM) low glucose supplemented with 10% FCS, 2 mM glutamine including 0.8 mg/mL neomycin (p22phox), and 2 μg/mL puromycin (gp91phox). Cells were transiently transfected with pCVM6, pCVM6-Pak1 wt, pCVM6-Pak1 L107F T423E, pCVM6-Pak1 (aa 1-201), or pCVM6-Pak1 (aa 206-545), alone or in combination with pJ3H-p47phox or pJ3H-p47phox S303, 304, 320, 328A using Lipofectamine Plus according to the manufacturer's instructions (Invitrogen). After 24 to 40 hours, cell lysates were prepared in 50 mM Tris (tris(hydroxymethyl)aminomethane, pH 7.5), 100 mM NaCl, 5 mM MgCl2, 1 mM EDTA (ethylenediaminetetraacetic acid), 1% nonidet P-40 (NP-40), and 10% glycerol, and subjected to immunoprecipitation with either anti-Myc (for Pak), anti-p22phox, or anti-HA (for p47phox) antibodies. Immunoblots were probed with the corresponding antibodies to detect coimmunoprecipitation. For some experiments, cells were labeled with [32P] H3PO4 (50 μCi/mL [1.85 MBq]) overnight in phosphate-free DMEM containing 0.5% dialyzed FBS, and phosphate incorporation into p47phox was evaluated after immunoprecipitation of the protein from cell lysates, gel electrophoresis, and subsequent autoradiography of the gel.
Phosphorylation assays
Phosphorylation of peptide sequences derived from the C-terminus of p47phox were carried out using recombinant, constitutively active GST-Pak1, as described,7,11 followed by scintillation counting to determine radioactivity bound to phosphocellulose filter units (Pierce, St Louis, MO). For 2-dimensional (2-D) phosphopeptide mapping, in vitro kinase reaction-swith purified, recombinant GST-Pak1 using GST, GST-p47phox, or GST-p47phox mutant proteins as substrates were performed. The purity of proteins was assessed by Coomassie blue staining, and protein was quantified with the bicinchoninic acid (BCA) assay system. The phosphorylation reactions were performed in kinase buffer (50 mM Hepes [pH 7.5], 10 mM MgCl2, 2 mM MnCl2, 0.2 mM dithiothreitol [DTT], 50 μM ATP) with 10 μCi (0.37 MBq) [32P]ATP/reaction and were analyzed using gel electrophoresis, blotting onto nitrocellulose, and detection by autoradiography. Band intensities were quantified by liquid scintillation counting. Tryptic digestion and phosphopeptide mapping were performed as described.16,17
Confocal microscopy
HL60 cells were differentiated with PMA on coverslips for 4 days. The cells were carefully washed in warm medium without phenol red and incubated with buffer or 1 μM fMLF at 37°C for 1 minute. The reaction mixture was removed and cells were immediately fixed for 20 minutes in 2% paraformaldehyde. Following fixation, the cells were washed twice for 10 minutes in PBS, permeabilized for 5 minutes in 0.5% Triton X-100/PBS, washed again with PBS, and blocked with an avidin/biotin blocking kit (Vector Laboratories, Youngstown, OH) for 30 minutes. Cells were then immediately incubated for 1 hour in combinations of affinity-purified anti-Pak antibody and anti-p47phox monoclonal antibody diluted in 2% FBS/PBS. Following this incubation, the cells were washed twice for 10 minutes in PBS, incubated for 1 hour with biotinylated donkey anti–rabbit immunoglobulin G (IgG; Jackson ImmunoResearch, West Grove, PA), washed twice for 10 minutes in PBS, incubated in the dark in fluorescent-conjugated goat anti–mouse IgG (Alexa Fluor 488; Molecular Probes) and streptavidinrhodamine red-X (Jackson ImmunoResearch), washed again with PBS, and fixed to glass slides using the Prolong Antifade Kit (Molecular Probes). Stained cells were examined with a × 63/1.4 NA objective under a Zeiss Axiovert S100TV confocal microscope (Carl Zeiss, Heidelberg, Germany) equipped with a krypton/argon mixed gas laser, visualized with Bio-Rad LaserSharp (v3.2) software (Bio-Rad, Hercules, CA), and processed using Adobe Photoshop (Adobe Systems, San Jose, CA). For human macrophages, stimulation with PMA was 3 minutes, followed by the procedure as described above. These cells were incubated with affinity-purified anti-Pak1 and anti-p22phox monoclonal antibody. For transfected Cos7p22phox cells, anti-Myc monoclonal antibody and anti-p22phox polyclonal antibody were used.
Results
Pak-mediated regulation of superoxide generation by the phagocyte NADPH oxidase
The elucidation of intracellular signaling mechanisms in primary human neutrophils is hindered by the short life span and low transfection efficiency of neutrophils, and functional studies are primarily performed using pharmacologic inhibitors or inferring data from myeloid precursor cell lines, such as K562 cells. Since neither of these approaches is suitable for studies involving Pak function, we used a protein transduction method to study the involvement of Pak in O2·- generation. This approach relies on a short polybasic sequence derived from the HIV Tat protein to introduce proteins into intact cells.18-20
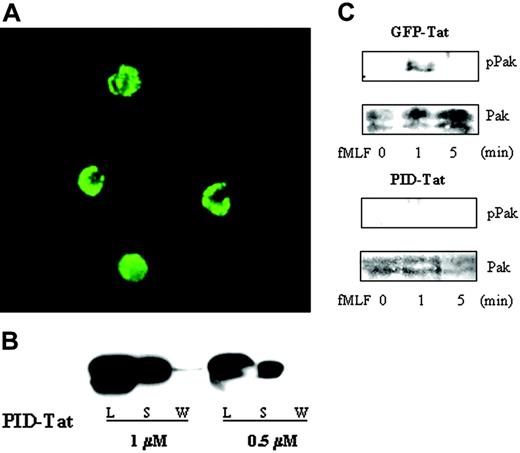
Currently, the best method to suppress receptor-stimulated Pak kinase activity without interfering with other downstream effectors of active Rac or Cdc42 is the expression of PID, which inhibits activation of 3 Pak isoforms (Paks 1-3).21-23 FITC-labeled PID-Tat fusion protein was incubated with human neutrophils, and cellular uptake was evaluated by confocal microscopy after removal of externally bound protein. Nearly 100% of the neutrophils showed high levels of incorporated FITC-PID-Tat, which was predominantly cytosolic (Figure 1A). Furthermore, immunoblotting for supernatants and cell lysates from PID-Tat–treated neutrophils confirmed uptake of the fusion protein (Figure 1B).
Cellular uptake and biologic activity of PID-Tat protein in human neutrophils. (A) Neutrophils (2 × 105 cells) were incubated with 1 μM FITC-labeled PID-Tat, and cellular uptake into live cells was analyzed by confocal microscopy as described in “Materials and methods.” (B) Neutrophils (5 × 105 cells) were incubated with 0.5 or 1 μM PID-Tat, followed by centrifugation. The supernatants (S) were collected, the cells were washed with PBS and the wash was collected (W), and the cell pellets were lysed in sample buffer (L). Samples were run on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with an anti-Pak antibody. (C) Neutrophils (2 × 105 cells) were preincubated with GFP-Tat or PID-Tat and then stimulated with 1 μM fMLF for 1 or 5 minutes. Cell lysates of stimulated or unstimulated cells were subjected to SDS-PAGE and probed with anti–phospho Pak antibody (top panel) followed by anti-Pak antibody (bottom panel).
Cellular uptake and biologic activity of PID-Tat protein in human neutrophils. (A) Neutrophils (2 × 105 cells) were incubated with 1 μM FITC-labeled PID-Tat, and cellular uptake into live cells was analyzed by confocal microscopy as described in “Materials and methods.” (B) Neutrophils (5 × 105 cells) were incubated with 0.5 or 1 μM PID-Tat, followed by centrifugation. The supernatants (S) were collected, the cells were washed with PBS and the wash was collected (W), and the cell pellets were lysed in sample buffer (L). Samples were run on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with an anti-Pak antibody. (C) Neutrophils (2 × 105 cells) were preincubated with GFP-Tat or PID-Tat and then stimulated with 1 μM fMLF for 1 or 5 minutes. Cell lysates of stimulated or unstimulated cells were subjected to SDS-PAGE and probed with anti–phospho Pak antibody (top panel) followed by anti-Pak antibody (bottom panel).
Since Tat fusion proteins are prepared by expression and purification from E coli, the potential for a formyl-methionine addition to the amino terminal sequence exists.24 To investigate the possibility of N-terminal modifications on purified Tat proteins, we analyzed their amino terminal start sequence MRGS (methionine-arginine-glycine-serine). According to studies by Hu et al and others, the formyl group will be removed from methionine in this sequence, while the methionine itself could be partially retained.25-30 To evaluate this possibility, we performed functional competition binding studies on neutrophils using FITC-labeled fMLF and Tat protein. Cells were incubated with either fluorescently labeled peptide or Tat protein on ice and analyzed by flow cytometry. This procedure will prevent immediate uptake of Tat protein or internalization of the occupied formyl peptide receptor (FPR). The differences in percentage of fluorescent cells incubated with FITC-fMLF peptide alone or with FITC-fMLF peptide after Tat protein treatment was between 4% and 5%, which is in the range of background binding. Thus, these results indicate that Tat fusion proteins expressed from the described plasmid sequence and purified according to the published protocols will not bind to the FPR and alter FPR-mediated signaling by virtue of E coli–generated modifications.
To determine whether PID-Tat was properly refolded and retained its expected biologic activity, neutrophils were treated with PID-Tat or control GFP-Tat and stimulated with fMLF. Normal activation of Pak was detected in GFP-Tat–transduced neutrophils, whereas Pak activation was completely inhibited when PID-Tat was incorporated into neutrophils (Figure 1C). The peak of Pak activation occurred one minute after receptor stimulation and was followed by rapid down-regulation. This time course closely resembles Pak activation in nontransduced cells7 and shows, together with the PID-mediated inhibition of this response, that introduction of PID-Tat fusion protein is a valid approach to elucidate Pak function in neutrophils.
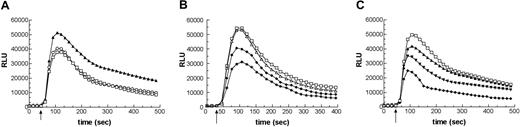
To investigate the role of Pak in regulating O2·- generation by the NADPH oxidase, we transduced cells with PID-Tat, as well as dominant-negative PID L107F-Tat and a dominant-negative Rac2 T17N-Tat mutant. It should be noted that we encountered substantial variation in respect to yield and aggregation of the PID and PID mutant constructs. These properties of PID-Tat proteins did not allow protein concentrations above 1 to 2 μM or freezer storage and necessitated continuous purification and immediate use of the proteins. Since preincubation of GFP-Tat with neutrophils for 10 minutes at RT before addition of fMLF resulted in slightly increased O2·- production (10%-20% depending on the protein and neutrophil preparation) (Figure 2A), the oxidative burst observed after incubation with GFP-Tat was used as the experimental control. Incubation of neutrophils with Rac2 T17N-Tat, a dominant-negative Rac mutant, decreased superoxide dismutase (SOD)–inhibitable O2·- production by approximately 50%, while transduction of cells with PID-Tat protein at the same concentration showed a 30% reduction (Figure 2B). In contrast, no inhibition was observed in neutrophils pretreated with inactive PID mutant protein (PID L107F-Tat) before fMLF stimulation (Figure 2B). The inhibition of fMLF-induced O2·- generation by PID-Tat protein was strictly dose dependent (Figure 2C), reaching close to 50% inhibition at a concentration of 2 μM PID-Tat. Under the same conditions, the oxidative burst remained unchanged when PMA was used as stimulus (data not shown). These results are consistent with our previous data indicating that PMA-mediated NADPH oxidase activation is Pak-independent.7,8
The NADPH oxidase protein p47phox is a target for active Pak
A correlation between the serine-threonine kinase activity of Pak and serine phosphorylation of p47phox was suggested by our previous data.7 Since stimulus-induced phosphorylation of multiple serine residues at the C-terminus of p47phox is essential for oxidase activation,31-33 we tested multiple serine-containing peptides located in the C-terminal region of p47phox to investigate potential Pak phosphorylation sites. Recombinant, constitutively active Pak phosphorylated 3 different peptides containing 6 serine residues located from amino acids 300 to 331 in p47phox (Figure 3A). Furthermore, coexpression of active Pak and p47phox in Cos cells resulted in incorporation of radioactive phosphorus into p47phox (data not shown), and full-length recombinant p47phox protein was phosphorylated by active GST-Pak in vitro (Figure 3B), indicating substantial Pak-mediated phosphorylation of intact p47phox. Phospho–amino acid analysis of the radioactive p47phox band indicated phosphorylation of serine residues, and tryptic digests of phosphorylated p47phox, followed by 2-D phosphopeptide mapping, revealed multiple phosphorylation sites (Figure 3C). We used a combination of 2-D mapping followed by high-performance liquid chromatography (HPLC), matrix-assisted laser desorption/ionization–mass spectrometry (MALDI-MS), and mutational analysis to identify the location of these phosphoserines in p47phox and found that serines 303, 304, 320, and 328 were targets of Pak-induced phosphorylation. Consistent with our peptide-based experiments (Figure 3A), the highest incorporation of 32P was detected at serine 328. Note that we also found several phosphopeptide spots that originated from incomplete cleavage products of the same peptide. To confirm the phosphorylation sites identified, we generated a quadruple p47phox S→A mutant protein and showed that this protein was not phosphorylated by active GST-Pak in vitro nor was it phosphorylated in vivo in Cos cells coexpressing constitutively active Pak (Figure 3B and data not shown).
Inhibition of fMLF-induced superoxide anion generation by PID-Tat protein. Human neutrophils were incubated in the absence or presence of the following proteins: BSA, GFP-Tat, PID-Tat, PID L107F-Tat, or Rac2 T17N-Tat. fMLF (1 μM)–stimulated O2·- generation was measured in 3 to 5 × 105 cells by chemiluminescence. (A) Cells were preincubated with buffer (□), 1 mg/mL bovine serum albumin (○), or 1 μM GFP-Tat (▴). (B) Cells were preincubated with 1 μM GFP-Tat (▵), PID L107F-Tat (□), PID-Tat (♦), or Rac2 T17N-Tat (*). (C) Cells were preincubated with 2 μM PID L107F-Tat (□), 0.5 μM PID-Tat (▴), 1 μM PID-Tat (▾), or 2 μM PID-Tat (♦). The arrow indicates addition of fMLF. The depicted graphs are representative of 3 separate experiments.
Inhibition of fMLF-induced superoxide anion generation by PID-Tat protein. Human neutrophils were incubated in the absence or presence of the following proteins: BSA, GFP-Tat, PID-Tat, PID L107F-Tat, or Rac2 T17N-Tat. fMLF (1 μM)–stimulated O2·- generation was measured in 3 to 5 × 105 cells by chemiluminescence. (A) Cells were preincubated with buffer (□), 1 mg/mL bovine serum albumin (○), or 1 μM GFP-Tat (▴). (B) Cells were preincubated with 1 μM GFP-Tat (▵), PID L107F-Tat (□), PID-Tat (♦), or Rac2 T17N-Tat (*). (C) Cells were preincubated with 2 μM PID L107F-Tat (□), 0.5 μM PID-Tat (▴), 1 μM PID-Tat (▾), or 2 μM PID-Tat (♦). The arrow indicates addition of fMLF. The depicted graphs are representative of 3 separate experiments.
Phosphorylation of p47phox by p21-activated kinase (Pak). (A) Peptides derived from the C-terminus of human p47phox were incubated with recombinant GST-Pak1 and [32P]ATP, as described. The numbers under the bars indicate the location of the peptides in p47phox (amino acids). Results are depicted as the mean ± SD of cpm measurement on 4 different fields per peptide. (B) In vitro kinase assays were performed as described using GST-Pak1 alone (lane 1), recombinant wild-type GST-p47phox (lane 2), or recombinant GST-p47phox quadruple mutant S303/304/320/328A (lane 3). (C) 2-D map of phosphopeptides derived from trypsin-digested GST-p47phox (left) or GST-p47phox S303/304/320/328A (right) after phosphorylation by GST-Pak1. The original (ori) application spot of the sample is indicated by an arrow.
Phosphorylation of p47phox by p21-activated kinase (Pak). (A) Peptides derived from the C-terminus of human p47phox were incubated with recombinant GST-Pak1 and [32P]ATP, as described. The numbers under the bars indicate the location of the peptides in p47phox (amino acids). Results are depicted as the mean ± SD of cpm measurement on 4 different fields per peptide. (B) In vitro kinase assays were performed as described using GST-Pak1 alone (lane 1), recombinant wild-type GST-p47phox (lane 2), or recombinant GST-p47phox quadruple mutant S303/304/320/328A (lane 3). (C) 2-D map of phosphopeptides derived from trypsin-digested GST-p47phox (left) or GST-p47phox S303/304/320/328A (right) after phosphorylation by GST-Pak1. The original (ori) application spot of the sample is indicated by an arrow.
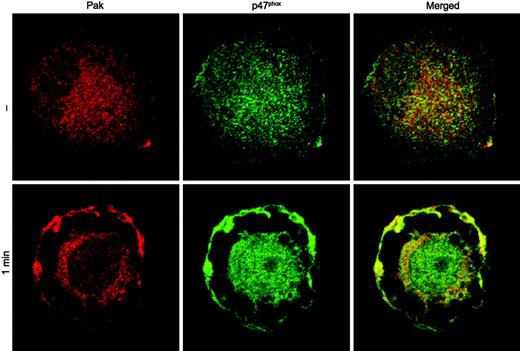
Colocalization of Pak with p47phox in fMLF-stimulated macrophage-like cells. HL60 cells differentiated with phorbol ester to a macrophage-like phenotype were incubated with buffer (—) or 1 μM fMLF (1 min). Fixed and permeabilized cells were incubated with anti-Pak and anti-p47phox antibodies and visualized using confocal microscopy. A representative image from 2 independent experiments is shown.
Colocalization of Pak with p47phox in fMLF-stimulated macrophage-like cells. HL60 cells differentiated with phorbol ester to a macrophage-like phenotype were incubated with buffer (—) or 1 μM fMLF (1 min). Fixed and permeabilized cells were incubated with anti-Pak and anti-p47phox antibodies and visualized using confocal microscopy. A representative image from 2 independent experiments is shown.
It is conceivable that Pak activity is required for the phosphorylation of NADPH oxidase components other than p47phox, and Ahmed et al reported that Pak phosphorylated p67phox protein fragments.34 However, we did not detect substantial phosphorylation of p67phox by activated GST-Pak in vitro using recombinant p67phox or in Cos cells coexpressing p67phox and active Pak (data not shown).
Protein kinases can associate with their phosphorylation targets, as we have previously shown for Pak and PGAM.11 Since recombinant p47phox protein bound nonspecifically to various affinity beads (U.G.K., unpublished observations, 1999), we evaluated the potential interaction of Pak with p47phox in intact cells. Wild-type Pak, as well as dominant-negative and constitutively active Pak mutants were coexpressed with wild-type p47phox in Cos cells, followed by immunoprecipitation of Pak from cell lysates. Independently of the Pak construct used, we were not able to detect stable complex formation between Pak and p47phox. Therefore, we analyzed possible colocalization of Pak with p47phox in HL-60 cells, which had been differentiated with phorbol ester to a macrophage-like cell. Confocal microscopy analysis of differentiated HL-60 cells, treated with fMLF to stimulate Pak and the NADPH oxidase, showed stimulus-dependent colocalization of Pak and p47phox in plasma membrane regions (Figure 4). Thus, while Pak and p47phox do not appear to form a stable complex, their colocalization is consistent with the conclusion that p47phox is a physiologic substrate of Pak.
Pak associates with the NADPH oxidase protein p22phox upon activation
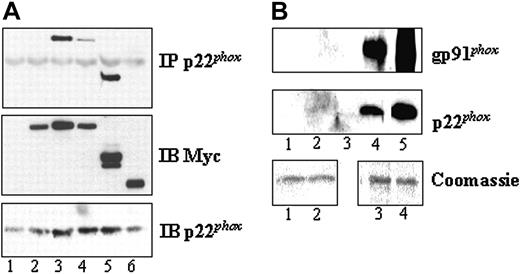
Since stimulation with chemoattractant led to a significant translocation of Pak to the plasma membrane, but apparently not to a stable interaction with p47phox, we hypothesized that Pak might interact directly with flavocytochrome b558. To evaluate this possibility, we transfected Cos7 cell lines stably expressing gp91phox and p22phox or p22phox alone with Pak wild-type and mutant constructs. We found that constitutively active Pak1, either as Pak1 mutant or Pak1 C-terminal domain, associated specifically with p22phox but not with gp91phox (Figure 5A). Furthermore, experiments using Cos7 cells stably expressing p22phox alone confirmed that active Pak interacts with p22phox independently of the presence of gp91phox (data not shown). A direct association of active Pak with flavocytochrome b558 was further established by the specific binding of the purified flavocytochrome b to purified, kinase-active GST-Pak1 but not to GST protein (Figure 5B).
Biochemical and structural studies have demonstrated that partial phosphorylation of p47phox relieves an autoinhibitory intramolecular interaction, which then permits phospho-p47phox binding to the cytoplasmic tail of p22phox.33 Thus, binding of p47phox and active Pak to p22phox must occur simultaneously without competition for the same binding site. To address this issue, we cotransfected active Pak1 or the constitutively active C-terminus of Pak1 with p47phox into flavocytochrome b–expressing Cos7 cells and treated the cells with either vehicle control or PMA to induce p47phox translocation to the plasma membrane. Immunoprecipitation of p22phox revealed that the interaction of Pak with p22phox was not affected by the presence of p47phox or PMA stimulation of the cells (Figure 6A). To ensure p47phox binding to p22phox under our conditions, the association of p47phox with p22phox was determined. Our data clearly demonstrate a substantial increase in p47phox/p22phox complex formation when the cells were stimulated with PMA (Figure 6B).
Association of Pak with p22phox. (A) Cos cells expressing functional flavocytochrome b were transfected with vector (lane 1), wild-type Myc-Pak1 (lane 2), constitutively active Myc-Pak1 F107 E423 (lane 3), dominant-negative Myc-Pak1 A299 (lane 4), Myc-Pak1 (aa 206-545) (lane 5), or Myc-Pak1 (aa 1-205) (lane 6). Anti-p22phox immunoprecipitates were prepared after 24 hours and probed with anti-Myc antibody for Pak detection (top panel). The middle and the bottom panels show Myc-Pak1 protein expression levels and p22phox protein in the IP, respectively. Representative of 3 independent experiments and verified twice in Cos cells stably expressing p22phox alone. Immunoblots of cell lysates are indicated by IB. (B) GST beads (lanes 1-2) or GST-Pak1 beads (lanes 3-4) were incubated with buffer (lanes 1,3) or purified flavocytochrome b (lanes 2,4). After washing, proteins bound to the beads were eluted and immunoblotted with anti-gp91phox or anti-p22phox antibodies. Control blots for the flavocytochrome b are shown in lane 5. Equal amounts of recombinant GST or GST-Pak1 bound to beads were verified by Coomassie blue staining (bottom panels). A representative data set of 4 independent experiments is shown.
Association of Pak with p22phox. (A) Cos cells expressing functional flavocytochrome b were transfected with vector (lane 1), wild-type Myc-Pak1 (lane 2), constitutively active Myc-Pak1 F107 E423 (lane 3), dominant-negative Myc-Pak1 A299 (lane 4), Myc-Pak1 (aa 206-545) (lane 5), or Myc-Pak1 (aa 1-205) (lane 6). Anti-p22phox immunoprecipitates were prepared after 24 hours and probed with anti-Myc antibody for Pak detection (top panel). The middle and the bottom panels show Myc-Pak1 protein expression levels and p22phox protein in the IP, respectively. Representative of 3 independent experiments and verified twice in Cos cells stably expressing p22phox alone. Immunoblots of cell lysates are indicated by IB. (B) GST beads (lanes 1-2) or GST-Pak1 beads (lanes 3-4) were incubated with buffer (lanes 1,3) or purified flavocytochrome b (lanes 2,4). After washing, proteins bound to the beads were eluted and immunoblotted with anti-gp91phox or anti-p22phox antibodies. Control blots for the flavocytochrome b are shown in lane 5. Equal amounts of recombinant GST or GST-Pak1 bound to beads were verified by Coomassie blue staining (bottom panels). A representative data set of 4 independent experiments is shown.
Pak/p22phox binding is independent of p47phox association with flavocytochrome b558. (A) Cos cells expressing flavocytochrome b were cotransfected with vector (lane 1), Myc-Pak1 F107, E423 (lane 2), or Myc-Pak1 (aa 206-545) (lane 3), and wild-type p47phox (lanes 1-3). After 30 hours, the cells were treated with vehicle (dimethyl sulfoxide [DMSO]) or PMA (1 ng/mL) for 10 minutes before lysis. The top panel shows Myc-Pak1 in p22phox immunoprecipitates (IP) by probing with anti-Myc antibody. Myc-Pak1 and p47phox expression levels are shown in the middle and bottom panel (immunoblot IB). (B) Anti-p22phox immunoprecipitates were blotted and probed with anti-p47phox antibody after PMA stimulation or vehicle (DMSO) treatment. A representative data set of 3 independent experiments is shown.
Pak/p22phox binding is independent of p47phox association with flavocytochrome b558. (A) Cos cells expressing flavocytochrome b were cotransfected with vector (lane 1), Myc-Pak1 F107, E423 (lane 2), or Myc-Pak1 (aa 206-545) (lane 3), and wild-type p47phox (lanes 1-3). After 30 hours, the cells were treated with vehicle (dimethyl sulfoxide [DMSO]) or PMA (1 ng/mL) for 10 minutes before lysis. The top panel shows Myc-Pak1 in p22phox immunoprecipitates (IP) by probing with anti-Myc antibody. Myc-Pak1 and p47phox expression levels are shown in the middle and bottom panel (immunoblot IB). (B) Anti-p22phox immunoprecipitates were blotted and probed with anti-p47phox antibody after PMA stimulation or vehicle (DMSO) treatment. A representative data set of 3 independent experiments is shown.
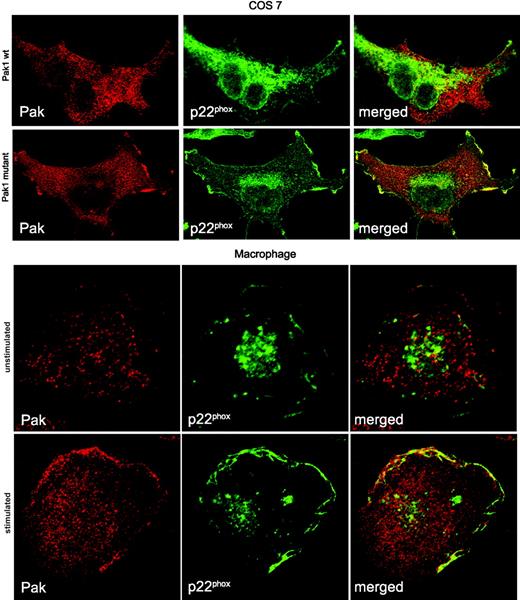
Active Pak colocalizes with p22phox in the plasma membrane. Cos cells expressing p22phox were transfected with wild-type Myc-Pak1 or Myc-Pak1 F107, E423 for 24 hours and prepared for confocal microscopy. Human monocytes were differentiated into macrophages in vitro and either left untreated or stimulated with 1 μM fMLF for 3 minutes. Localization of proteins in all panels was assessed by confocal microscopy after fixation and costaining for Pak and p22phox. A representative image of 2 independent experiments is shown.
Active Pak colocalizes with p22phox in the plasma membrane. Cos cells expressing p22phox were transfected with wild-type Myc-Pak1 or Myc-Pak1 F107, E423 for 24 hours and prepared for confocal microscopy. Human monocytes were differentiated into macrophages in vitro and either left untreated or stimulated with 1 μM fMLF for 3 minutes. Localization of proteins in all panels was assessed by confocal microscopy after fixation and costaining for Pak and p22phox. A representative image of 2 independent experiments is shown.
The active NADPH oxidase complex localizes in immune cells to the plasma membrane or phagolysosome. To establish the cellular compartment of the Pak/p22phox association, we initially determined the localization of wild-type Pak or constitutively active Pak in flavocytochrome b–expressing Cos cells. Wild-type Pak was widely dispersed over the cytoplasm, whereas p22phox was localized to endomembranes, vesicles, and some plasma membrane sites. Introduction of kinase-active Pak1 initiated the translocation of a pool of Pak1 to the plasma membrane, where active Pak colocalized with p22phox (Figure 7, top panel). Control experiments using p22phox-deficient NCI H292 cells clearly demonstrated that both wild-type and kinase-active Pak localize in unstimulated cells to the cytosol (data not shown). To assess the localization of Pak in primary, chemoattractant-stimulated cells, human monocytes were isolated and differentiated to macrophages in vitro. In fMLF-stimulated macrophages, a substantial increase of plasma membrane–bound p22phox was observed in areas resembling membrane ruffles (Figure 7, bottom panel). Concurrently, Pak translocated from the cytosol to the periphery, overlapping to a large extent with p22phox staining. The fMLF-stimulated colocalization of Pak with p22phox was most pronounced in well-spread, highly ruffling macrophages and resembled the colocalization of Pak with p47phox in fMLF-stimulated macrophage-like cells (Figure 4).
Discussion
Primary, terminally differentiated hematopoietic cells are exceedingly refractory to gene transfer. Neutrophils in particular have a very short life span and display negligible protein synthesis under quiescent conditions. To address this problem, methods for protein transduction into intact cells have recently been developed. More specifically, Dowdy and others demonstrated that recombinant proteins fused to a Tat leader sequence will enter the majority of mammalian cell types and retain their biologic functions upon intracellular refolding (Vocero-Akbani et al13 ; Ho et al19 ; and Schwarze et al20 ). Using the protein transduction domain of HIV-1 Tat as a carrier for recombinant proteins, we observed an uptake efficiency of almost 100% in myeloid cells. Therefore, this approach allows altering signaling pathways in neutrophils when specific pharmacologic inhibitors are not available. In this study, we used Tat-mediated entry of an inhibitory domain of Pak to investigate the role of Pak in regulating O2·- generation by the phagocyte NADPH oxidase.
We addressed the effects of Pak in the context of chemotactic receptor signaling stimulated by bacterially derived N-formyl peptide agonist (fMLF), an event where rapid and transient Pak activation coincides with a burst of O2·-. Inhibition of Pak kinase activity in human neutrophils reduced fMLF-stimulated O2·- generation substantially and in a dose-dependent fashion, while a Tat fusion protein with the inactive Pak inhibitory domain did not affect O2·- generation. Thus, these data demonstrate that Pak activation not only correlates with oxidase activation but is directly involved in regulation of this enzyme complex.
The critical role of Rac2 in regulation of the NADPH oxidase has been established in cell-free studies, using neutrophils from Rac2-null mice, and through a dominant-negative Rac2 mutation identified in a pediatric patient with recurrent bacterial infections.5 Interestingly, Rac2 T17N-Tat protein transduced into neutrophils did not abolish O2·- production as predicted. The 50% to 60% inhibition of the fMLF-induced oxidative burst by dominant-negative Rac2 N17-Tat may be explained by incomplete refolding of a fraction of the Tat proteins to their active conformation inside the cell, thereby altering the bioavailability of the protein. Immunofluorescence microscopy of transduced PID-Tat protein showed some degree of intracellular protein aggregation, a phenomenon that could decrease the amount of bioactive protein. Additionally, the propensity of some Tat fusion proteins to form aggregates in solution did not allow us to use PID-Tat protein above a concentration of 1 to 2 μM.
A role for Pak in O2·- generation was proposed by our initial studies in neutrophils, by studies using Rac effector domain mutants in a Cos cell model, and by partial purification of a protein kinase with similar biochemical features.7,35,36 One of several potential mechanisms to explain how Pak regulates O2·- production in neutrophils involves the phosphorylation of NADPH oxidase components. While we did not observe Pak-mediated phosphorylation of p67phox or p22phox, we detected a high level of p47phox phosphorylation. The significance of p47phox phosphorylation in conformational changes of the protein, association with other oxidase components, and the overall output of O2·- is well documented, and multiple serines have been identified as phosphorylation sites in stimulated, intact neutrophils.37 Ago et al demonstrated in cell-free assay systems and in a model cell line that phosphorylation of serines 303, 304, and 328 is required for O2·- production.38 In the present study, we used in vitro phosphorylation and coexpression experiments in radiolabeled cells to identify serines 303, 304, 320, and 328 of p47phox as amino acids targeted for phosphorylation by Pak.
Several protein kinases have been implicated previously in the phosphorylation of p47phox, including protein kinase C isoforms, mitogen-activated protein (MAP) kinases, phosphatidic acid–activated kinase, and the phosphatidylinositol-3 kinase target Akt.39-43 In the present study, we conclusively show that Pak-mediated phosphorylation of p47phox occurs at multiple sites relevant for O2·- generation and contributes to the regulation of oxidase activity in intact cells. However, because of the multiple kinases involved in p47phox phosphorylation in neutrophils and the potential for some redundancy in this process, it was not possible to provide absolute confirmation of the essentiality of Pak-mediated phosphorylation of p47phox phosphorylation in oxidase activation in intact, fMLF-stimulated neutrophils. Indeed, it is clear that p47phox activation can be initiated by phosphorylation of only a subset of sites that is known to be phosphorylated in vivo, and removal of individual kinases or kinase targets does not necessarily eliminate p47phox function. Likewise, treatment alone with individual kinases in vitro can fully activate p47phox.41-43 Thus, it is plausible that the neutrophil uses multiple kinases, including Pak, to ensure rapid and efficient phosphorylation of p47phox, leading to rapid assembly of the oxidase. Furthermore, the essential role of Rac in oxidase activation and its demonstrated regulation of Pak strongly implicated Pak as a physiologically relevant mediator in the phosphorylation events.7,35 Combined with these previous studies, the data presented here provide further direct evidence for a key role of Pak in NADPH oxidase regulation in intact cells via its involvement in p47phox phosphorylation. While Tat transduction methodology resulted in a significant 40% to 50% reduction in oxidase activity, the low efficiency of neutrophil labeling with 32P and concomitant preactivation during this procedure did not allow us to detect subtle differences in p47phox phosphopeptides in stimulated neutrophils. Additionally, we were also not able to generate HL60 cell lines stably expressing PID, presumably due to the requirement of Pak activity for cell proliferation. Thus, future studies in neutrophils obtained from Pak isoform knock-out animals will be required to absolutely delineate the regulatory role of Pak in NADPH oxidase activation and its relation to the multiple kinases known to phosphorylate p47phox in vitro and in vivo.
Our studies suggest that Pak may actually play multiple roles in the regulation of the phagocyte oxidase. It is likely that Rac activation and movement of active Rac to the plasma membrane stimulate Pak activity and possibly also affect its localization. Notably, a direct association of catalytically active Pak with p22phox at the plasma membrane was observed in this study. Stimulus-dependent translocation of Pak and association with flavocytochrome b558 may either precede p47phox translocation leading to additional phosphorylation at the membrane or may occur simultaneously. In light of structural data33 indicating that certain phosphorylations are required for p47phox association with p22phox, Pak-mediated p47phox phosphorylations and translocation of both proteins are likely part of a rapid, highly synchronized process. One can envision the presence of a large multimeric signaling complex on membranes of activated neutrophils, where flavocytochrome b558 and potentially Rac2 serve as the anchor, and proteins are held together by multiple protein-protein interactions. Additionally, Paks may regulate the NADPH oxidase at several levels, including phosphorylation of oxidase components, glycolytic enzymes, and structural or regulatory elements of the cytoskeleton, as well as by association with protein binding partners in specific membrane compartments, where formation of signaling networks occurs.
Prepublished online as Blood First Edition Paper, August 11, 2005; DOI 10.1182/blood-2005-03-0859.
K.D.M. and M.-J.K. contributed equally to this work.
K.D.M. and M.-J.K. performed research; M.T.Q. and M.C.D. contributed essential reagents and revised the article; U.G.K. designed and performed research, analyzed data, and wrote the article.
Supported by National Institutes of Health grants AI35947 (U.G.K.), AR42426 (M.T.Q.), HL45635 (M.C.D.), T32 DK07022 (M.-J.K.), and M01 RR00833 (to the General Clinical Research Center at The Scripps Research Institute).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Steve Dowdy for reagents and helpful discussions. We are grateful to Wolfgang Fischer for HPLC peptide sequence analysis and Charles King for MALDI-MS analysis. We also thank Abina Mulcahy and Monica Valo for technical assistance, Becky Diebold and Jonathan Chernoff for technical advice, and Katrina Schreiber for administrative assistance.

![Figure 3. Phosphorylation of p47phox by p21-activated kinase (Pak). (A) Peptides derived from the C-terminus of human p47phox were incubated with recombinant GST-Pak1 and [32P]ATP, as described. The numbers under the bars indicate the location of the peptides in p47phox (amino acids). Results are depicted as the mean ± SD of cpm measurement on 4 different fields per peptide. (B) In vitro kinase assays were performed as described using GST-Pak1 alone (lane 1), recombinant wild-type GST-p47phox (lane 2), or recombinant GST-p47phox quadruple mutant S303/304/320/328A (lane 3). (C) 2-D map of phosphopeptides derived from trypsin-digested GST-p47phox (left) or GST-p47phox S303/304/320/328A (right) after phosphorylation by GST-Pak1. The original (ori) application spot of the sample is indicated by an arrow.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/12/10.1182_blood-2005-03-0859/2/m_zh80240587500003.jpeg?Expires=1765935129&Signature=Y5GMI2P6DoXEK9qRMpMjqnyicm4WfCdlGHrWrt6LUC0wW2QnIRtNGaesx0bY8v2i987UVxerMiIAtvVbNmwz9ETSa2hWwsaAmU1ZgYnKSrLt4W96mDgNTJVZQAmZkKnzQUkuRcxEGC46frAiA-l2UU7w3f9pRNq2wujMA3FdSz-x9H9dMrTY2sniccmr-Q4DjqUpO2IkAibQdqYQIH-EdYTw-9JQGhN~3O16MjQpOlkZ8vMVzOUycxXptv9TYHVvTzHaF7WKgHMPZ~Ifqs3OXgN0KIN0-lgUHOuVEDlzkKY8I0omrLPTwLku9laCWreOk1KcLSZ4dAsz8x9XldwXEA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Pak/p22phox binding is independent of p47phox association with flavocytochrome b558. (A) Cos cells expressing flavocytochrome b were cotransfected with vector (lane 1), Myc-Pak1 F107, E423 (lane 2), or Myc-Pak1 (aa 206-545) (lane 3), and wild-type p47phox (lanes 1-3). After 30 hours, the cells were treated with vehicle (dimethyl sulfoxide [DMSO]) or PMA (1 ng/mL) for 10 minutes before lysis. The top panel shows Myc-Pak1 in p22phox immunoprecipitates (IP) by probing with anti-Myc antibody. Myc-Pak1 and p47phox expression levels are shown in the middle and bottom panel (immunoblot IB). (B) Anti-p22phox immunoprecipitates were blotted and probed with anti-p47phox antibody after PMA stimulation or vehicle (DMSO) treatment. A representative data set of 3 independent experiments is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/12/10.1182_blood-2005-03-0859/2/m_zh80240587500006.jpeg?Expires=1765935129&Signature=YRk62O2UoXKQ3ycw38sLEuPpDRhWZ5b7YQ4IZODeDaeWr6Np4brrTby5T0MQOEmWoAhKH5zvxOJwyHMurZzMQOlH7uulxW94gb9XiFLDafbh-IleDl51KC8idUTuDau2QNlsPThLmcZJlpo~4K7ddT4mjX~GzV6VEqYrXq1GoPm~0i6J8MP4Qr8w~0~Mt6kD~BQUgpzvfZ9UypVp7osY3NJ9zDp4XuRCPqZt3BsbT~sMhVaEEiGge20HhKYSKYDKrcSxSc6mbSWX-t7f8qXbrPTLdthEnSWj8cQjk4tYYZ1RqNCPUtSvU3KwPbqm2Zuda3YdSqZzI3980c81dqpoOg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal