Peripheral-blood mononuclear cells (PBMCs) mobilized with AMD3100, a CXCR4 antagonist, combined with granulocyte colony-stimulating factor (G-CSF) have reconstituted autologous hematopoiesis in cancer patients following myeloablative conditioning. The engraftment potential of PBMCs mobilized with AMD3100 alone, however, has remained unproven. We therefore studied AMD3100-mobilized PBMCs in a canine model. Four dogs received 920 cGy total body irradiation (TBI) before infusion of autologous AMD3100-mobilized PBMCs (median CD34 cell count, 3.9 × 106/kg). Neutrophil (> 0.5 × 109/L [500/μL]) and platelet (> 20 ×/109/L [> 20 000/μL]) recoveries occurred at medians of 9 (range, 7-10) days and 25 (range, 23-38) days, respectively, after TBI, and all dogs had normal marrow function at 1 year after transplantation. To evaluate the long-term engraftment potential of AMD3100-mobilized PBMCs, 5 dogs were given 920 cGy TBI followed by infusion of AMD3100-mobilized PBMCs (median CD34 cell dose, 4.7 × 106/kg) from their dog leukocyte antigen (DLA)-identical littermates. Neutrophil and platelet recoveries occurred at medians of 8 (range, 8-10) days and 26 (range, 26-37) days, respectively, after TBI. With a median follow-up of 53 (range, 33-61) weeks, recipients' marrow function was normal, and blood-donor chimerism levels were 97% to 100%. In summary, both autologous and allogeneic AMD3100-mobilized PBMCs led to prompt and durable engraftment in dogs after 920 cGy TBI.
Introduction
Under steady-state conditions, CD34+ hematopoietic progenitor and stem cells circulate in the blood of animals and humans at frequencies too low to allow for efficient collection of numbers sufficient to ensure timely hematopoietic reconstitution after myeloablative therapy. The frequencies of CD34+ cells in the blood are considerably increased both in response to various growth factors and during the recovery phase following myelosuppressive chemotherapy. Granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral-blood mononuclear cells (PBMCs) are routinely used as a source of hematopoietic stem cells for transplantation and, owing to the earlier neutrophil and platelet engraftments and shortened hospital stay associated with the use of this product, are often preferred over bone marrow.1-8 In the setting of autologous hematopoietic cell transplantation (HCT), difficulties in mobilizing and harvesting sufficiently large numbers of hematopoietic stem cells may occur, particularly in patients who have been exposed to repetitive cycles of myelotoxic chemo- and/or radiation therapy.9 Alternative strategies aimed at improving CD34+ cell mobilization including use of novel growth factors or growth factor combinations have been explored in clinical studies.10-15 With respect to efficacy and/or safety of the mobilization procedure, however, none of these strategies has proven superior to G-CSF used alone or in combination with chemotherapy.
Recently, several studies have demonstrated the importance of the interaction between stromal-cell-derived factor-1α (SDF-1α) and its receptor, CXCR4, in stem-cell mobilization and engraftment.16-22 SDF-1α, a member of the CXC-chemokine family, is expressed on bone marrow stromal cells and is present as a soluble cytokine in the marrow microenvironment. Its receptor, CXCR4, is expressed on many different types of hematopoietic cells including megakaryocytes and CD34+ cells.23-25 The bicyclam AMD3100 is a potent, selective, and reversible antagonist of the CXCR4 chemokine receptor and disrupts the binding of CXCR4 to SDF-1, thereby mobilizing hematopoietic cells into the blood.26
Studies involving animals and humans have shown that AMD3100 treatment resulted in mobilization of hematopoietic progenitor cells27-31 and that combining AMD3100 with G-CSF had additive effects.32 A more recent study demonstrated that PBMCs mobilized with a combination of AMD3100 and G-CSF stably reconstituted autologous hematopoiesis in patients with cancer following myeloablative conditioning.33 The long-term engraftment potential of PBMCs mobilized with AMD3100 alone, however, has remained unproven. In theory, even though yields of CD34+ cells and clonogenic progenitors appear to be similar with AMD3100 compared with G-CSF mobilization, their quality could differ, and exclusive use of AMD3100-mobilized PBMCs could be associated with eventual graft failure.
We therefore studied the short- and long-term engraftment potentials of both autologous and allogeneic AMD3100-mobilized PBMCs in a canine model. In a 3-step approach, we first evaluated the mobilization kinetics of CD34+ and clonogenic progenitor cells in dogs given a single dose of AMD3100. This information was then used to determine the optimal time period for collecting mobilized cells by leukapheresis. We next investigated whether transplantation of autologous, and then allogeneic, dog leukocyte antigen (DLA)-identical AMD3100-mobilized PBMCs would lead to timely and sustained engraftment in dogs following a myeloablative dose of 920 cGy total body irradiation (TBI).
Materials and methods
Laboratory animals
The Institutional Animal Care and Use Committee at the Fred Hutchinson Cancer Research Center (FHCRC), which is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, approved this study. Elevated enclosed runs were used for housing, and dogs were maintained in social groups wherever possible. All dogs were enrolled in a veterinary preventive medicine program that included routine anthelmintics and a standard immunization series against canine distemper, parvovirus, adenovirus type 2, parainfluenza virus, coronavirus, and rabies. In addition, 2 intradermal doses of a custom-prepared formalin-inactivated canine papilloma virus vaccine were administered. Four dogs were selected for the mobilization experiments, 4 dogs underwent autologous transplantation, and 5 DLA-identical littermate pairs were used for allogeneic transplants. DLA-identical donor and recipient pairs were selected by matching for highly polymorphic major histocompatibility complex (MHC)-associated classes I and II microsatellite markers34,35 ; in addition, specific DLA-DRB1 allele identity was confirmed by direct sequencing.36 At the time of the experiments, the dogs had a median weight of 10 (range, 7.2-16.1) kg and were a median 9.6 (range, 6.1-85.7) months old.
Investigational drug
AMD3100 was supplied as a solution of 50 mg/mL per 1 mL glass ampule. The study drug was stored at -20°C. Upon use in the experiments, it was thawed and subsequently stored at 4°C. AMD3100 was administered at 4 mg/kg as a single subcutaneous injection without premedication. After injection of AMD3100, dogs were closely monitored for 6 hours for the occurrence of adverse events.
Monoclonal antibodies
For flow cytometry, monoclonal antibodies against canine CD34 (1H6, immunoglobulin G1 [IgG1]),37 CD3 (CA17.6F9, IgG2b),38 CD4 (CA13.1.E4, IgG1),39 CD8 (CA9.JD3, IgG2a),39 and CD14 (TUK4, IgG2a; purchased from DaKo, Carpinteria, CA) were used. The monoclonal antibodies were either unconjugated or conjugated to biotin, fluorescein isothiocyanate (FITC), or phycoerythrin (PE). Isotype-matched control antibodies were used in parallel as negative controls. Dr Peter Moore (School of Veterinary Medicine, University of California, Davis) kindly provided antibodies to CD3.
Flow cytometry
For the mobilization experiments, 4 dogs received single subcutaneous injections of AMD3100 (4 mg/kg) followed by collection of heparinized peripheral-blood samples before and then 1, 2, 4, 6, 8, 10, and 24 hours after AMD3100 injection. For the autologous and allogeneic transplants, heparinized peripheral-blood samples were collected before and at 4 and/or 6 hours after AMD3100 injection, and from the leukapheresis products. Flow cytometry was carried out as previously described.40 Both the flow cytometry and colony-forming unit assay results were used to determine the optimal time for leukapheresis.
Colony-forming unit assay
Peripheral blood was obtained for the colony-forming unit (CFU) assay at the various time points described under “Flow cytometry.” CFU assays were carried out as previously described.41
Pharmacokinetics
Peripheral blood was obtained for the pharmacokinetics studies at the various time points described under “Flow cytometry.” AMD3100 levels in dog plasma were determined by liquid-chromatography/mass spectrometry following liquid-liquid extraction. Briefly, plasma samples were extracted with methyl t-butyl either following pH-adjustment and addition of internal standard, followed by back extraction into 0.5% trifluoroacetic acid (TFA) in water. Reverse-phase gradient chromatography was performed using a C8-column and a mobile-phase containing 0.1% TFA in water and acetonitrile. AMD3100 was quantitated by electrospray ionization (ESI) mass spectrometry using AMD3100 calibrators (0.01 to 15 μg/mL) that were prepared in a manner identical to that used for samples. Noncompartmental pharmacokinetic parameters were calculated using WinNonlin V4.01 (Pharsight, Mountain View, CA).
Studies of CXCR4 expression
PBMCs were isolated by centrifugation on Ficoll-Hypaque from blood obtained 6 hours after AMD3100 administration and cultured in Iscoves modified Dulbecco medium, 10% fetal bovine serum (FBS), 55 μM β-mercaptoethanol, and 1% vol/vol phytohemagglutinin (PHA) at 37°C, 5% CO2 for 3 days. The cells were transferred to fresh medium supplemented with 2 ng/mL interleukin-2 for an additional 10 days. CXCR4 expression was measured by flow cytometry using the FITC-labeled anti-CXCR4 antibody, 12G5. For the SDF-1α ligand binding assay, cells were incubated in phosphate-buffered saline containing 5 mM MgCl2, 1 mM CaCl2, and 0.25% bovine serum albumin (BSA, pH 7.4) for 3 hours at 4°C with 100 pM 125I-SDF-1α. Unbound ligand was removed by filtration and bound 125I-SDF-1α was measured by liquid scintillation counting. Competition studies were performed with either cold SDF-1 or AMD3100. For calcium flux measurements, the cells were loaded with the calcium-indicator, Fluo-4/AM (1 μM). Changes in intracellular calcium concentration upon SDF-1α addition were monitored using the FLEXstation (Molecular Devices, Sunnyvale, CA) at 525 nm (excitation = 485 nm). For inhibition studies, the cells were preincubated with AMD3100 for 15 minutes at 37°C and calcium flux was initiated by addition of 10 nM SDF-1α.
Hematopoietic-cell transplantation
Dogs were given single subcutaneous injections of AMD3100 (4 mg/kg; 1 DLA-identical donor [G423] inadvertently received 6.5 mg/kg) followed by 3- to 4-hour leukapheresis using the COBE Spectra, version 6 cell separator (Gambro, Lakewood, CO), initiated 6 to 7 hours after AMD3100 administration. After completion of the leukapheresis, recipients received myeloablative TBI with 920 cGy, delivered at 7 cGy/min from a high-energy linear accelerator (Varian CLINAC 4, Palo Alto, CA),42 which was followed by infusion of the autologous or allogeneic AMD3100-mobilized leukapheresis products. Day 0 was designated as the day of TBI and hematopoietic stem cell grafting. Recipients of allogeneic grafts received cyclosporine (5 mg/kg, orally twice daily) from days - 1 to + 35 in order to prevent graft-versus-host disease (GVHD).43
For autologous and allogeneic recipients, engraftment was monitored by peripheral-blood counts and donor chimerism (allogeneic recipients only). Neutrophil recovery after the postradiation nadir was defined as the first of 3 consecutive days with a neutrophil count exceeding 0.5 × 109/L (500 cells/μL). Platelet recovery was defined as the first of 5 consecutive days with an unsupported platelet count higher than 20 × 109/L (20 000 μL). For allogeneic recipients, hematopoietic engraftment was assessed by chimerism analyses of peripheral blood and bone marrow granulocyte and mononuclear cells. Chimerism was assessed by fluorescent variable number tandem repeat (VNTR) assays using ABI Prism 310 Genetic Analyzer and Gene Scan 3.1 Software (Applied BioSystems, Foster City, CA). Probes for canine microsatellite polymorphisms were used that distinguished donor from recipient cells.44,45 Chimerism analysis was performed by Dr Michael Harkey (supported by core National Institutes of Health [NIH] grant DK56465).
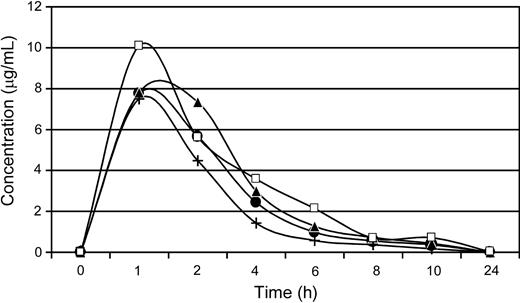
Plasma concentrations of AMD3100 in dogs after a single subcutaneous administration of 4 mg/kg. E343 (•), G035 (▴), G272 (+), and G105 (□) depict dog identification numbers.
Plasma concentrations of AMD3100 in dogs after a single subcutaneous administration of 4 mg/kg. E343 (•), G035 (▴), G272 (+), and G105 (□) depict dog identification numbers.
Immune reconstitution
In vitro immune functions were measured by standard assays of lymphocyte proliferation, including the mixed lymphocyte culture and concanavalin A (ConA, 1 μg/mL) evaluation as previously described.46 In addition, peripheral-blood absolute lymphocyte subsets (CD4 and CD8) were assessed using flow cytometry as described under “Flow cytometry.”
Results
Pharmacokinetics
For the 4 dogs given single subcutaneous injections of AMD3100 (4 mg/kg), concentration-time curves demonstrated a concentration peak within 1 hour, and clearance within 24 hours after drug administration (Figure 1). Maximal plasma concentrations ranged from approximately 7.8 to 10.3 μg/mL, with a low degree of variability in the pharmacokinetics of AMD3100 observed between dogs.
Peripheral blood cell counts of dogs (n = 4) following a single subcutaneous dose of AMD3100 (4 mg/kg). (A) White blood cell counts; (B) absolute neutrophil counts; (C) CD34+ cell counts; (D) colony-forming unit (CFU) counts. E343 (•), G035 (▴), G272 (+), and G105 (□) depict dog identification numbers.
Peripheral blood cell counts of dogs (n = 4) following a single subcutaneous dose of AMD3100 (4 mg/kg). (A) White blood cell counts; (B) absolute neutrophil counts; (C) CD34+ cell counts; (D) colony-forming unit (CFU) counts. E343 (•), G035 (▴), G272 (+), and G105 (□) depict dog identification numbers.
CXCR4 is expressed on canine PBMCs
In addition to flow cytometry, CXCR4 expression was confirmed by homologous competition SDF-1α ligand binding, which showed a dissociation constant (Kd) of 109 nM. Furthermore, blood samples from 6 dogs were evaluated in calcium-flux studies. Four of the 6 dogs tested showed a positive calcium flux response upon stimulation with human SDF-1α (average effective concentration [EC50] of 4.1 ± 1.9 nM ± SE), which confirmed signaling through CXCR4. SDF-1α-stimulated calcium flux was inhibited by the CXCR4-specific inhibitor, AMD3100, with an average median inhibitory concentration (IC50) of 836.4 ± 404.4 nM (n = 3).
AMD3100 mobilizes leukocytes, CD34+ cells, and clonogeneic progenitors
A single administration of AMD3100 (4 mg/kg) uniformly caused leukocytosis with peak increases in total white blood cell and absolute neutrophil counts occurring 8 to 10 hours after administration for the majority of the dogs (Figure 2). Lymphocyte and monocyte counts also increased by medians of 1.5- and 4-fold, respectively (not shown). There were no significant changes in the hematocrits or platelet counts. In addition, we found a 3- to 10-fold and 2- to 4-fold increase in the numbers of circulating CD34+ and clonogeneic progenitor cells, respectively, with peaks occurring between 8 to 10 hours following AMD3100 administration for most dogs. One dog (E343) had an early peak of circulating CD34+ cells at 2 hours (Figure 2). All dogs tolerated AMD3100 without any appreciable short-term or long-term adverse effects. Based on these results, a 3- to 4-hour leukapheresis initiated 6 to 7 hours after AMD3100 administration was considered to be optimal with respect to maximizing the yield of CD34+- and progenitor-cell collections.
Myeloablative conditioning followed by transplantation of AMD3100-mobilized PBMCs resulted in timely and durable engraftment: autografts
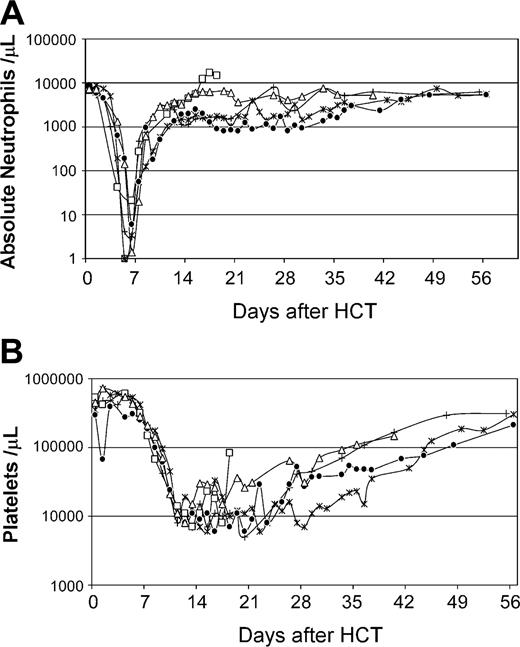
For recipients of autologous AMD3100-mobilized PBMCs (n = 4), the median doses per kilogram recipient weight of total nucleated cells and CD34+ cells were 14.4 (range, 4-23) × 108 and 3.9 (range, 2.0-8.7) × 106, respectively (Table 1). Neutrophil and platelet recoveries occurred at medians of 9 (range, 7-10) days and 25 (range, 23-38) days, respectively, after HCT (Figure 3). Bone marrow aspirates obtained between days 21 and 28 after transplantation demonstrated moderate (n = 1), hypo (n = 2), or normal cellularity (n = 1) with megakaryopoiesis between 50% to 80% of normal. At the time of death 1 year after HCT, all 4 dogs had normal marrow morphology and cellularity, and normal peripheral-blood counts.
Cellular composition of leukapheresis products and engraftment data from dogs given 920 cGy TBI followed by transplantation of either autologous or allogeneic AMD3100-mobilized PBMCs
Dog no. . | TNC, × 108/kg . | CD34, × 106/kg . | CD3, × 108/kg . | CD4, × 108/kg . | CD8, × 108/kg . | CD14, × 108/kg . | Engraftment . | Clinical GVHD . | Survival, d . | Cause of death . |
|---|---|---|---|---|---|---|---|---|---|---|
| Autologous HCT | ||||||||||
| G275 | 15.6 | 3.3 | 2.6 | 1.6 | 0.6 | 4.3 | Yes | NA | 365 | End of study |
| G248 | 13.2 | 4.5 | 2.7 | 1.6 | 0.8 | 2.5 | Yes | NA | 365 | End of study |
| G294 | 23.0 | 8.7 | 0.6 | 0.4 | 0.1 | 4.2 | Yes | NA | 365 | End of study |
| G315 | 4.0 | 2.0 | NE | NE | NE | 0.3 | Yes | NA | 365 | End of study |
| DLA-identical HCT | ||||||||||
| G383 | 8.0 | 2.1 | 1.4 | 0.7 | 0.5 | 0.6 | Yes* | None | 430† | Alive |
| G378 | 10.7 | 6.1 | 4.4 | 2.2 | 1.3 | 2.6 | Yes* | None | 414† | Alive |
| G422 | 12.1 | 4.7 | 3.6 | 2.1 | 1.3 | 2.7 | Yes* | ? Acute S, G | 18‡ | Pancreatitis, canine HSV |
| G375 | 4.5 | 1.5 | 2.3 | 1.2 | 0.6 | 1.0 | Yes* | None | 324† | Alive |
| G455 | 31.4 | 8.2 | 0.5 | 0.4 | 0.1 | 2.5 | Yes* | Chronic S | 233† | Alive |
Dog no. . | TNC, × 108/kg . | CD34, × 106/kg . | CD3, × 108/kg . | CD4, × 108/kg . | CD8, × 108/kg . | CD14, × 108/kg . | Engraftment . | Clinical GVHD . | Survival, d . | Cause of death . |
|---|---|---|---|---|---|---|---|---|---|---|
| Autologous HCT | ||||||||||
| G275 | 15.6 | 3.3 | 2.6 | 1.6 | 0.6 | 4.3 | Yes | NA | 365 | End of study |
| G248 | 13.2 | 4.5 | 2.7 | 1.6 | 0.8 | 2.5 | Yes | NA | 365 | End of study |
| G294 | 23.0 | 8.7 | 0.6 | 0.4 | 0.1 | 4.2 | Yes | NA | 365 | End of study |
| G315 | 4.0 | 2.0 | NE | NE | NE | 0.3 | Yes | NA | 365 | End of study |
| DLA-identical HCT | ||||||||||
| G383 | 8.0 | 2.1 | 1.4 | 0.7 | 0.5 | 0.6 | Yes* | None | 430† | Alive |
| G378 | 10.7 | 6.1 | 4.4 | 2.2 | 1.3 | 2.6 | Yes* | None | 414† | Alive |
| G422 | 12.1 | 4.7 | 3.6 | 2.1 | 1.3 | 2.7 | Yes* | ? Acute S, G | 18‡ | Pancreatitis, canine HSV |
| G375 | 4.5 | 1.5 | 2.3 | 1.2 | 0.6 | 1.0 | Yes* | None | 324† | Alive |
| G455 | 31.4 | 8.2 | 0.5 | 0.4 | 0.1 | 2.5 | Yes* | Chronic S | 233† | Alive |
TNC indicates total nucleated cell; NA, not applicable; NE, not evaluable; ?, possible; S, skin GVHD; G, gut GVHD; and HSV, herpes simplex virus.
All animals tested had engraftment of donor origin demonstrated by VNTR
Alive
Dead
All dogs in the autologous transplant group tolerated AMD3100 without adverse effects. Two of the 4 dogs undergoing transplantation developed neutropenic fevers after HCT and were given empiric antibiotics. One dog was diagnosed with a soft tissue infection of the forearm 7 days after transplantation, which promptly responded to antibiotics. None of the dogs had hemorrhagic complications. Three of the 4 dogs were given prophylactic whole-blood transfusions at the time of their scheduled bone marrow aspirates and biopsies. The median weight loss was 15% (range, 10%-18%) during the first month after autologous HCT.
Recovery of peripheral blood cell counts of dogs (n = 4) given 920 cGy TBI and infusions of autologous AMD3100-mobilized PBMCs. (A) Absolute neutrophil counts; (B) platelet counts. G275 (X), G248 (⋄), G294 (□), and G315 (▪) depict dog identification numbers.
Recovery of peripheral blood cell counts of dogs (n = 4) given 920 cGy TBI and infusions of autologous AMD3100-mobilized PBMCs. (A) Absolute neutrophil counts; (B) platelet counts. G275 (X), G248 (⋄), G294 (□), and G315 (▪) depict dog identification numbers.
Myeloablative conditioning followed by transplantation of AMD3100-mobilized PBMCs resulted in timely and durable engraftment: allografts
Donors. The median doses of total nucleated cells and CD34+ cells infused per kilogram recipient weight were 10.7 (range, 4.5-31.4) × 108 and 4.7 (range, 1.5-8.2) × 106, respectively. The cellular composition of AMD3100-mobilized leukapheresis products is summarized in Table 1. All donor dogs in the allogeneic transplant group tolerated AMD3100 without adverse effects.
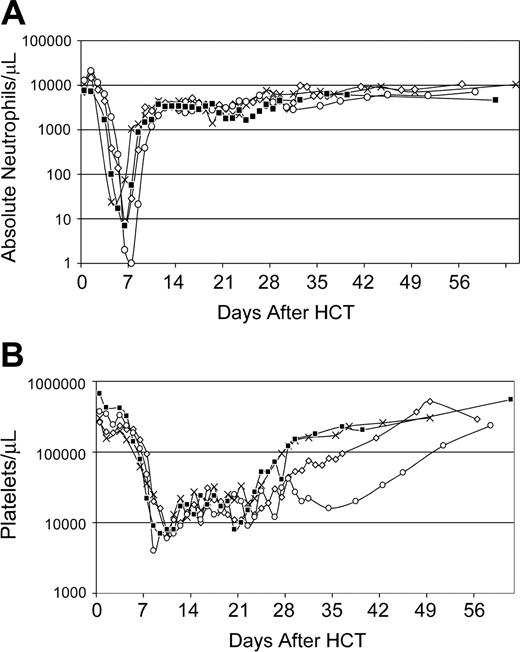
Recipients. Five dogs were given myeloablative conditioning with 920 cGy TBI followed by infusion of AMD3100-mobilized PBMCs from DLA-identical littermates. Postgrafting cyclosporine (5 mg/kg orally, twice daily) was given from days - 1 to + 35. Neutrophil and platelet recoveries occurred at medians of 8 (range, 8-10) days and 26 (range, 21-37) days, respectively, after HCT (Figure 4). Dog G422 was humanely killed on day 18 after transplantation. At the time of death, neutrophil counts were 14.72 × 109/L (14 720 cells/μL), platelet counts were below 20 × 109/L (20 000/μL), and donor peripheral blood mononuclear and granulocyte chimerism levels were 89% and 99%, respectively. With a median follow-up among the 4 living recipients of 53 (range, 33-61) weeks, donor peripheral-blood mononuclear-cell chimerism levels were 97% to 100% in all 4 dogs (Figure 5). In addition, analyses of sorted peripheral-blood CD3+ T cells and granulocytes demonstrated 100% donor chimerism levels in all 4 dogs at a median of 37 (range, 17-45) weeks after transplantation. In 3 of the 4 dogs tested (G375 was unevaluable), bone marrow mononuclear cell and granulocyte chimerism levels, and lymph node chimerism levels were 99% or more donor at a median of 37 (range, 17-45) weeks after transplantation.
Recovery of peripheral blood cell counts of dogs (n = 5) given 920 cGy TBI and infusions of allogeneic AMD3100-mobilized PBMCs. (A) Absolute neutrophil counts; (B) platelet counts. G383 (*), G378 (+), G422 (□), G375 (○), and G455 (⋄) depict dog identification numbers. G422's day-18 platelet count reflects the effect of transfusions.
Recovery of peripheral blood cell counts of dogs (n = 5) given 920 cGy TBI and infusions of allogeneic AMD3100-mobilized PBMCs. (A) Absolute neutrophil counts; (B) platelet counts. G383 (*), G378 (+), G422 (□), G375 (○), and G455 (⋄) depict dog identification numbers. G422's day-18 platelet count reflects the effect of transfusions.
Percent donor peripheral-blood mononuclear-cell chimerism in 5 dogs given 920 cGy TBI and infusions of allogeneic AMD3100-mobilized PBMCs. G383 (*), G378 (+), G422 (□), G375 (•), and G455 (▵) depict dog identification numbers.
Percent donor peripheral-blood mononuclear-cell chimerism in 5 dogs given 920 cGy TBI and infusions of allogeneic AMD3100-mobilized PBMCs. G383 (*), G378 (+), G422 (□), G375 (•), and G455 (▵) depict dog identification numbers.
Following allogeneic HCT, 2 of the 5 dogs developed neutropenic fevers requiring treatment with empiric antibiotics. Another dog was given antibiotics for a superficial skin infection that developed during neutropenia. The clinically suspected diagnosis of pancreatitis in dog G422, who was humanely killed on day 18, was subsequently confirmed by necropsy. Cultures of the dog's pancreas, kidneys, and duodenum were positive for canine herpes simplex virus. In addition, there was evidence of possible skin and gut GVHD on autopsy. However, a clear distinction between gut GVHD and viral infection was difficult to make. All dogs required between 1 and 2 whole-blood transfusions after transplantation, which were indicated because of petechiae, skin hematoma, hematuria, and/or hematochezia in the setting of low platelet counts. Furthermore, 3 of the 5 dogs were given prophylactic whole-blood transfusions before their scheduled bone marrow aspirates and biopsies. The median weight loss was 17% (range, 13%-31%) during the first month after allogeneic HCT. With a median follow-up of 53 (range, 33-61) weeks, one dog (G455) developed biopsy-proven skin GVHD, 4 months after HCT, and was started on cyclosporine (5 mg orally twice daily), which resulted in prompt resolution of symptoms.
Immune reconstitution was assessed for the living recipients at time of last follow-up. Specifically, absolute lymphocyte-cell subsets and T-cell reactivity in the mixed lymphocyte culture reaction were tested. Results demonstrated normal CD4 to CD8 counts in peripheral blood (23%-31% and 9%-22%, respectively) compared with normal control dogs and recipients of marrow allografts.47 In addition, the mixed lymphocyte reaction stimulation indices to either third party (14.0-42.6) or to the mitogen concanavalin A (22.2-35.4) were similar to concurrently obtained values in PBMCs from a normal control dog (Table 2).
Mixed lymphocyte culture results
. | Stimulating cells* . | . | |
|---|---|---|---|
| Responding . | Allogeneic-3rd party . | Concanavalin A . | |
| G383 | 42.6 | 28.7 | |
| G378 | 19.0 | 22.2 | |
| G375 | 16.6 | 35.4 | |
| G455 | 14.0 | 34.6 | |
| G324, normal control† | Median, 9.4 (range, 3.0-16.9) | 39.3 | |
. | Stimulating cells* . | . | |
|---|---|---|---|
| Responding . | Allogeneic-3rd party . | Concanavalin A . | |
| G383 | 42.6 | 28.7 | |
| G378 | 19.0 | 22.2 | |
| G375 | 16.6 | 35.4 | |
| G455 | 14.0 | 34.6 | |
| G324, normal control† | Median, 9.4 (range, 3.0-16.9) | 39.3 | |
Irradiated with 2000 cGy; data shown are stimulating indices
Normal control dog responding to stimulating cells from the 4 AMD3100 recipients
Comparison of AMD3100 and G-CSF: cell composition of leukapheresis products
The median doses of total nucleated cells and CD34+ cells per kilogram recipient weight harvested from 9 dogs mobilized with AMD3100 (autologous, n = 4; allogeneic, n = 5) were 12.1 (range, 4.0-31.4) × 108 and 4.5 (range, 1.5-8.7) × 106, respectively. In comparison, median doses of total nucleated cells and CD34+ cells per kilogram recipient weight were 7.3 (range, 5.7-9.3) × 108 and 4.1 (range, 2.0-7.0) × 106, respectively, in 10 dogs that underwent PBMC collection following 5 days of G-CSF treatment (10 μg/kg per day given subcutaneously in 2 divided doses). Similar median numbers of AMD3100 or G-CSF-mobilized CD3 and CD14+ cells were found (data not shown).
Comparison of AMD3100 and G-CSF: kinetics of engraftment after 920 cGy TBI
Engraftment kinetics among 4 dogs given 920 cGy TBI followed by infusion of autologous AMD3100-mobilized PBMCs were compared with historic controls (n = 5) given G-CSF-mobilized PBMCs.48 The median times to neutrophil and platelet recoveries were 9 (range, 7-10) days and 25 (range, 23-38) days, respectively, with AMD3100-mobilized PBMCs versus 16 (range 14-23) days and 41 (range, 40-46) days, respectively, with G-CSF-mobilized PBMCs. There were 2 limitations of this comparison: (1) different leukapheresis procedures were used, and, importantly, (2) recipients of G-CSF-mobilized PBMCs received only 1 × 108 PBMCs/kg, whereas there was no limit in the number of AMD3100-mobilized PBMCs given to the autologous recipients (median total nucleated cells infused per kilogram recipient weight were 14.4 [range 4-23] × 108). Due to these limitations, statistical comparisons were not made.
Engraftment kinetics among 5 dogs given 920 cGy TBI followed by infusion of AMD3100-mobilized PBMCs from DLA-identical littermates were compared with historic controls (n = 5) given G-CSF-mobilized PBMCs (Sandmaier et al49 ; B.M.S., unpublished data, May-September 1994). The 5 DLA-identical littermate recipients in the AMD3100 group received cyclosporine for immunosuppression after transplantation, while the 5 dogs in the G-CSF group were not given postgrafting immunosuppression. The median total nucleated cells per kilogram recipient weight infused for the 5 dogs mobilized with AMD3100 were 10.7 (range, 4.5-31.4) × 108 compared with 32.6 (range, 19.2-52.5) × 108 for the 5 dogs mobilized with G-CSF. The median times to neutrophil and platelet recoveries were 8 (range, 8-10) days and 26 (range, 21-37) days, respectively, with AMD3100-mobilized PBMCs versus 8 (range, 8-9) days and 14 (range, 12-36) days, respectively, with G-CSF-mobilized PBMCs. Log-rank tests,50 which accounted for both the timing and the incidence of engraftment, did not reveal statistically significant differences in recoveries of neutrophils and platelets among the 2 groups of recipients (P = .32 and P = .13, respectively); however, larger numbers of dogs are needed to confirm this result.
Discussion
Similar to what has been shown in other animal models and in humans, our experiments demonstrated that a single dose of AMD3100 resulted in leukocytosis accompanied by mobilization of CD34+ and clonogenic progenitor cells. Pharmacokinetic experiments demonstrated a rapid peak and subsequent clearance of AMD3100 within 24 hours of injection. In addition, AMD3100 administration to dogs was tolerated without noticeable immediate or long-term adverse effects. Calcium flux studies confirmed that dog PBMCs express functional CXCR4, which could be inhibited by AMD3100.
The engraftment kinetics of granulocytes and platelets were indistinguishable between dogs conditioned with 920 cGy TBI followed by infusions of either autologous AMD3100-mobilized PBMCs or those from DLA-identical littermates. Median days to neutrophil and platelet recoveries were 9 and 25 days, respectively, for autologous dogs, and 8 and 26 days, respectively, for allogeneic dogs. Of importance, allograft recipients had 97% to 100% donor hematopoietic chimerism levels in myeloid and lymphoid compartments of blood at a median observation time of 53 (range, 33-61) weeks after HCT. These findings unequivocally demonstrated that AMD3100-mobilized PBMCs conferred long-term hematopoietic reconstitution. One of the 5 allograft recipients developed probable acute GVHD of skin and gut, and 1 dog developed chronic skin GVHD that promptly responded to immunosuppressive therapy.
When comparing mobilization in dogs given a single dose of AMD3100 with that of dogs given 5 days of G-CSF, we found similar median numbers of total nucleated cells, CD34+ cells, T cells, and monocytes during 3- to 4-hour leukaphereses after AMD3100 and during 2- to 3-hour leukaphereses after G-CSF. Furthermore, the engraftment kinetics of neutrophils and platelets in recipients given AMD3100-mobilized PBMCs from DLA-identical littermates were comparable with those among historic controls given G-CSF-mobilized PBMCs.49
In summary, a single injection of AMD3100 resulted in efficient mobilization of CD34+ and clonogeneic progenitor cells in dogs. The numbers of CD34+ cells, T cells, and monocytes collected by leukapheresis after AMD3100 mobilization were similar to cell yields obtained after G-CSF. Of importance, AMD3100-mobilized PBMCs resulted not only in timely but also durable hematopoietic reconstitution. The toxicity of the transplant procedure did not appear to be increased, which supports further investigation of AMD3100 in human studies.
Prepublished online as Blood First Edition Paper, August 16, 2005; DOI 10.1182/blood-2005-05-1937.
Supported in part by National Institutes of Health (NIH) grants CA78902, CA15704, HL36444, HL62923, DK56465, DK064715, and K12 CA076930. This study was also supported in part by research funding from AnorMED to R.S.
L.B. designed and performed AMD3100 experiments, analyzed data, and wrote the article. M.M. contributed to the experimental design, performed the G-CSF leukapheresis experiments, and edited the article. M.-T.L. contributed to the experimental design and helped with analysis of flow cytometry data. G.B. initiated collaboration and supplied AMD3100. R.M. performed pharmacokinetic studies. S.F. performed studies of CXCR4 expression. J.L. performed studies of CXCR4 expression. B.M.S. supplied unpublished G-CSF allogeneic transplant data. R.S. designed and supported research, provided oversight, and edited the paper.
Several of the authors (G.B., R.M., S.F., J.L.) are employed by a company (AnorMED, Inc.) whose product (AMD3100) was studied in the present work.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Gretchen Johnson, Erlinda B. Santos, Stacy Zellmer, Jennifer Baker, Patrice Stroup, Sam Shin, Carol Loretz, Serina Gisburne, John Gass, and Gloria Lau for their technical help in conducting the studies; Michele Spector, DVM, and the technicians of the animal health resources for their excellent care of and dedication to the dogs; George Sale, MD, of the Seattle Cancer Care Alliance Department of Pathology for analysis of bone marrow and autopsy histologies; Jim Evermann, MS, PhD, of the College of Veterinary Medicine, Washington State University, for viral identification; Wendy Leisenring, ScD, of the Fred Hutchinson Cancer Research Center, Department of Statistics for log-rank test; Michael Harkey, PhD, and Ludmilla Golubev for the VNTR analysis; Amgen for the generous supply of canine G-CSF; and Bonnie Larson, Helen Crawford, and Sue Carbonneau for help with article preparation.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal