In this issue of Blood, 2 companion articles by the Italiano lab give insight into the microtubule-mediated transport required for platelet production.
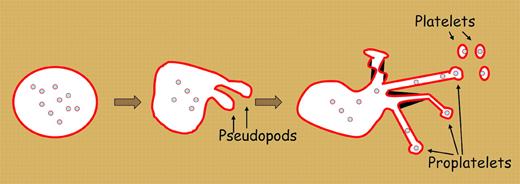
We are approaching the 100th anniversary of James Homer Wright's seminal study of feline bone marrow that led him to postulate that platelets are fragments of megakaryocytes.1 Despite this length of time, there is still controversy about the mechanism of platelet formation. There is some evidence to suggest that platelets are formed by fragmentation of the megakaryocyte cytoplasm. But several recent studies support the theory first proposed by Radley and Scurfield2 that platelet production begins with an elaborate dance of the megakaryocyte requiring the extension of thick pseudopods (see figure). These pseudopods extend further and further, and become quite thin, forming structures called proplatelets. Tablin et al3 showed that proplatelets were filled with a cellular supportive network called microtubules. Microtubules serve as a highway to transport materials and information between the center and the periphery of the cell. Microtubules are composed of interlocking tubulin that are slowly generated and rapidly disassembled in most cells. Poisons of the microtubular system, such as vinca alakaloids, impair proplatelet and platelet production. Mice lacking a microtubule building block, β1-tubulin, have reduced megakaryocyte proplatelets and produce only round platelets (but, curiously, still do make some platelets).4
Platelets derive from megakaryocyte proplatelets. Microtubules aggregate at one end of the cell cortex of a megakaryocyte as the cell extends pseudopods. As pseudopods extend further, they becomes thinner and ultimately branch and split into proplatelets. Platelet granules and organelles are transported along microtubules through the proplatelets into the nascent platelets. Ultimately, platelets release off the ends of the proplatelets.
Platelets derive from megakaryocyte proplatelets. Microtubules aggregate at one end of the cell cortex of a megakaryocyte as the cell extends pseudopods. As pseudopods extend further, they becomes thinner and ultimately branch and split into proplatelets. Platelet granules and organelles are transported along microtubules through the proplatelets into the nascent platelets. Ultimately, platelets release off the ends of the proplatelets.
Recent eloquent papers have made substantial inroads into our understanding of platelet production. Italiano and colleagues have shown through a series of experiments using ex vivo–cultured megakaryocytes that this process is initiated at one end of the megakaryocyte called the erosion pole, where microtubules consolidate into cortical bundles.5 These bundles slide along each other and ultimately form the coils of microtubules that are unique to proplatelets. The proplatelets then bend, split, and ultimately release budding platelets from their ends. This suggests that packets of platelet organelles need to be transported within proplatelets to deliver to the nascent platelets.
The article by Patel and colleagues has given a detailed description of how the cytoskeletal engine organizes microtubules to power proplatelet elongation. Since proplatelets can be several millimeters long, the megakaryocyte needs to assemble a relatively long microtubular system. Long microtubular highways could be generated by either elongation of microtubule polymers resulting from the assembly of additional tubulin or by the sliding of adjacent microtubules to make long networks composed of multiple microtubules. The work by Patel et al demonstrates that microtubule sliding is the predominant mechanism for elongation, while assembly plays a minor role.
The article by Richardson and colleagues addresses the mechanism used by megakaryocytes to pack granules and organelles into budding platelets. As expected, they found that these organelles move along the microtubules. Curiously, they found that the megakaryocyte microtubule highway is a 2-way street, allowing organelles to move (perhaps randomly) back and forth to the proplatelets. It is compelling that about a third of the organelles are in motion at any given time. Ultimately, the organelles are captured at the proplatelet ends. The need for this active process finally presents clear proof that platelets are not preassembled within the cytoplasm of megakaryocytes, but are assembled de novo at the ends of proplatelets. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal