Immunophenotyping disclosed CD10 negativity in 70 of 2408 cases of B-lineage acute lymphoblastic leukemia (ALL), although other criteria followed classification of pre-B ALL (eg, cytoplasmic immunoglobulin positivity). These blasts showed high myeloid antigen expression (60% CD65 positivity) and reacted with antibody 7.1 in 95% of the cases. MLL-AF4 fusion transcripts or an 11q23/MLL rearrangement or both were evident in 46 of 56 samples (82%). Although 83% of the patients achieved complete remission, the remission duration remained remarkably low: 141 days for MLL rearrangement-positive and 245 days for MLL rearrangement-negative CD10- pre-B ALL. Thus, the overall survival probability 3 years after diagnosis was 0.34 ± 0.20 SE in MLL-rearrangement-negative versus 0.12 ± 0.06 SE in MLL rearrangement-positive CD10- pre-B ALL. Our data identify CD10- cytoplasmic immunoglobulin-positive pre-B ALL as a rare (2.2%) but distinct immuno-subtype of adult ALL that is characterized by a high MLL rearrangement rate and a worse outcome.
Introduction
Immunologic subtyping and molecular genetic analysis allow risk-adapted treatment of acute lymphoblastic leukemia (ALL). One poor-prognosis subgroup of adult B-lineage precursor ALL is characterized by translocations involving the mixed lineage leukemia (MLL) gene on chromosome 11q23. These translocations occur in approximately 3% to 6% of all adult ALL and correlate with a younger age, a higher leukocyte count, and a CD19+/CD10-/cytoplasmic(cy)IgM- immunophenotype (pro-B ALL) coexpressing myeloid marker.1-3
Recent reports have shown that 11q23 translocations occur in up to 8% of T-ALLs, which were characterized by a poor clinical outcome.4,5 Here, we present evidence of another high-risk group with frequent MLL gene translocations involving 82% (46 or 56) of the distinct CD19+/CD10-/cyIgM+ pre-B ALL subset.
Study design
Dual-staining immunophenotyping of 3168 newly diagnosed ALL specimens was performed with commercially available fluorochrome monoclonal antibody conjugates (Dako, Hamburg, Germany; BD Biosciences, San Jose, CA; Beckman-Coulter, Fullerton, CA) identifying pro-B ALL, TdT+/CD19+/CD10-/cyIgM-/surface(S)Ig-; c-ALL, TdT+/CD19+/CD10+/cyIgM-/SIg-; and pre-B ALL, TdT+/CD19+/CD10+/cyIgM+/SIg-.1,6,7 Ambiguous cases of CD10+ B-lineage marker-positive blasts (5%-15% CD10 positivity) were rechecked by a second antibody (BD Biosciences, Dako). Only consistently negative samples (< 20% positive cells) were considered for CD10 negativity. Molecular detection of MLL-AF4 and BCR-ABL fusion as well as cytogenetic analysis were carried out as described elsewhere.6-11 Fluorescence in situ hybridization (FISH) used directly labeled dual-color break-apart MLL rearrangement probes (Vysis, Downers Grove, IL).7 At least 200 nuclei were scored, setting the cut-off value for false-positive nuclei at 10%. Patients were treated according the German Multicenter Adult ALL (GMALL) trials,1,6,12 allocating MLL-AF4+ patients to the high-risk arm.13 The median follow-up was 25.6 months (range, 1.8-70.4 months) for survivors. The protocols were reviewed and approved by institutional review boards at each of the participating sites, and all patients provided informed consent prior to enrollment.
Results and discussion
Prevalence of CD10- pre-B ALL within B-lineage ALL
Immunophenotyping identified 382 pro-B ALL, 335 pre-B ALL, and 1621 c-ALL specimens. In addition, 70 ALL specimens were positive for TdT, CD19, and cyIgM but did not express CD10 in an analysis with 2 antibodies. These samples were assigned to the unusual subtype of CD10- pre-B ALL.
Prevalence of MLL-AF4 transcripts and 11q23/MLL translocations in B-lineage ALL
A 50% prevalence of MLL-AF4 fusions (112 of 211 positive samples) was evident in pro-B ALL.
All CD10+ pre-B ALL specimens were negative for MLL-AF4 fusion transcripts (n = 52 tested with reverse transcription-polymerase chain reaction [RT-PCR]) or an 11q23 translocation (n = 45 analyzed with FISH or cytogenetics). Similarly, c-ALL (CD10+cyIgM-) correlated with an exclusively MLL-AF4- (0 of 77 positive samples) and t(4;11)- (264 samples analyzed by chromosome banding) genotype.
In 57 CD10- pre-B ALL sufficient cells were available for molecular or cytogenetic analysis. One patient was excluded because molecular genetic analysis was performed only at late relapse. All 56 evaluable CD10- pre-B ALL samples were BCR-ABL-. Forty-two samples revealed MLL rearrangement (RT-PCR 27, cytogenetics t(4;11) 3, t(11;19) 1, FISH 1, RT-PCR/cytogenetics 9, FISH/RT-PCR 1). Four samples were assigned MLL rearrangement positivity including one specimen with an MLL-AF4 fusion (RT-PCR) and a normal karyotype, and 3 samples with an MLL deletion (n = 1) or an MLL translocation detected with FISH and a MLL-AF4- RT-PCR result. Ten patients were MLL rearrangement-negative (RT-PCR 3, cytogenetics 1, FISH 1, FISH/RT-PCR 2, cytogenetics/RT-PCR 1, cytogenetics/FISH/RT-PCR 2).
Features and outcome of CD10 pre-B ALL
MLL rearrangement-positive patients were characterized by a higher median white blood cell count (70 150/μL versus 9200/μL; P = .001) and their blasts showed more frequent CD65 expression (67% versus 22%; P = .01; Table 1). The presence of neuroglial antigen 2 chondroitin sulfate proteoglycan (NG2) was indicative of an MLL rearrangement in CD10- pre-B ALL (P = .02).
Clinical and immunologic features of 56 CD10- pre-B ALL
Characteristics . | CD10– pre-B ALL MLL– . | CD10– pre-B ALL MLL+ . | Total . | P . |
|---|---|---|---|---|
| No. | 10 | 46 | 56 | |
| Sex | ||||
| Male | 1 | 15 | 16 | NS |
| Female | 9 | 32 | 41 | NS |
| Median age, y, (range) | 42.5 (18-78) | 39.5 (15-71) | 40 (15-78) | NS |
| WBC count, × 106/L* | ||||
| Less than 30 000 | 8 | 9 | 17 | NS |
| Greater than 30 000 | 1 | 36 | 37 | <.001 |
| Range | 1600-43 600 | 2600-735 000 | 1600-735 000 | – |
| Median | 9200 | 70 150 | 50 000 | .001 |
| Immunophenotype, n/total | ||||
| CD34 | 8/10 | 18/46 | 26/56 | .02 |
| TdT | 10/10 | 40/46 | 50/56 | NS |
| CD33 greater than 20%† | 2/10 | 3/45 | 5/55 | NS |
| CD65 greater than 20%* | 2/9 | 30/45 | 32/54 | .01 |
| NG2 greater than 20%‡ | 1/2 | 18/18 | 19/20 | .002 |
Characteristics . | CD10– pre-B ALL MLL– . | CD10– pre-B ALL MLL+ . | Total . | P . |
|---|---|---|---|---|
| No. | 10 | 46 | 56 | |
| Sex | ||||
| Male | 1 | 15 | 16 | NS |
| Female | 9 | 32 | 41 | NS |
| Median age, y, (range) | 42.5 (18-78) | 39.5 (15-71) | 40 (15-78) | NS |
| WBC count, × 106/L* | ||||
| Less than 30 000 | 8 | 9 | 17 | NS |
| Greater than 30 000 | 1 | 36 | 37 | <.001 |
| Range | 1600-43 600 | 2600-735 000 | 1600-735 000 | – |
| Median | 9200 | 70 150 | 50 000 | .001 |
| Immunophenotype, n/total | ||||
| CD34 | 8/10 | 18/46 | 26/56 | .02 |
| TdT | 10/10 | 40/46 | 50/56 | NS |
| CD33 greater than 20%† | 2/10 | 3/45 | 5/55 | NS |
| CD65 greater than 20%* | 2/9 | 30/45 | 32/54 | .01 |
| NG2 greater than 20%‡ | 1/2 | 18/18 | 19/20 | .002 |
Each column gives the number of patients with a specific feature. The χ2 test was used to compare the clinical parameters of MLL rearrangement-positive and -negative patients. Median values were compared by the 2-sided Wilcoxon Mann-Whitney test.
NS indicates not significant; WBC, white blood cell; and –, not done.
Data missing for 2 patients
Data missing for 1 patient
Data missing for 36 patients
Forty patients with CD10- pre-B ALL were evaluable for the treatment response and outcome. Seven additional patients were excluded from the GMALL trials due to age (n = 2), prior malignancies (n = 2), or other reasons (n = 3). Nine patients were not treated and documented within the GMALL trials.
A complete remission (CR) was achieved in 6 of 7 (86%) MLL rearrangement-negative and in 27 of 33 (82%) MLL rearrangement-positive patients (P = .06). Five (18%) MLL rearrangement-positive patients failed to respond to induction therapy, and one MLL rearrangement-negative patient died within 56 days (early death).
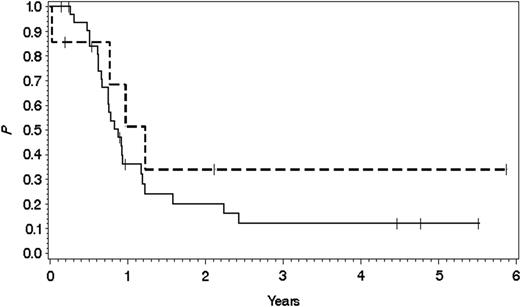
Overall survival probability in MLL rearrangement-positive versus MLL rearrangement-negative CD10- pre-B ALL. Overall survival probability in 7 patients with MLL rearrangement-negative CD10- pre-B ALL (0.34 ± 0.20 SE; top broken line) and in 33 with MLL rearrangement-positive CD10- pre-B ALL (0.12 ± 0.06 SE; bottom solid line) after treatment in the GMALL studies 04/89, 05/93, 06/00, and 07/03. Median follow-up was 354 days for MLL rearrangement-negative versus 278 days for MLL rearrangement-positive patients.
Overall survival probability in MLL rearrangement-positive versus MLL rearrangement-negative CD10- pre-B ALL. Overall survival probability in 7 patients with MLL rearrangement-negative CD10- pre-B ALL (0.34 ± 0.20 SE; top broken line) and in 33 with MLL rearrangement-positive CD10- pre-B ALL (0.12 ± 0.06 SE; bottom solid line) after treatment in the GMALL studies 04/89, 05/93, 06/00, and 07/03. Median follow-up was 354 days for MLL rearrangement-negative versus 278 days for MLL rearrangement-positive patients.
CR was maintained by 2 of 6 (33.3%) MLL rearrangement-negative and 7 of 27 (26%) MLL rearrangement-positive patients. Thus, the probability of remission duration after 3 years was 0.37 ± 0.29 SE in MLL rearrangement-negative versus 0.28 ± 0.13 SE in MLL rearrangement-positive patients. Eight patients (7 MLL rearrangement-positive) underwent stem-cell transplantation in the first CR and 5 of these patients survived (all MLL rearrangement-positive).
The presence of a CD10- pre-B ALL immunophenotype was indicative of a poor outcome (279 days; median overall survival 278 days in MLL rearrangement-positive versus 354 days in MLL rearrangement-negative patients). The overall survival probability 3 years after diagnosis accounted for 0.34 ± 0.20 SE in MLL rearrangement-negative versus 0.12 ± 0.06 SE in MLL rearrangement-positive patients (Figure 1). For patients who achieved CR, the survival probability 3 years after diagnosis was 0.40 ± 0.22 SE in MLL rearrangement-negative versus 0.14 ± 0.08 SE in MLL rearrangement-positive CD10- pre-B ALL.
Studies on immunologic markers and other cell-phenotype expressions have provided valuable clues for diagnosing and classifying ALL according to cell-lineage affiliation and differentiation.1,14 Numerous reports have been published on chromosomal translocations and mutations in human leukemias (for an overview, see Pui et al15 ) and have promoted the development of risk-adapted therapies.13
The results of our analysis underline the importance of a distinct CD10- pre-B ALL subtype characterized by a high prevalence of MLL rearrangements indicating involvement of a primary genetic event.16 Interestingly, 90% of the cases in which the blasts were tested reacted with antibody 7.1,7 adding NG2 expression to the main characteristics of MLL rearrangement-positive CD10- IgM+ pre-B ALL (100% positivity).
Regarding the therapeutic outcome, a CR could be achieved in more than 80% of MLL rearrangement-positive CD10- pre-B ALL, which goes beyond the results obtained for MLL rearrangement-positive pro-B ALL in the GMALL trials 03/87 and 04/891 and correlates favorably with the CR rate given for standard-risk BCR-ABL- pre-B ALL and c-ALL patients in the GMALL 05/93 trial.6 With one exception, all MLL rearrangement-positive patients with CD10- pre-B ALL received high-dose cytosine-arabinoside induction therapy but were nevertheless found to have a clearly lower chance of maintaining CR than those with, for example, BCR-ABL- pre-B and c-ALL or even MLL rearrangement-positive pro-B ALL, the latter comprising a well-known therapeutic high-risk group.1 The adverse outcome is illustrated by a median overall survival rate within and below the range of values reported for BCR-ABL+ ALL patients6 and may be influenced by the high white blood cell counts of most MLL rearrangement-positive CD10- pre-BALL.13
CD10-cyIgM+ pre-B ALL identifies a distinct immunophenotypic entity of partly differentiated precursor B-lineage ALL with intracytoplasmic immunoglobulin expression. Recognition of this subtype is important because it was observed in 17% of all pre-B ALL specimens and is characterized by a high prevalence of MLL-AF4 fusion transcripts. The overall survival was low in MLL rearrangement-positive patients and although our data did not analyze different therapeutic options, stem-cell transplantation in the first CR might improve the results. On the other hand, for the small subgroup of MLL rearrangement-negative CD10-cyIgM+ pre-B ALL survival ranged within the treatment results achieved in standard-risk adult ALL.6 Further molecular analysis should help to elucidate the status of CD10- pre-B ALL compared to other MLL rearrangement-positive acute leukemias, which have been related to 2 distinct expression profiles17,18 and are thought to result from an MLL translocation-induced arrest at an early stage of B-cell development.18
Appendix
Institutions participating in the GMALL study group are as follows: Augsburg: Zentralklinik (D. Renner, G. Schlimock); Bonn: Universitätsklinik (T. Sauerbruch,A. Glasmacher); Duisburg: St Johannes-Hospital (C. Aul, A. Giagounidis); Düren: Krankenhaus Düren (J. Karow, M. Engels); Düsseldorf: Universitätsklinik (R. Haas, A. Heyll); Essen: Klinik der GHS (U. Dührsen, C. Rosenthal); Universitätsklinik (S. Seeber, M.R. Nowrousian); Frankfurt: Universitätsklinik (D. Hoelzer, B. Wassmann, O.G. Ottmann); Göttingen: Universitätsklinikum (L. Trümper, F. Griesinger); Graz: Universitätsklinik (W. Linkesch, C. Tinchon); Greifswald: Ernst-Moritz-Arndt-Universität (G. Dölken, M. Schwenke); Hamburg: Krankenhaus St Georg (N. Schmitz, T. Colberg); Krankenhaus Altona (D. Braumann, P. Hoelzer); Hamm: Evangelische Krankenhaus (L. Balleisen; A. Grote-Metke); Hannover: Medizinische Hochschule (A. Ganser, H. Diedrich); Heidelberg: Universitätsklinik (A. Ho, G. Egerer); Jena: Universitätsklinik (K. Höffken, H.-J. Fricke); Kaiserslautern: Westpfalz Klinikum (H. Link, F.-G. Hagmann); Kassel: Klinikum (W.-D. Hirschmann, B. Ritter); Kiel: Universitätsklinik (K. Kneba, F. Gieseler); Lübeck: Universitätsklinik (T. Wagner, S. Peters); Ludwigshafen am Rhein: Klinikum (M. Uppenkamp, B. Claus); Mainz: Universitätsklinik (C. Huber, J. Beck); Mannheim: Klinikum (R. Hehlmann, A. Weiss); Marburg: Klinikum (A. Neubauer, M. Jänike); München: Klinikum Rechts der Isar (C. Peschel, F. Schneller); Universitätsklinik Grosshadern (R. Hiddemann; Lenz); Münster: Universitätsklinik (W.E. Berdel, M. Stelljes); Neubrandenburg: Klinikum (H. Rühle, N. Grobe); Nürnberg Klinikum Nord (W. Gallmeier, H. Wandt), Offenburg Klinikum (F. Hirsch, I. Dresel); Potsdam Ernst von Bergmann Klinik (R. Pasold, F. Rothman); Regensburg Universitätsklinik (R. Andreesen, M. Gnad); Stuttgart Bürgerhospital (H.C. Benöhr, W. Grimminger); Tübingen Universitätsklinik (L. Kanz, M. Schmalzinger); Ulm Universitätsklinik (H. Döhner, M. Schmid); Würzburg Universitätsklinik (K. Wilms, M. Wilhelm).
Prepublished online as Blood First Edition Paper, August 25, 2005; DOI 10.1182/blood-2005-05-1866.
A complete list of the members of the GMALL study group appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The excellent technical assistance of P. Havemann, A. Sindram, and B. Komischke is gratefully acknowledged.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal